Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5203
Peer-review started: January 27, 2021
First decision: February 11, 2021
Revised: February 21, 2021
Accepted: May 15, 2021
Article in press: May 15, 2021
Published online: July 6, 2021
Processing time: 147 Days and 16.9 Hours
Retroperitoneal hemorrhage (RPH) is a rare and severe complication in patients undergoing extracorporeal membrane oxygenation (ECMO). Clinical diagnosis is difficult.
Three cases of RPH patients with corona virus disease-19 (COVID-19) were included in this study. All three suffered from respiratory failure, were treated with veno-venous or veno-arterial-venous ECMO, and experienced RPH during ECMO treatment. Two of the COVID-19 cases were diagnosed after the patients experienced abdominal pain. The other patient exhibited decreases in the ECMO circuit flow rate and hemoglobin level. Two cases were treated by transcatheter arterial embolization, and one was treated conservatively. The hemorrhage in each of the three cases did not deteriorate. Satisfactory treatment results were achieved for the three patients because of prompt diagnosis and treatment.
Although the incidence of RPH during ECMO treatment is low, the risk is increased by anticoagulant use and local mechanical injury. If declines in blood flow velocity and hemoglobin are detected, RPH should be considered, and prompt aggressive therapy should be started.
Core Tip: Retroperitoneal hemorrhage (RPH) is a rare complication during the application of extracorporeal membrane oxygenation (ECMO). Corona virus disease-19 (COVID-19) is often accompanied by coagulation disorders, and the use of heparin in patients with severe COVID-19 patients during ECMO treatment increases the bleeding risk. Three COVID-19 patients who experienced RPH during ECMO treat
- Citation: Zhang JC, Li T. Delayed retroperitoneal hemorrhage during extracorporeal membrane oxygenation in COVID-19 patients: A case report and literature review. World J Clin Cases 2021; 9(19): 5203-5210
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5203.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5203
Extracorporeal membrane oxygenation (ECMO) provides gas exchange and supports cardiac function in patients with acute respiratory distress syndrome (ARDS) or cardiac failure[1]. Since the publication of the Conventional Ventilatory Support vs Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial results in 2009, veno-venous ECMO has been widely used to treat severe ARDS[2,3], bur as an important salvage therapy, ECMO can be difficult to perform and maintain. A significant challenge for ECMO management is achieving and mainta
COVID-19 is currently spreading worldwide, and has a mortality rate as high as 10% in the intensive care units (ICU)[6] of some countries. No specific treatments have yet been developed for COVID-19, and the majority of deaths are caused by respiratory failure[7]. The application of ECMO in COVID-19 patients with severe respiratory failure is currently controversial[8,9]. In our center, twelve COVID-19 patients were treated with ECMO. Of those, seven eventually had the ECMO removed and are recovering. Three COVID-19 patients who experienced retroperitoneal hemorrhage (RPH) during ECMO are described in this report. A literature review on similar presentations is provided, and the experience of the present authors in treatment is also summarized.
Three patients were admitted to the hospital with fever complicated by manifestations of pulmonary infection.
Three patients came to the clinic for treatment because of fever with symptoms of respiratory infection of several days duration. Chest computed tomography (CT) revealed the presence of ground-glass opacities in the lungs. SARS-CoV-2 was confirmed by a nasopharyngeal specimen that was PCR- positive.
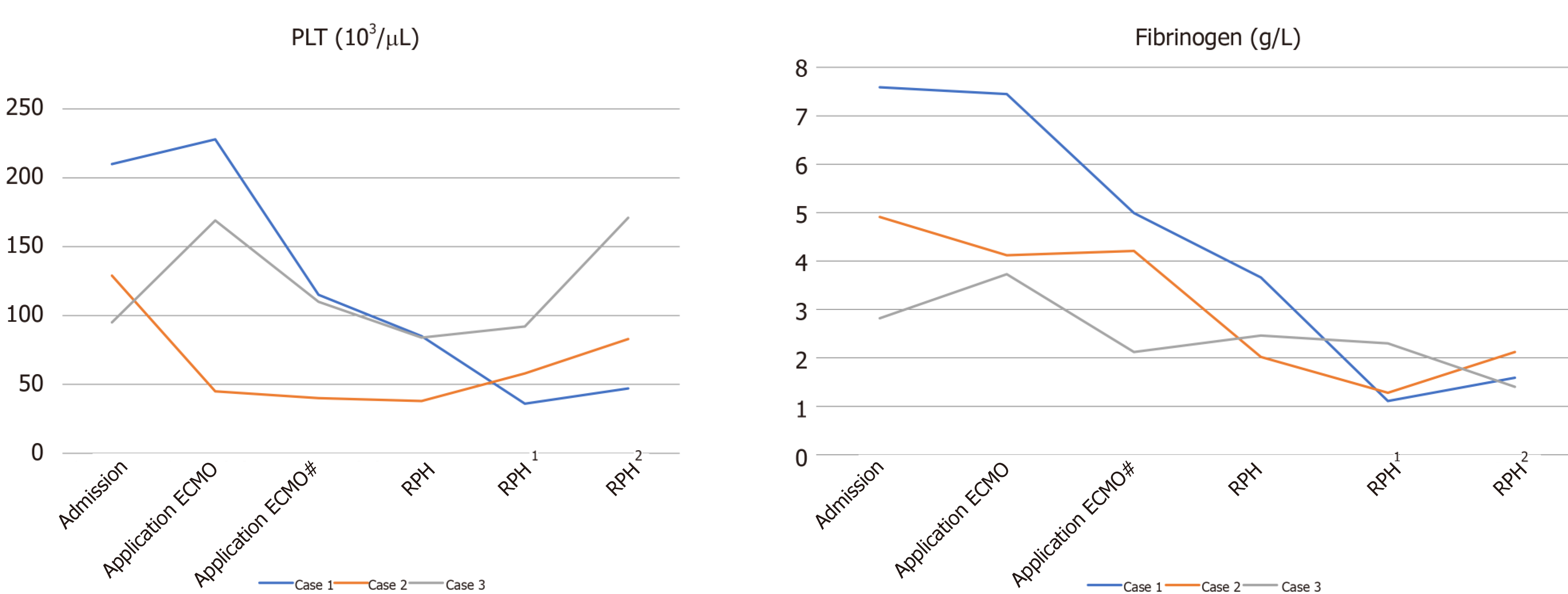
Case 1: A 71-year-old man was subjected to salvage ECMO on day 10 after COVID-19 identification. The parameters for the ECMO settings are shown in Table 1, and the clinical course of the patient is shown in Figure 1. On the day 4 of ECMO, the assessment was satisfactory, tracheal intubation was replaced with awake ECMO, and rehabilitation exercise was initiated. The training involved sitting daily and passive lower limb functional training.
| Case 1 | Case 2 | Case 3 | |
| Mode | V-V | V-A-V | V-V |
| Return cannula location | Right femoral vein | Left femoral vein/ left femoral artery | Right femoral vein |
| Return cannula size (F) | 19 | 19/17 | 19 |
| Drainage cannula location | Right internal jugular vein | Right internal jugular vein | Right internal jugular vein |
| Drainage cannula size(F) | 22 | 22 | 22 |
| Initial bolus venous injection heparin dosage (U/kg) | 25 | 25 | 50 |
| Objective APTT (s) | 40-60 | 40-60 | 60-80 |
| ECMO start running to bleeding time (d) | 8 | 9 | 18 |
Case 2: An 81-year-old woman underwent salvage ECMO after 28 d of confirmed COVID-19. The ECMO settings are shown in Table 1. Following heart failure with veno-venous ECMO, veno-arterial-venous ECMO was given on the day 2 to day 6. A 17F left femoral artery tube was used. The arterial end was closed with a vascular stapler, and the operation mode was changed to veno-venous ECMO. The sedation level was maintained at a Richmond Agitation Sedation Scale (RASS) score of −2 to −3. The clinical course is shown in Figure 1.
Case 3: A 62-year-old man experienced salvage ECMO after 31 d of confirmed COVID-19. The ECMO settings are shown in Table 1. Digestive tract bleeding occurred during ECMO for 13 d, and rectal cancer was diagnosed. Radical rectal cancer surgery was performed with ECMO support. After 11 d of ECMO, mechanical ventilation was withdrawn and replaced by awake ECMO. Rehabilitation exercises included sitting on the bedside, use of an electric bicycle, and limb rehabilitation exercises. The clinical course is shown in Figure 1.
Cases 1 and 2 had histories of hypertension, and case 2 had a history of diabetes. Case 3 had a history of bloody stools and during hospitalization was found to have rectal cancer.
None of the patients had a history of drug allergies or genetic diseases.
The symptoms on admission included cough, expectoration of white sputum, and body temperatures of 37.8ºC to 38.5°C. Coarse breath sounds in both lungs with wet rales distributed at the base were heard on auscultation.
Case 1: After 8 d of ECMO, the patient complained of abdominal pain, and a blood evaluation revealed a hemoglobin (Hb) of 60 g/L, hematocrit of 26.2%, and a lactic acid concentration of 18 mmol/L, accompanied by low ECMO circuit flow rates.
Case 2: After 9 d of ECMO, the circuit flow rate was low and was accompanied by a decrease of Hb from 84 g/L to 53 g/L. The patient’s hematocrit was 23.4%.
Case 3: The patient’s vital signs included a decrease in blood pressure to 85-100/50-65 mmHg, the heart rate was 110-135 bpm, Hb dropped from 77 g/L to 44 g/L, and the hematocrit was 21.1%.
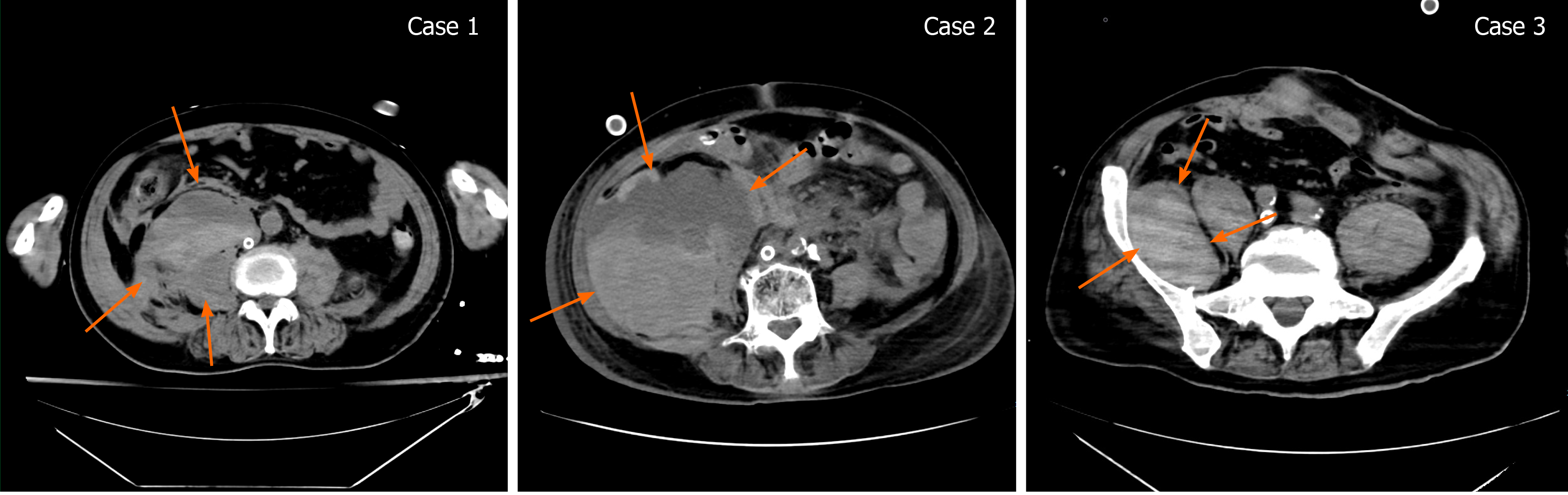
Case 1: After 8 d of ECMO, the patient complained of abdominal pain. An abdominal CT found an RPH of 10.4 cm × 8.6 cm × 13.2 cm (Figure 2).
Case 2: After 9 d on ECMO, the circuit flow rate was low and accompanied by a decrease of Hb. A CT scan revealed an RPH of 10.6 cm × 10.3 cm × 17.3 cm (Figure 2).
Case 3: The oxygenator was replaced after 16 d of ECMO. After 18 d, the patient complained of abdominal pain. The vital signs included a decrease in blood pressure to 85-100/50-65 mmHg and a heart rate of 110-135 bpm. Ultrasound revealed edema of the psoas muscle, and a CT scan revealed an RPH with a size of 8.2 cm × 4.2 cm × 6.5 cm (Figure 2).
Critically ill with COVID-19, ECMO support, and RPH.
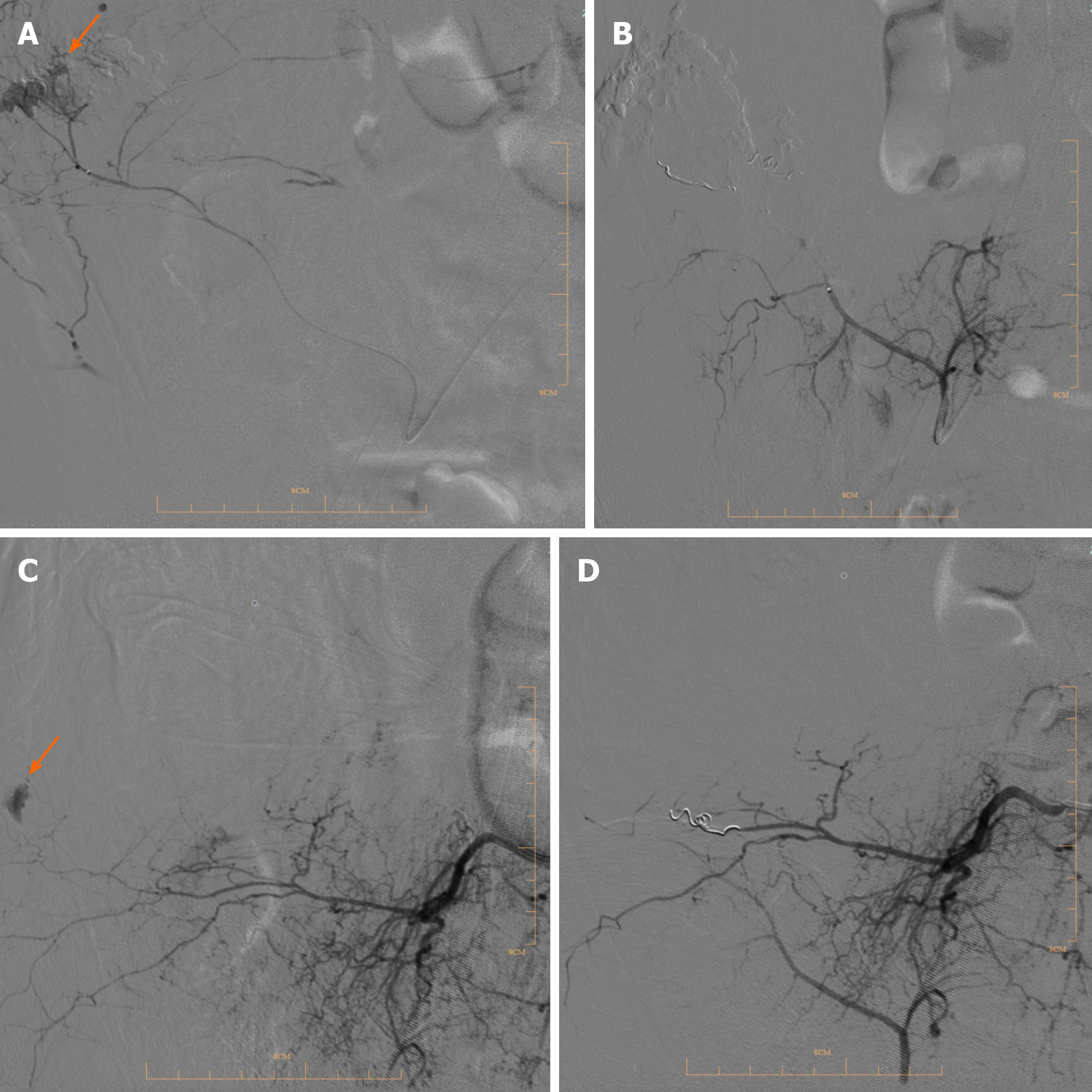
Case 1: RBCs (14 units) and fresh-frozen plasma (10 units) were transfused and anticoagulation was stopped. Digital subtraction angiography (DSA) results revealed bleeding in the right lumbar artery (Figure 3A), which was treated by performing transcatheter embolization (Figure 3B).
Case 2: Red blood cells (6 units) and fresh-frozen plasma (6 units) were transfused, and anticoagulation was stopped. DSA revealed an arterial hemorrhage in the right lower right lumbar artery (Figure 3C) that was treated by transcatheter arterial embolization (TAE) (Figure 3D).
Case 3: The heparin infusion was suspended at the same time that eight units of RBCs were given. Out-of-bed rehabilitation was suspended. Ultrasound and an abdominal CT scan revealed a stable hemorrhage.
After treatment, none of the RPHs become worse. Follow-up ultrasound and CT scans did not find any deterioration and confirmed that the hemorrhages were stable. Heparin and rehabilitation exercises were resumed 7 d after the discovery of hematoma in case 3. There was no significant change in the RPH after follow-up until discharge.
Severe COVID-19 is associated with abnormal coagulation, and patients with abnormal hemostasis were identified early in the pandemic[10-13]. Hemorrhage has not been found to be a distinctive feature of the disease. Hemostasis-related laboratory findings include minimal prolongation of prothrombin time (PT), mild reduction in platelet count, increased fibrinogen levels and no schistocytes[14]. Subsequent studies have observed a high incidence of pulmonary congestion with microvascular thrombosis, and vascular occlusion events such as limb ischemia and stroke[15]. Treatment recommendations include early intensive anticoagulant therapy in hospitalized COVID-19 patients with the expectation that concurrent anticoagulant therapy may reduce mortality[16-19].
A recent study reported an overall hemorrhage rate of 4.8% (7.6% in critically ill patients) and a massive hemorrhage rate of 2.3% (5.6% in critically ill patients) in COVID-19 patients[20]. Occult hemorrhage events can go undetected because of difficulties in imaging diagnosis in critically ill patients, which in combination with clinical findings can result in an underestimation of the hemorrhage rate. Few hemorrhage events have been reported in COVID-19 patients, and were usually associated with the use of antiplatelet drugs and anticoagulant therapy. Previous studies, such as one by Zhang et al[21] have reported that the risk of massive hemorrhage in COVID-19 patients is associated with platelet or fibrinogen reduction. Investigation of abnormal coagulation in COVID-19 patients has focused on thrombotic events, but hemorrhage remains an important prognostic factor in severe COVID-19.
SARS-CoV-2 virus infection can cause coagulation disorders in COVID-19 patients, making heparin anticoagulation difficult in patients treated with ECMO. Hemorrhage in ECMO patients is more common at surgical incision or ECMO intubation sites and in the nose and airways[22]. Hemorrhage from cannula and surgical sites remains a significant problem and is associated with poor ECMO survival rates[23]. In addition, although being a rare complication in spontaneous hemorrhage, RPH has a significantly high mortality rate. Reports of RPH can be attributed to the occurrence of difficult cannulation or mal-cannulation, with RPHs related to those events occurring within 5 d of initiating ECMO[24]. RPHs have not often been observed after 7 d on ECMO.
The hypercoagulable state of COVID-19 patients increases platelet and fibrinogen consumption in the ECMO circuit, which eventually leads to hemorrhage diathesis, such as RPH. Excessive consumption of coagulation factors and platelets in ECMO patients can be prevented by the application of heparin, inappropriate use can increase the risk of hemorrhage. The three COVID-10 patients in this study were elderly patients who underwent ECMO for severe ARDS. Low platelet levels (< 50 103/μL) during ECMO have been acknowledged as a reason for RPH formation. All three patients had low platelet counts before the development of RPH[25].
Consumption of coagulating substances is also regarded as an important factor influencing hemorrhage events in ECMO patients. In case 3, the ECMO oxygenator failed and was replaced before the RPH occurred. Replacement gave rise to a transient consumption of coagulation substances, which may have been associated with the subsequent hemorrhage events, and should cause alarm.
In addition to antithrombotic therapy, other causes of retroperitoneal hemorrhage include trauma and benign and malignant renal tumors[26]. The three cases of RPH exhibited multiple diffuse bleeding, in which trauma should be considered first. The recovery period of COVID-19 was long, and protective clothing increased the difficulty of caretaking by the nursing staff. Inadequate attention to body position, particularly the waist and back, and the frequent prone position required for the treatment of patients and imaging examinations can cause long-term compression of the waist and back and affect blood circulation[27]. Another possible issue is hemorrhage from rehabilitation injuries, including frequent body position changes, weight training, and lower limb exercise. Hence, to reduce the incidence of RPH, specific procedures should be developed for elderly patients with ECMO.
RPH during ECMO has been previously reported, but there is a scarcity of discussion on the etiology and early diagnosis[28,29]. The three cases occurred at our center at the same time, and the pathogenesis and possible causes were analyzed. These three patients received care and active support in the ICU, including blood transfusions, coagulopathy reversal, and radiation interventions. The case 1 and case 2 patients achieved hemostasis within 2h following transcatheter embolization. The case 3 patient was treated by corrected blood coagulation to reduce postural injury and recover from injury. If patients present with a significant decrease in hemoglobin and exhibit shock, prompt TAE is recommended[30].
COVID-19 can cause coagulation disorders that may be exacerbated by hemorrhage and thrombotic events that are associated with ECMO. ECMO treatment for COVID-19 patients, especially elderly patients, should be implemented in accordance with the specific circumstances of the individual.
Suggested management includes a combination of awake ECMO and rehabilitation for elderly patients, standard anticoagulation reduction, close monitoring of changes in blood coagulation function and hemoglobin and routine ultrasound examination to detect the occurrence of retroperitoneal hemorrhage. If ECMO patients undergo changes in position, then a patient transfer board and other aided measures are recommended. If patients complain of lower back pain with decreased Hb or decreased ECMO flow, retroperitoneal hemorrhage should be considered.
Manuscript source: Unsolicited manuscript
Specialty type: Critical Care Medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chrcanovic BR, Gaid D S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Xing YX
| 1. | White A, Fan E. What is ECMO? Am J Respir Crit Care Med. 2016;193:P9-P10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2740] [Cited by in RCA: 2332] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 3. | Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, Harvey C, Cordingley JJ, Price S, Vuylsteke A, Jenkins DP, Noble DW, Bloomfield R, Walsh TS, Perkins GD, Menon D, Taylor BL, Rowan KM. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 615] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 4. | Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A; EOLIA Trial Group; REVA, and ECMONet. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018;378:1965-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1417] [Cited by in RCA: 1485] [Article Influence: 212.1] [Reference Citation Analysis (0)] |
| 5. | Asakura H, Ogawa H. Overcoming bleeding events related to extracorporeal membrane oxygenation in COVID-19. Lancet Respir Med. 2020;8:e87-e88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, Cattaneo S, Cereda D, Colombo S, Coluccello A, Crescini G, Forastieri Molinari A, Foti G, Fumagalli R, Iotti GA, Langer T, Latronico N, Lorini FL, Mojoli F, Natalini G, Pessina CM, Ranieri VM, Rech R, Scudeller L, Rosano A, Storti E, Thompson BT, Tirani M, Villani PG, Pesenti A, Cecconi M; COVID-19 Lombardy ICU Network. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 1054] [Article Influence: 210.8] [Reference Citation Analysis (0)] |
| 7. | Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3736] [Cited by in RCA: 3197] [Article Influence: 639.4] [Reference Citation Analysis (0)] |
| 8. | Henry BM. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 9. | MacLaren G, Fisher D, Brodie D. Preparing for the Most Critically Ill Patients With COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation. JAMA. 2020;323:1245-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 10. | Arachchillage DRJ, Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1233-1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 11. | Obi AT, Barnes GD, Wakefield TW, Brown S, Eliason JL, Arndt E, Henke PK. Practical diagnosis and treatment of suspected venous thromboembolism during COVID-19 pandemic. J Vasc Surg Venous Lymphat Disord. 2020;8:526-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |
| 13. | Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA. 2020;324:799-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 589] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 14. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3992] [Cited by in RCA: 4040] [Article Influence: 808.0] [Reference Citation Analysis (0)] |
| 15. | Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 926] [Article Influence: 185.2] [Reference Citation Analysis (0)] |
| 16. | Thachil J, Srivastava A. SARS-2 Coronavirus-Associated Hemostatic Lung Abnormality in COVID-19: Is It Pulmonary Thrombosis or Pulmonary Embolism? Semin Thromb Hemost. 2020;46:777-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 17. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3488] [Cited by in RCA: 3408] [Article Influence: 681.6] [Reference Citation Analysis (0)] |
| 18. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18201] [Article Influence: 3640.2] [Reference Citation Analysis (0)] |
| 19. | Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, Muscarella G, Orlandi M. Pulmonary Embolism or Pulmonary Thrombosis in COVID-19? Thromb Haemost. 2020;120:1230-1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 20. | Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1669] [Cited by in RCA: 2051] [Article Influence: 410.2] [Reference Citation Analysis (0)] |
| 21. | Zhang C, Zhang Z, Mi J, Wang X, Zou Y, Chen X, Nie Z, Luo X, Gan R. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore). 2019;98:e15833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Thomas J, Kostousov V, Teruya J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin Thromb Hemost. 2018;44:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Sunga KL, Bellolio MF, Gilmore RM, Cabrera D. Spontaneous retroperitoneal hematoma: etiology, characteristics, management, and outcome. J Emerg Med. 2012;43:e157-e161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Ranney D, Hatch S, Bonadonna D, Daneshmand M. ECMO Flow as a Sign of Intraabdominal Hemorrhage After Prolonged CPR. ASAIO J. 2019;65:e55-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Balle CM, Jeppesen AN, Christensen S, Hvas AM. Platelet Function During Extracorporeal Membrane Oxygenation in Adult Patients. Front Cardiovasc Med. 2019;6:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Sugiura G, Bunya N, Yamaoka A, Okuda H, Saito M, Mizuno H, Inoue H, Narimatsu E. Delayed retroperitoneal hemorrhage during veno-venous extracorporeal membrane oxygenation: a case report. Acute Med Surg. 2019;6:180-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Ibrahim W, Mohamed A, Sheikh M, Shokr M, Hassan A, Wienberger J, Afonso LC. Antiplatelet Therapy and Spontaneous Retroperitoneal Hematoma: A Case Report and Literature Review. Am J Case Rep. 2017;18:85-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Zissin R, Ellis M, Gayer G. The CT findings of abdominal anticoagulant-related hematomas. Semin Ultrasound CT MR. 2006;27:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Sy E, Sklar MC, Lequier L, Fan E, Kanji HD. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. J Crit Care. 2017;39:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 30. | Tani R, Sofue K, Sugimoto K, Katayama N, Hamada MAS, Maruyama K, Horinouchi H, Gentsu T, Sasaki K, Ueshima E, Koide Y, Okada T, Yamaguchi M, Murakami T. The utility of transarterial embolization and computed tomography for life-threatening spontaneous retroperitoneal hemorrhage. Jpn J Radiol. 2019;37:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |