Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4559
Peer-review started: November 7, 2020
First decision: December 13, 2020
Revised: December 26, 2020
Accepted: February 4, 2021
Article in press: February 4, 2021
Published online: June 26, 2021
Processing time: 215 Days and 11.6 Hours
Careful selection of hepatocellular carcinoma (HCC) patients prior to chemoembolization treatment is a daily reality, and is even more necessary with new available therapeutic options in HCC.
To propose two new models to better stratify patients and maximize clinical benefit: “6 and 12” and “pre/post-TACE-predict” (TACE, transarterial chemoembolization).
We evaluated and compared their performance in predicting overall survival with other systems {Barcelona Clinic Liver Cancer (BCLC), Albumin-Bilirubin (ALBI) and NIACE [Number of tumor(s), Infiltrative HCC, alpha-fetoprotein, Child-Pugh (CP), and performance status]} in two HCC French cohorts of different stages enrolled between 2010 and 2018.
The cohorts included 324 patients classified as BCLC stages A/B (cohort 1) and 137 patients classified as BCLC stages B/C (cohort 2). The majority of the patients had cirrhosis with preserved liver function. “Pre-TACE-predict” and “6 and 12” models identified three distinct categories of patients exhibiting different prognosis in cohort 1. However, their prognostic value was no better than the BCLC system or NIACE score. Liver function based on CP and ALBI grades significantly impacted patient survival. Conversely, the “post-TACE-predict” model had a higher predictive value than other models. The stratification ability as well as predictive performance of these new models in an intermediate/advanced stage population was less efficient (cohort 2).
The newly proposed “Pre-TACE-predict” and “6 and 12” models offer an interesting stratification into three categories in a recommended TACE population, as they identify poor candidates, those with partial control and durable response. The models' contribution was reduced in a population with advanced stage HCCs.
Core Tip: Management of hepatocellular carcinoma (HCC) has significantly changed over the past few years. The introduction of new systemic therapies, including immune checkpoint inhibitors has improved survival, especially in patients with advanced stage HCC. Careful selection of patients prior to transarterial chemoembolization (TACE) is crucial. “Up-to-seven criteria” have been proposed for subclassification of intermediate stages. More recently, two models (“6 and 12”; “pre-TACE-predict”) have been developed to improve patient stratification and refine prognosis, overcoming the limitations of points-based scores. In this retrospective multi-center French study, we evaluated and compared these two new models to validate their prognostic value and applicability in clinical current practice.
- Citation: Adhoute X, Larrey E, Anty R, Chevallier P, Penaranda G, Tran A, Bronowicki JP, Raoul JL, Castellani P, Perrier H, Bayle O, Monnet O, Pol B, Bourliere M. Expected outcomes and patients’ selection before chemoembolization—“Six-and-Twelve or Pre-TACE-Predict” scores may help clinicians: Real-life French cohorts results. World J Clin Cases 2021; 9(18): 4559-4572
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4559.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4559
Careful selection of patients with hepatocellular carcinoma (HCC) before performing transarterial chemoembolization (TACE) treatment remains a daily reality. HCC therapeutic management in the West is based on the Barcelona Clinic Liver Cancer (BCLC) system endorsed by the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) guidelines[1,2]. However limitations in its ability both to guide this procedure and more generally to manage intermediate-stage HCCs are recognized[3]. Despite serious improvements in patient selection, TACE modalities, efficacy [using modified response evaluation criteria in solid tumors (mRECIST)][4] and discontinuation criteria[5], this treatment usually fails to achieve sustained control of the disease, despite an objective response in about 50% of patients[6]. Despite subclassification propositions for intermediate stages[7,8] using up-to-seven criteria arbitrarily, clinicians have also continued to promote scores both to refine individual prognosis and guide therapeutic decisions. “Pre- and post-TACE-predict” models[9] have been developed accordingly, in consideration of TACE daily practices, based on a very large cohort of non-surgical HCC patients, some beyond the intermediate stage. Using continuous variables, to overcome the limitations of points-based scores, and online calculators, it offers a four-group stratification with survival prediction superior to the Hepatoma Arterial-embolization Prognostic score[10] and its different versions[11]. The “six-and-twelve”[12] model is another new score designed to stratify HCC patients recommended for TACE treatment. Based on simple addition, it defines three distinct prognosis populations (the sum is ≤ 6; or > 6 but ≤ 12; or > 12), with one over twelve who will not benefit from TACE treatment. To date, no model has really proved suitable as a prognostic tool and/or as an aid to the decision-making process. We do not yet have biomarkers or driver mutations to help us select beyond classic criteria such as tumor size and number. The scores could be helpful, and their relevance is supported by emerging treatment options[13] and trials currently underway evaluating the combination of TACE plus immune checkpoint inhibitors (ICIs). Recently, the combination of TACE with sorafenib, used as an anti-angiogenic agent before the procedure, has demonstrated a benefit on progression-free survival (PFS)[14]. A simple model, which identifies one group that benefits sustainably from TACE treatment, a second one that benefits only partially by delaying progression and a third with no benefits, i.e. TACE-refractory patients, could provide valuable guidance for clinicians. The aims of this study are to assess and compare the prognostic value of these two recent models with other commonly used systems and to further explore their suitability in two French cohorts including patients with HCCs of different stages.
This retrospective study was performed on two French cohorts of HCC patients undergoing conventional (c) TACE treatment. The first cohort was a multicenter cohort (Marseille, Nancy, cohort 1) with 324 patients displaying comparable BCLC staging to those in the study by Wang et al[12], and the second cohort was a single-center cohort (Nice, cohort 2) with 137 patients displaying comparable BCLC staging to those in the study by Han et al[9]. Patients enrolled were screened from January 2010 to December 2018. HCC diagnosis was either based on imaging according to EASL[1]/AASLD[2] criteria, or histology if no cirrhosis or typical imaging of HCC was found. We selected all treatment-naïve patients not curable by resection or ablation, and those who received TACE for HCC recurrence after curative therapies. We excluded patients treated by pre-operative TACE (before resection or liver transplantation), those who received TACE in combination with other therapies (ablation or systemic therapy), those with impaired performance status (PS) > 1 and/or liver function [Child-Pugh (CP) B 8/9], and those with metastatic disease. Decision of TACE was adopted in all three centers following a multidisciplinary team discussion. Candidates selected for this retrospective analysis were therefore: (1) those with multinodular HCC featuring arterial enhancement, PS 0, CP A or B7 grade; (2) those with early HCC according to the stage treatment migration concept[1]; and (3) those with segmental or more peripheral portal venous invasion and/or slight impairment of PS (PS 1), CP A or B7 grade classified as stage C in the BCLC system. Baseline and follow-up demographic, clinical, biological and radiological characteristics were collected prospectively and analyzed retrospectively following a similar process in all three centers that regularly collaborate in the field of HCC. The local institutional review board in each center approved the study protocol.
Treatment procedure: A conventional TACE procedure was applied in each center. The protocol combined an emulsion of epirubicin (20-50 mg) and iodized oil (3-10 mL), followed by embolization with absorbable gelatin sponge particles, adjusted to the tumor volume. Selective embolization of tumor-feeding vessels was performed until blood flow discontinuation on angiography. This supra-selective approach was prioritized and systematically applied when a portal venous invasion was present. A second TACE session was delivered six to eight weeks later in case of partial disease control. Conversely, treatment was discontinued when clear progression or serious adverse events occurred. Additional TACE sessions were scheduled "on demand", following radiological and AFP results every 12 wk. Response to TACE was evaluated, after each procedure, on dynamic computed tomography (CT) and/or magnetic resonance imaging, according to the mRECIST[4], based on a measurement of the tumor's enhanced viable component. Homogeneous and dense-deposited areas of iodized oil (lipiodol) in the tumor on CT were further considered as criteria related to tumor response. Similar treatment and follow-up regimes were conducted in all three centers.
Risk stratification based on “pre-TACE-predict”, “6 and 12” and NIACE[15] [Number of tumor(s), Infiltrative HCC, AFP, CP class, and Eastern Cooperative Oncology Group (ECOG) PS] scores, Albumin-Bilirubin (ALBI)[16] grade and “post-TACE-predict” model were calculated (Table 1).
| Prognostic systems | Constituents | Risk stratification | |
| Pre-TACE-predict | Pre-TACE score was calculated according to the following equation = 0.313 × tumor number (0 = single, 1 = multifocal) + 1.252 × log10 tumor size (cm) + 0.230 × baseline log10 AFP (ng/mL) + [-0.0176 × baseline albumin (g/L)] + 0.458 × baseline log10 bilirubin (mcmol/L) + 0.437 × VI (0 = no, 1 = yes) + 0.149 × HBV (0 = no, 1 = yes) + 0.333 × alcoholic (0 = no, 1 = yes) + 0.211 × other etiology if not HCV/HBV/alcoholic (0 = no, 1 = yes) | C-1: ≤ 0.94, C-2: > 0.94 to ≤ 1.47, C-3: > 1.47 to ≤ 2.10, C-4: > 2.10 | |
| Post-TACE-predict | Post-TACE score was calculated according to the following equation 0.207 × tumor number (0 = single, 1 = multifocal) + 1.129 × log10 tumor size (cm) + 0.147 × baseline log10 AFP (ng/mL) + 0.750 × baseline log10 bilirubin (mcmol/L) + 0.447 × VI (0 = no, 1 = yes) + 0.469 × PR (0 = no, 1 = yes) + 1.143 × SD (0 = no, 1 = yes) + 1.354 × PD (0 = no, 1 = yes) | C-1: ≤ 1.82, C-2 > 1.82 to ≤ 2.49, C-3: > 2.49 to ≤ 3.37, C-4: > 3.37 | |
| 6 and 12 | 6 and 12 score = tumor size in cm + tumor number | ≤ 6/6-12/> 12 | |
| NIACE | Tumor nodules ≥ 3, Infiltrative vs Nodular HCC, AFP ≥ 200 ng/mL, Child-Pugh grade A/B, PS ≥ 1 | 1 point, 1.5 / 0 point(s), 1.5 points, 0/1.5 points, 1.5 points | ≤ 1/1.5-3/> 3 |
| ALBI | The ALBI score was calculated according to the following equation = 0.66 × log10 bilirubin - 0.085 × albumin (bilirubin level in mcmol/L and albumin level in g/L) | Grade 1: ≤ -2.60, Grade 2: > -2.60 to ≤ -1.39, Grade 3: > -1.39 | |
Continuous variables are presented as medians (interquartile range) and categorical data as frequencies (percentage). Overall survival (OS) was the endpoint used. Survival time was defined as the time interval between HCC diagnosis and death or time of last follow-up. Proportionality of the subdistribution hazards was assessed both by inspecting Schoenfeld-type residuals and by testing the correlation of these residuals with time[17]. OS were compared between staging systems using Kaplan-Meier estimates and compared using the Log-Rank test for overall comparison, and the Sidak test adjusted for multiple comparisons. Cox proportional hazards regression was performed and hazard ratios were retrieved with their 95% confidence interval (CI). One, two, and three-year predictive accuracies of staging systems were assessed using the area under receiver operating characteristic curve (AUROC). AUROCs were compared between staging systems using the Delong approach, having defined Pre-TACE-Predict and Post-TACE-Predict as references[18]. The Concordance (C)-index was also assessed and compared to determine the performance of staging systems across time. The larger the C-index, the more accurate the prognostic prediction was. C-index values were compared among each staging system using the Delong approach. All P values were considered significant at α-level = 0.05. All calculations were performed using the SAS V9.4 statistical software (SAS Institute Inc., Cary, NC, United States).
A description of patients is provided in Table 2. Both cohorts contained mostly males (85% and 92% for cohort 1 and cohort 2, respectively). Median age was 68 (62-74) years in cohort 1 and 67 (57-75) years in cohort 2. All HCC patients with cirrhosis had well-preserved liver function before first TACE session. HCCs were primarily linked to hepatitis C virus infection, high-risk alcohol consumption or non-alcoholic steatohepatitis. Liver cancers appeared multinodular in more than 50% of cases. These two cohorts varied according to BCLC staging, ECOG PS, tumor size, and mean number of TACE sessions.
| Demographic variables | Marseille/Nancy cohort, n = 324 | Nice cohort, n = 137 |
| Age, median (Q1-Q3), yr | 68 (62-74) | 67 (57-75) |
| Gender n (%) | ||
| Male/Female | 276 (85)/48 (15) | 126 (92%)/11 (8) |
| Liver disease n (%) | ||
| HCV/HBV/Alcoholism/MS/other | 129 (40)/14 (4)/122 (38)/42 (13)/17 (5) | 55 (40)/8 (6)/44 (32)/11 (8)/19 (14) |
| ECOG (PS-0/1) n (%) | 324 (100) | 69 (50)/68 (50) |
| Cirrhosis n (%) | 311 (96) | 118 (86) |
| Tumor variables | ||
| Tumor size, mm, median (Q1-Q3) | 35 (25-50) | 46 (25-70) |
| Nodule (s): n (%) 1/2/3/4/≥ 5 | 95 (29)/72 (22)/80 (25)/38 (12)/39 (12) | 20 (15)/22 (16)/63 (46)/32 (23)/0 |
| Vascular invasion | 0 | 24 (18%) |
| Laboratory variables | ||
| AFP, ng/mL, median (Q1-Q3) | 16.3 (6.0-120.3) | 18 (1-600) |
| PT (%), median (Q1-Q3) | 76 (64-88) | 90 (79-100) |
| Albumin (g/L), median (Q1-Q3) | 35 (28-38) | 37 (33-41) |
| Total bilirubin (mcmol/L), median (Q1-Q3) | 19.0 (13.7-28.7) | 11 (8-19) |
| Platelet count, 109/L, Median (IQR) | 106 (82-153) | - |
| Child-Pugh grade n (%) A/B7 | 249 (77)/75 (23) | 126 (92)/11 (8) |
| ALBI grade 1/ 2/3 n (%) | 64 (20)/230 (71)/30 (9) | 52 (38)/79 (58/6 (4) |
| BCLC stage A/B/C n (%) | 145 (45)/179 (55)/0 | 0/58 (42)/79 (58) |
| “6 and 12” score allocation n (%) | ||
| ≤ 6/6-12/> 12 | 154 (48)/163 (50)/7 (2) | 51 (37)/73 (53)/13 (10) |
| NIACE score allocation n (%) | ||
| ≤ 1/1.5-3/> 3 | 168 (52/134 (41)/22 (7) | 41 (30)/72 (52)/24 (18) |
| 1Pre-TACE allocation n (%) | ||
| C-1/C-2/C-3/C-4 | 47 (15)/144 (44/109 (34)/23 (7) | 32 (23)/35 (26)/47 (34)/23 (17) |
| TACE session, mean (SD) | 2.7 ± 1.8 | 4.0 ± 2.3 |
| Radiological response (after first TACE) | ||
| CR/PR/SD2/PD n (%) | 176 (54)/67 (21)/15 (5)/66 (20) | 34 (25)/88 (64)/6 (4)/9 (7) |
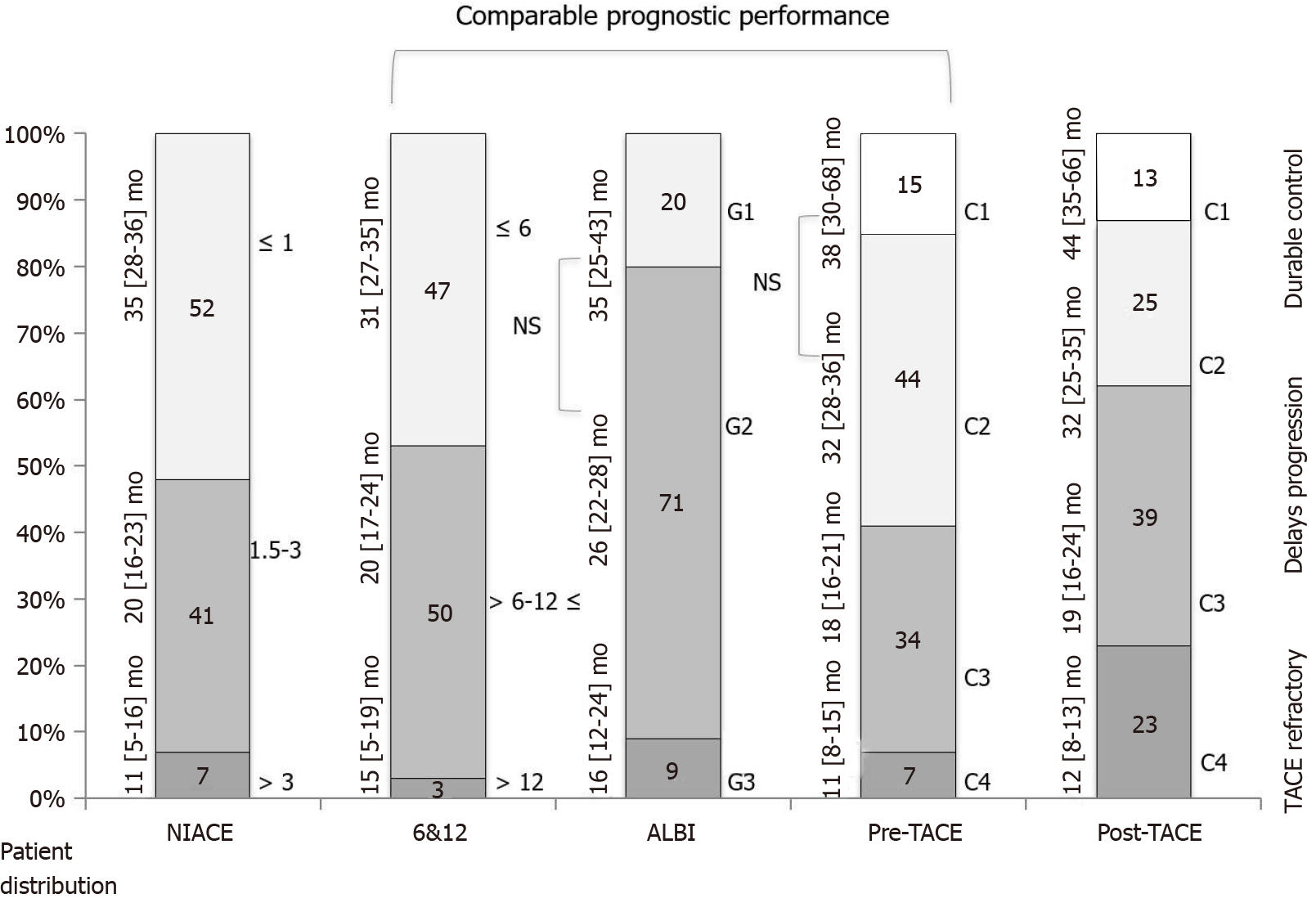
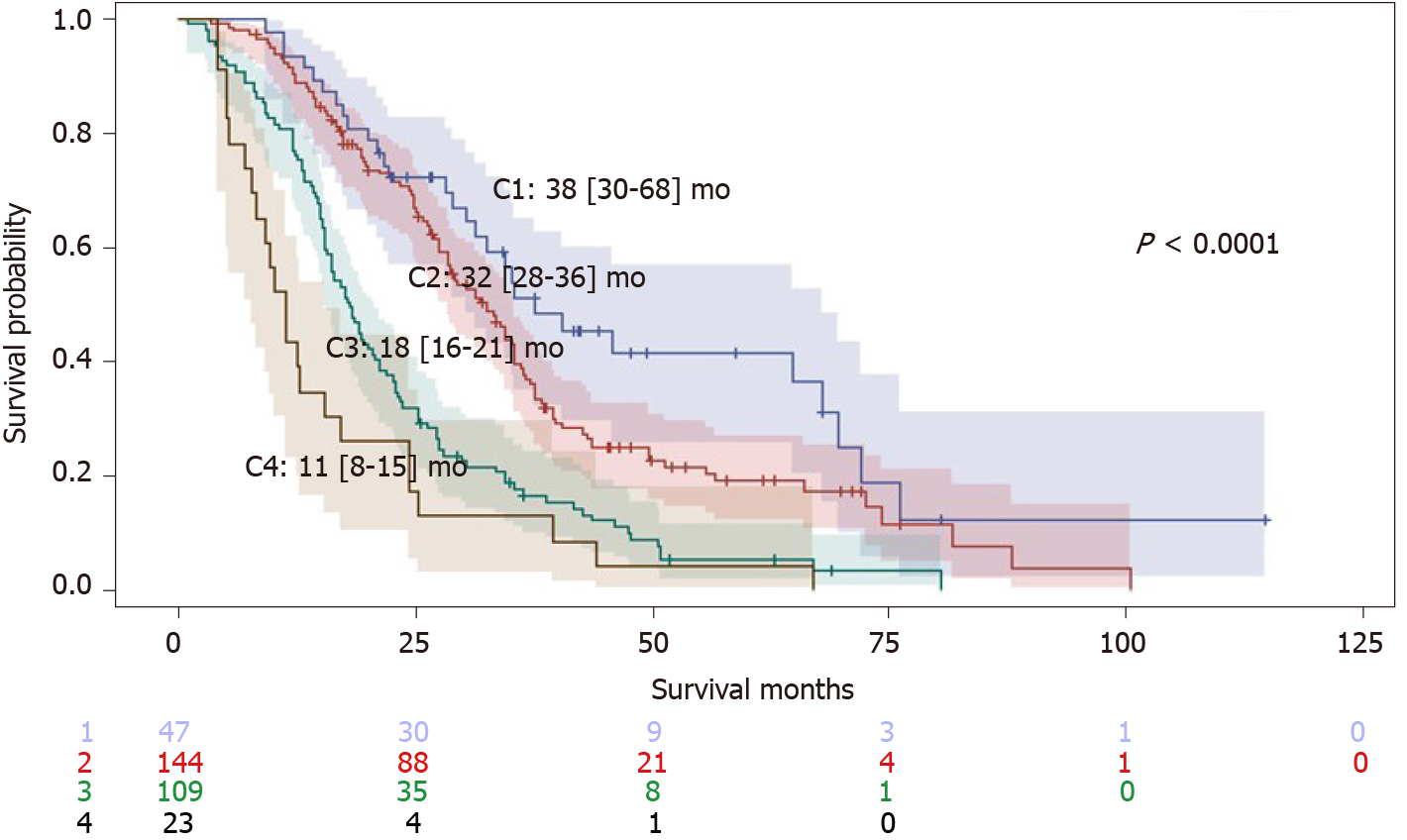
Median duration of follow-up time in that first group was 24.4 (15.0-36.8) mo. A total of 81% of patients died during this period. Kaplan-Meier analyses found that OS distribution among BCLC staging, the “6 and 12” and NIACE scores subgroups varied significantly in this multicenter cohort including early and intermediate stage HCCs (P < 0.0001 for all) (Table 3 and Figure 1). The “pre-TACE-predict” model also identified subgroups of different prognosis with survival ranging from 38 (30-68) (category 1) to 11 (8-15) mo (category 4) (P < 0.0001). However, the two lowest risk categories did not show a statistically significant difference in survival (Table 3 and Figure 2). Liver function also influenced survival outcomes; median OS varied between CP classes [CP-A, 27 (25-31) mo; CP-B7, 21 (15-24) mo (P = 0.0003)], and ALBI grades [grade 1, 35 (25-43) mo; grade 2, 26 (22-28) mo; grade 3, 16 (12-24) mo (P = 0.0029)].
| Scoring/Staging systems | OS (95%CI), mo | P value (log-rank) | Sidak1 | Hazard ratio (95%CI) | P value |
| 2Pre-TACE-predict | < 0.0001 | ||||
| Category 1 (n = 47) | 38 (30-68) | Ref | Ref | ||
| Category 2 (n = 144) | 32 (28-36) | 0.9707 | 1.45 (0.96-2.19) | 0.0769 | |
| Category 3 (n = 109) | 18 (16-21) | < 0.0001 | 2.99 (1.97-4.54) | < 0.0001 | |
| Category 4 (n = 23) | 11 (8-15) | < 0.0001 | 4.87 (2.81-8.47) | < 0.0001 | |
| 2Post-TACE-Predict | < 0.0001 | ||||
| Category 1 (n = 74) | 44 (35-66) | Ref | Ref | ||
| Category 2 (n = 125) | 32 (25-35) | 0.0725 | 1.94 (1.35-2.78) | 0.0003 | |
| Category 3 (n = 80) | 19 (16-24) | < 0.0001 | 4.33 (2.92-6.41) | < 0.0001 | |
| Category 4 (n = 44) | 12 (8-13) | < 0.0001 | 14.0 (8.89-22.15) | < 0.0001 | |
| “6 and 12” score | < 0.0001 | ||||
| Sum ≤ 6 (n = 154) | 31 (27-35) | Ref | Ref | ||
| Sum 6-12 (n = 163) | 20 (17-24) | 0.0009 | 1.55 (1.21-1.99) | 0.0005 | |
| Sum > 12 (n = 7) | 15 (5-19) | < 0.0001 | 3.80 (1.76-8.21) | 0.0007 | |
| BCLC staging | < 0.0001 | ||||
| A (n = 145) | 35 (29-38) | - | Ref | ||
| B (n = 179) | 19 (17-23) | - | 1.88 (1.47-2.41) | < 0.0001 | |
| NIACE score | < 0.0001 | ||||
| ≤ 1 (n = 168) | 35 (28-36) | Ref | Ref | ||
| 1.5-3 (n = 134) | 20 (16-23) | < 0.0001 | 1.92 (1.49-2.48) | < 0.0001 | |
| > 3 (n = 22) | 11 (5-16) | < 0.0001 | 6.23 (3.87-10.02) | < 0.0001 | |
| Child-Pugh class | 0.0003 | ||||
| A (n = 249) | 27 (25-31) | - | Ref | ||
| B (n = 75) | 21 (15-24) | - | 1.66 (1.26-2.19) | 0.0003 | |
| ALBI grade | 0.0029 | ||||
| Grade 1 (n = 64) | 35 (25-43) | Ref | Ref | ||
| Grade 2 (n = 230) | 26 (22-28) | 0.1228 | 1.50 (1.06-2.11) | 0.0216 | |
| Grade 3 (n = 30) | 16 (12-24) | 0.0016 | 2.30 (1.41-3.75) | 0.0009 |
Performances of the “pre-TACE-predict” model and other systems to predict survival are reported in Table 4. Time-dependent AUROC values and concordance-indices of the “pre-TACE-predict” model were not significantly different to those of other systems (BCLC, CP and ALBI grades), except for NIACE score that provided a better prognostication ability [C-index (pre-TACE) 0.59 (0.56-0.61) vs (NIACE) 0.70 (0.64-0.77), P = 0.0004]. The performance of the “post-TACE-predict” model outperformed other systems (BCLC, CP and ALBI grades).
| Scoring/Stage systems | 1-yr AUROC | P (vs Ref) | 2-yr AUROC | P (vs Ref) | 3-yr AUROC | P (vs Ref) | C-index | P (vs Ref) |
| Pre-TACE-predict | 0.67(0.60-0.75) | Ref | 0.60(0.56-0.64) | Ref | 0.57(0.53-0.60) | Ref | 0.59(0.56-0.61) | Ref |
| “6 and 12” score | 0.65(0.56-0.74) | 0.6040 | 0.64(0.58-0.70) | 0.1425 | 0.63(0.57-0.70) | 0.0347 | 0.66(0.58-0.73) | 0.0806 |
| BCLC staging | 0.60(0.52-0.67) | 0.0549 | 0.64(0.58-0.69) | 0.1705 | 0.61(0.55-0.67) | 0.1537 | 0.61(0.54-0.68) | 0.4459 |
| NIACE score | 0.77(0.69-0.84) | 0.0254 | 0.69(0.63-0.75) | 0.0010 | 0.69(0.63-0.75) | < 0.0001 | 0.70(0.64-0.77) | 0.0004 |
| Child-Pugh class | 0.56(0.49-0.63) | 0.0399 | 0.55(0.51-0.60) | 0.1574 | 0.54(0.49-0.59) | 0.4784 | 0.59(0.55-0.64) | 0.8075 |
| ALBI grade | 0.63(0.57-0.69) | 0.3997 | 0.56(0.51-0.61) | 0.1972 | 0.55(0.49-0.61) | 0.7351 | 0.62(0.55-0.68) | 0.3909 |
| Post-TACE-predict | 0.81(0.76-0.87) | Ref | 0.73(0.68-0.78) | Ref | 0.73(0.67-0.78) | Ref | 0.74(0.68-0.80) | Ref |
| “6 and 12” score | 0.65(0.56-0.74) | < 0.0001 | 0.64(0.58-0.70) | 0.0011 | 0.63(0.57-0.70) | 0.0029 | 0.66(0.58-0.73) | 0.0145 |
| BCLC staging | 0.60(0.52-0.67) | < 0.0001 | 0.64(0.58-0.69) | 0.0005 | 0.61(0.55-0.67) | < 0.0001 | 0.61(0.54-0.68) | 0.0002 |
| NIACE score | 0.77(0.69-0.84) | 0.1673 | 0.69(0.63-0.75) | 0.1263 | 0.69(0.63-0.75) | 0.1927 | 0.70(0.64-0.77) | 0.2562 |
| Child-Pugh class | 0.56(0.49-0.63) | < 0.0001 | 0.55(0.51-0.60) | < 0.0001 | 0.54(0.49-0.59) | < 0.0001 | 0.59(0.55-0.64) | < 0.0001 |
| ALBI grade | 0.63(0.57-0.69) | < 0.0001 | 0.56(0.51-0.61) | < 0.0001 | 0.55(0.49-0.61) | < 0.0001 | 0.62(0.55-0.68) | 0.0051 |
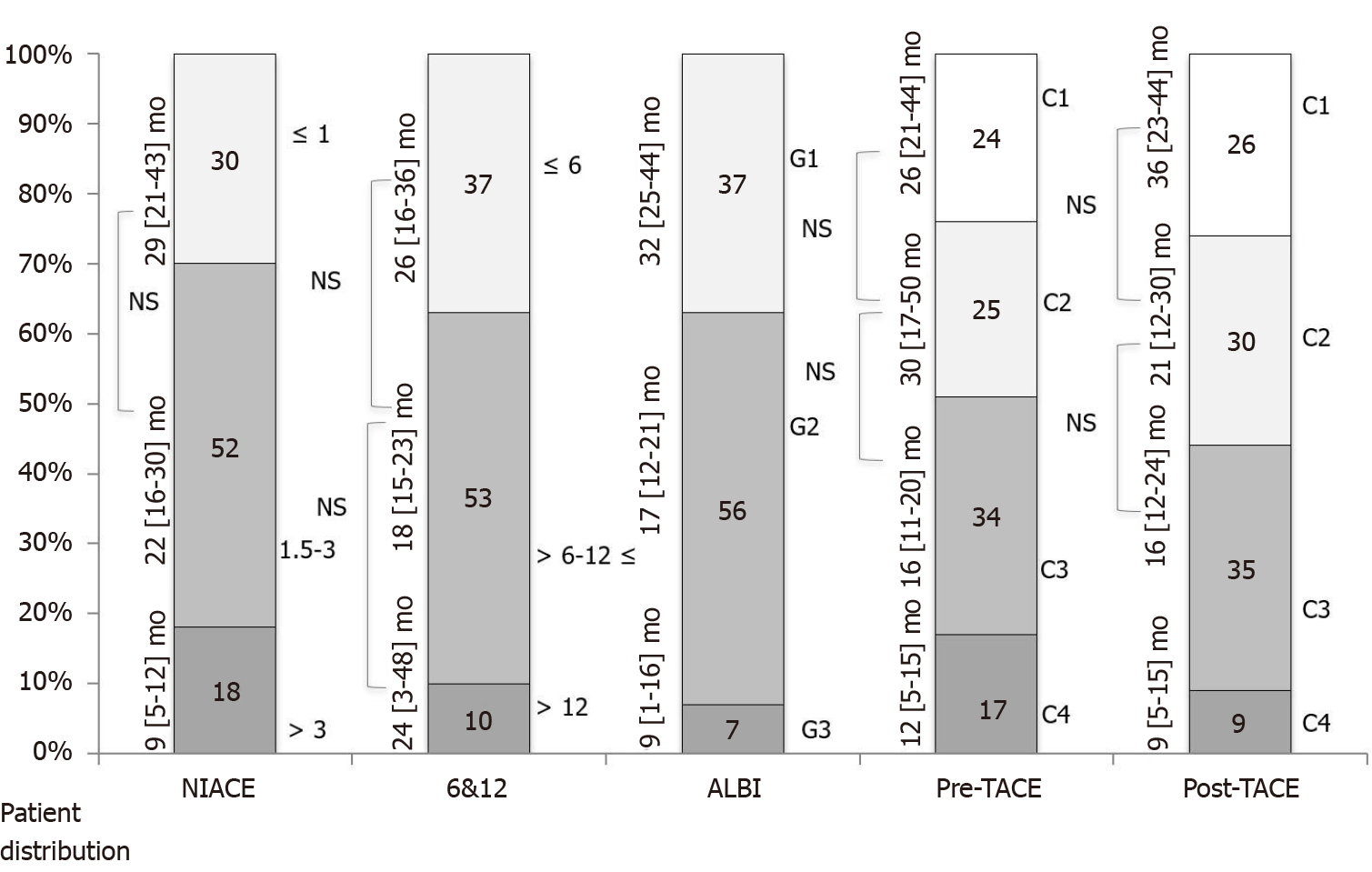
Median duration of follow-up time in the second group was 19 (10-32) mo. A total of 74% of patients died during this period. The “pre and post-TACE-predict” models score also identified subgroups of different prognosis with survival times ranging from 26 (21-44) mo (category 1) to 12 (5-15) mo (category 4) (P = 0.0032), and from 36 (23-44) mo to 9 (5-15) mo (P = 0.0022), respectively. However, there was some overlap between the three lowest risk categories when considering multiple comparisons (Table 5 and Figure 3). Kaplan-Meier analyses found that OS distribution among BCLC staging, CP and ALBI grades subgroups varied significantly. Conversely, OS distribution did not differ significantly across the “6 and 12” score subgroups within this cohort including intermediate and advanced HCCs. Time-dependent AUROC values and concordance-indices of the “pre- and post-TACE-predict” models were not significantly different to those of other systems (BCLC, NIACE score, CP and ALBI grades), except for the “6 and 12” score that provided a lower prognostic value [C-index (6 and 12) 0.52 (0.41-0.63) vs (pre-TACE) 0.63 (0.53-0.73), P = 0.0087; C-index (6 and 12) 0.52 (0.41-0.63) vs (post-TACE) 0.63 (0.52-0.73), P = 0.0232; respectively].
| Scoring/Staging systems | OS (95%CI), mo | P value (log-rank) | Sidak1 | Hazard ratio (95%CI) | P value |
| Pre-TACE-Predict | 0.0032 | ||||
| Category 1 (n = 32) | 26 (21-44) | Ref | Ref | ||
| Category 2 (n = 35) | 30 (17-50) | 0.8786 | 0.83 (0.46-1.52) | 0.5463 | |
| Category 3 (n = 47) | 16 (11-20) | 0.2611 | 1.56 (0.92-2.65) | 2.6614 | |
| Category 4 (n = 23) | 12 (5-15) | 0.0418 | 2.27 (1.24-4.14) | 0.0079 | |
| Post-TACE-Predict | 0.0022 | ||||
| Category 1 (n = 36) | 36 (23-44) | Ref | Ref | ||
| Category 2 (n = 38) | 21 (12-30) | 0.4851 | 1.47 (0.83-2.61) | 0.1843 | |
| Category 3 (n = 48) | 16 (12-24) | 0.0699 | 1.89 (1.10-3.23) | 0.0203 | |
| Category 4 (n = 15) | 9 (5-15) | 0.0022 | 3.68 (1.79-7.55) | 0.0004 | |
| “6 and 12” score | 0.8633 | ||||
| Sum ≤ 6 (n = 51) | 26 (16-36) | Ref | Ref | ||
| Sum 6-12 (n = 73) | 18 (15-23) | 0.8328 | 1.12 (0.74-1.70) | 0.5913 | |
| Sum > 12 (n = 13) | 24 (3-48) | 0.8867 | 1.09 (0.54-2.18) | 0.8159 | |
| BCLC staging | 0.0234 | ||||
| B (n = 58) | 29 (18-39) | - | Ref | ||
| C (n = 79) | 16 (13-21) | - | 1.58 (1.06-2.36) | 0.0253 | |
| NIACE score | < 0.0001 | ||||
| ≤ 1 (n = 41) | 29 (21-43) | Ref | Ref | ||
| 1.5-3 (n = 72) | 22 (16-30) | 0.5463 | 1.38 (0.86-2.19) | 0.1782 | |
| > 3 (n = 24) | 9 (5-12) | < 0.0001 | 3.69 (2.09-6.51) | < 0.0001 | |
| Child-Pugh class | < 0.0001 | ||||
| A (n = 126) | 23 (18-29) | - | Ref | ||
| B (n = 11) | 9 (6-11) | - | 4.74 (2.34-9.59) | < 0.0001 | |
| ALBI grade | < 0.0001 | ||||
| Grade 1 (n = 51) | 32 (25-44) | Ref | Ref | ||
| Grade 2 (n = 77) | 17 (12-21) | 0.0074 | 1.93 (1.26-2.97) | 0.0026 | |
| Grade 3 (n = 9) | 9 (1-16) | < 0.0001 | 6.82 (3.14-14.83) | < 0.0001 |
Firstly, our findings showed, as demonstrated previously[9], that the “pre and post-TACE-predict” models could stratify survival among recommended TACE candidates in cohort 1. We observed similar results for survival time compared to the original study[9] and similar performances for survival prediction. However, the two lowest risk categories exhibited no significant difference in survival when applying the “pre-TACE-predict” model (P = 0.9707) as reported in the validation cohorts in the study by Han et al[9]. Thus, this new model mainly identified three patient groups with different prognosis as the “6 and 12” score, and not four as mentioned previously. Moreover, the “pre-TACE-predict” model predictive value was comparable to that of other systems (BCLC staging, ALBI grade). The NIACE score was more powerful within this multicenter French cohort, but it encompasses an important feature, namely tumor appearance. TACE performances are based on nodule size, number and morphology. Patients with poorly limited HCCs are usually considered to be poor responders to TACE or with less sustained response compared to well-limited encapsulated HCC. Many reasons are involved: the lack of a safety margin related to the procedure, capsular tumor invasion and microsatellite lesions widely linked to these forms of HCCs[19] are usually both fed by the hepatic artery and portal vein[20]. Conversely, the “post-TACE-predict” model identified four different prognostic groups and not surprisingly outperformed other models; treatment response is an independent predictor of survival after TACE[21].
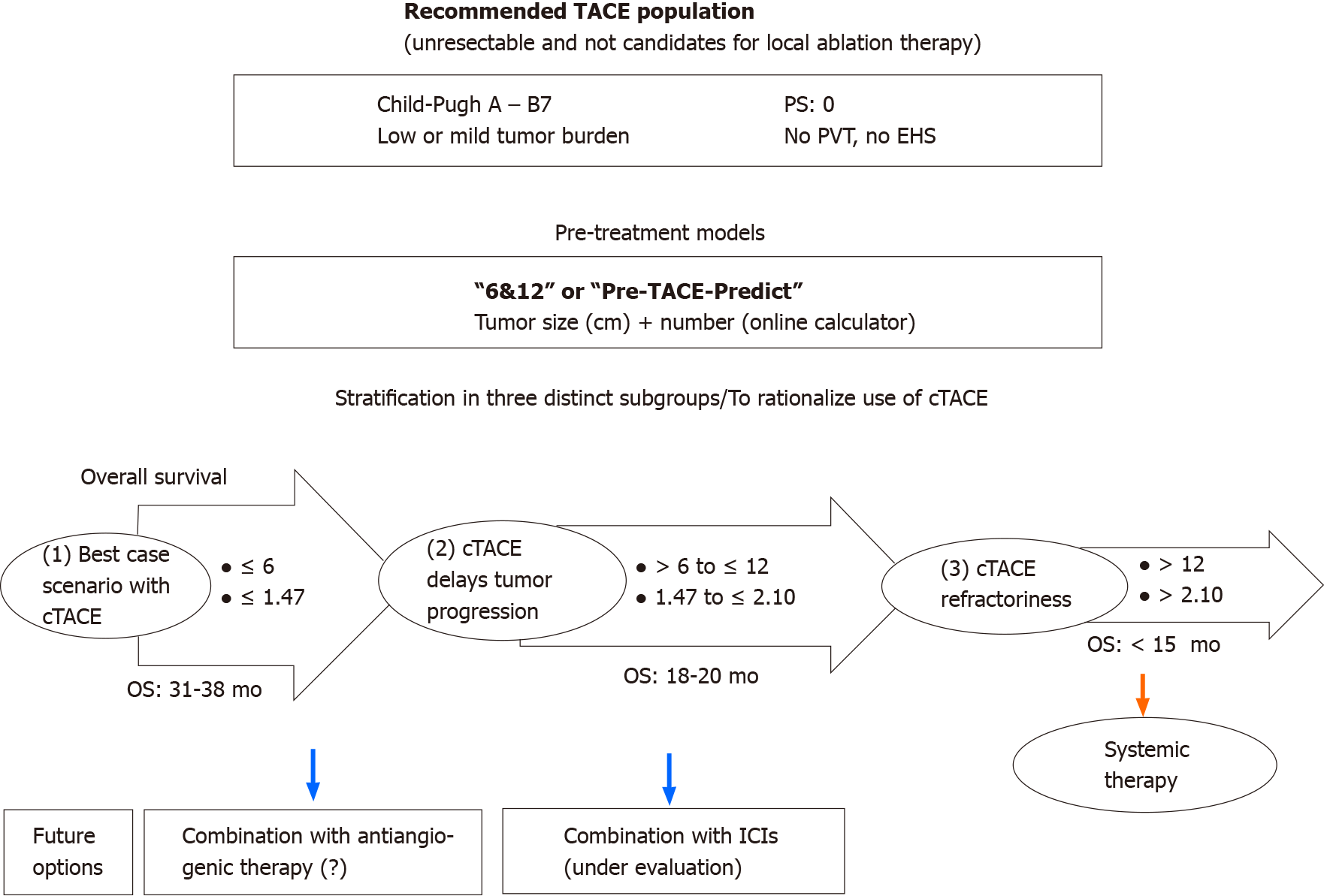
Secondly, this stratification into three categories proposed by the “pre-TACE-predict” or “6 and 12” models is attractive and reflects the different scenarios (Figure 4) with patients who sustainably benefit from TACE, while some intermediate stages still have a high risk of progression, and others may not achieve benefit from TACE and therefore may require earlier initiation of systemic treatment[13]. The different models, especially “pre-TACE-predict”, “6 and 12” and NIACE scores identify a population of patients who do not benefit from TACE and therefore are deemed TACE refractory, accounting for less than 10% of our population (Figure 1) vs 15% to 45% of patients in the study by Han et al[9], which means a change in our practices resulting in better patient selection. Conversely, these different models identified a first group of patients accounting for around 50% of our population who benefit sustainably from TACE, as this treatment may sometimes lead to complete tumor necrosis[22]. However, local and/or distant intrahepatic recurrences are commonly observed including in patients who achieved complete response following TACE[5]. These recurrences sometimes have a more invasive pattern[23], and became TACE resistant. Portal venous supply of HCC nodules, resulting from impaired arterial flow following repeated TACE, may help tumor survival[24]. This group may require additional treatment. Antiangiogenic therapy could enhance TACE efficacy[25]. In a new prospective randomized controlled study, Kudo et al[14] reported that combining cTACE plus sorafenib improves PFS based on a new definition of progression in patients with unresectable HCC. The authors propose another drug management; in contrast with other studies[26,27], sorafenib was introduced two to three weeks before the first TACE in an attempt to decrease vascular endothelial growth factor upregulation. This study requires further confirmation, since population size was limited and criteria for PFS are not yet consensual, but provides new insights, especially for patients who are considered TACE responders. The three scores also highlight an intermediate group (risk category 3 following “pre-TACE-predict”, or sum of tumor size and number over six and not exceeding twelve in the “6 and 12” score) accounting for one-third of the population (Figure 1) as reported in the original study[9]. This population, whose TACE delays tumor progression with an estimated median survival of 18 (16-21) mo, is well defined as opposed to the intermediate stage subclassifications (“outside up-to-seven criteria”). This group would deserve additional treatment to improve TACE efficacy, the whole purpose of the combined approaches with ICIs. TACE impacts the immune microenvironment of the tumor and may augment the effects of ICIs. Ongoing studies are currently underway[28].
Thirdly, the models' usefulness was reduced in our single center cohort including both intermediate and advanced stage HCCs (cohort 2) as in some cohorts that contributed to the development of the “pre-/post-TACE-predict” models[9]. The “6 and 12” score did not differentiate between subgroups with different prognosis. The low sample size may have affected this outcome, but other factors beyond tumor number or size influenced the prognosis in that population, especially vascular invasion[11]. The “6 and 12” model was developed based on a cohort of early (over 50%) and intermediate stage HCCs[12]. In a recent study, the “6 and 12” score survival prediction decreased within a population with slightly altered PS[29]. Thus, this model should be limited to TACE recommended candidates. It should also be stated that the performance of the “pre-TACE-predict” and NIACE scores was poorer for patient stratification with mainly two subgroups (Figure 3); thus, no model outperformed the BCLC system within this population. Moreover, the “post-TACE-predict” model identified only two subgroups of patients with different survival time. No study has shown a significant correlation between survival and radiological response following TACE treatment for HCCs with vascular invasion.
Fourthly, since pre-treatment scores contribution is comparable in a recommended TACE population combining a three-group stratification with therapeutic decision support, which model should be promoted? The simplicity of the “6 and 12” score by adding “the sum of largest tumor size and number” outperforms existing systems for daily medical practice even those with an online calculator. The recommendation for TACE results from a multi-disciplinary discussion with different clinicians who should both know and master at least one model. Other systems include some additional key features that may affect OS[15,30], but those parameters are not routinely recorded such as tumor morphology due to lack of consensus regarding radiological procedures and criteria that should be applied. However, liver function remains a key criterion in our cohorts of cirrhotic patients as CP and ALBI grades also identified subgroups with different prognosis (Figures 1 and 3). This is one of the limitations of the “6 and 12” model in our population.
Our study is limited by its retrospective design; therefore, selection and confounding biases are both possible. Moreover, sample size in cohort 2 was relatively small, and probably affected the results. A further limitation relies on the fact that we considered only the main treatment.
In sum, the two latest models (“6 and 12”, “pre-TACE-predict”) share comparable prognostic value with the BCLC system in our recommended TACE population. The newly proposed “pre-TACE-predict” model does not outperform the other models for predicting survival. However, it provides similar to the “6 and 12” score, an interesting three-subgroup stratification (long-term control, delay in progression and poor responders) especially when considering new available systemic therapies and future options. The usefulness of these scores was less apparent within our HCC cohort with advanced stages.
Transarterial chemoembolization (TACE) is currently recommended for intermediate stage hepatocellular carcinomas (HCCs), while in practice TACE is performed beyond the recommendations. New therapeutic options in advanced HCC require careful selection of patients prior to TACE treatment, since some patients may not benefit from this therapy and may impair their liver function.
Two recently developed models entitled "pre-TACE-predict” and “Six-and-Twelve” both easy-to-use and powerful, have been designed accordingly, to identify suitable and inappropriate candidates and thus help in the decision-making process.
To evaluate and compare the performance of both new models in survival prediction, and their potential contribution to patient treatment strategy.
This is a retrospective multicenter study performed on two French cohorts with HCC of different stages, including 324 patients classified as Barcelona Clinic Liver Cancer (BCLC) stages A/B (cohort 1) and 137 patients classified as BCLC stages B/C (cohort 2), respectively. All of these patients (treatment naïve or with recurrence after curative therapies) received conventional TACE treatment as the main therapy during a period from 01/2010 to 12/2018. Survival prediction was calculated based on these two new models and compared to the BCLC system and established prognostic scores [Albumin-Bilirubin grade, NIACE (Number of tumor, Infiltrative HCC, Child-Pugh, Alpha-fetoprotein, Eastern Cooperative Oncology Group Performance Status)] using concordance-index and area under the receiver operating characteristic curve across time.
The "pre-TACE-predict" model identified three rather than four groups of patients with different prognosis within a recommended TACE candidate cohort (cohort 1), similar to the “6 and 12” model. Its prognostic value was no higher than other systems, as opposed to the "post-TACE-predict" model that includes response to treatment. The contribution of both new models was reduced in our second cohort with advanced stage HCCs (cohort 2), as prognosis is influenced by variables other than tumor size or number and TACE efficacy is unclear in HCC with vascular invasion.
Both “pre-TACE-predict” and “6 and 12” models offer an interesting stratification into three groups in a recommended TACE population, by defining respectively a first group with durable control but prone to recurrence, a second partially controlled group prone to progression and a third group that do not benefit from this treatment.
With further refinement prior to chemoembolization, the “6 and 12” and “pre-TACE-predict” models allow us to consider the future scenarios of TACE therapy with (1) HCC patients who might benefit from adjuvant therapy to prevent recurrence (after a complete response to TACE); (2) others who might benefit from a combined therapy following a partial response to TACE; and (3) others who should be treated with a systemic exclusive therapy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rong GH S-Editor: Gao CC L-Editor: Webster JR P-Editor: Liu JH
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6065] [Article Influence: 866.4] [Reference Citation Analysis (3)] |
| 2. | Bruix J, Sherman M; Practice Guidelines Committee; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 3. | Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol. 2017;67:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 4. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3303] [Article Influence: 220.2] [Reference Citation Analysis (36)] |
| 5. | Labeur TA, Takkenberg RB, Klümpen HJ, van Delden OM. Reason of Discontinuation After Transarterial Chemoembolization Influences Survival in Patients with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2019;42:230-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 7. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 8. | Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi's Subclassification (Kinki Criteria). Dig Dis. 2015;33:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 9. | Han G, Berhane S, Toyoda H, Bettinger D, Elshaarawy O, Chan AWH, Kirstein M, Mosconi C, Hucke F, Palmer D, Pinato DJ, Sharma R, Ottaviani D, Jang JW, Labeur TA, van Delden OM, Pirisi M, Stern N, Sangro B, Meyer T, Fateen W, García-Fiñana M, Gomaa A, Waked I, Rewisha E, Aithal GP, Travis S, Kudo M, Cucchetti A, Peck-Radosavljevic M, Takkenberg RB, Chan SL, Vogel A, Johnson PJ. Prediction of Survival Among Patients Receiving Transarterial Chemoembolization for Hepatocellular Carcinoma: A Response-Based Approach. Hepatology. 2020;72:198-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 10. | Kadalayil L, Benini R, Pallan L, O'Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, Palmer DH, Meyer T. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 11. | Cappelli A, Cucchetti A, Cabibbo G, Mosconi C, Maida M, Attardo S, Pettinari I, Pinna AD, Golfieri R. Refining prognosis after trans-arterial chemo-embolization for hepatocellular carcinoma. Liver Int. 2016;36:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, Mu W, Yin G, Li H, Zhao H, Li J, Zhang C, Zhu X, Wu J, Gong W, Li Z, Lin Z, Pan X, Shi H, Shao G, Liu J, Yang S, Zheng Y, Xu J, Song J, Wang W, Wang Z, Zhang Y, Ding R, Zhang H, Yu H, Zheng L, Gu W, You N, Wang G, Zhang S, Feng L, Liu L, Zhang P, Li X, Chen J, Xu T, Zhou W, Zeng H, Huang W, Jiang W, Zhang W, Shao W, Li L, Niu J, Yuan J, Lv Y, Li K, Yin Z, Xia J, Fan D, Han G; China HCC-TACE Study Group. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol. 2019;70:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 13. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4707] [Article Influence: 941.4] [Reference Citation Analysis (2)] |
| 14. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 504] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 15. | Adhoute X, Pénaranda G, Raoul JL, Bollon E, Pol B, Letreut YP, Perrier H, Bayle O, Monnet O, Beaurain P, Muller C, Hardwigsen J, Lefolgoc G, Castellani P, Bronowicki JP, Bourlière M. NIACE score for hepatocellular carcinoma patients treated by surgery or transarterial chemoembolization. Eur J Gastroenterol Hepatol. 2017;29:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2014] [Article Influence: 201.4] [Reference Citation Analysis (0)] |
| 17. | Kohl M, Plischke M, Leffondré K, Heinze G. PSHREG: a SAS macro for proportional and nonproportional subdistribution hazards regression. Comput Methods Programs Biomed. 2015;118:218-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] |
| 19. | Kuroda C, Sakurai M, Monden M, Marukawa T, Hosoki T, Tokunaga K, Wakasa K, Okamura J, Kozuka T. Limitation of transcatheter arterial chemoembolization using iodized oil for small hepatocellular carcinoma. A study in resected cases. Cancer. 1991;67:81-86. [PubMed] |
| 20. | Tajima T, Honda H, Taguchi K, Asayama Y, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Shimada M, Masuda K. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178:885-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 22. | Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, Ravaioli M, D'Errico-Grigioni A, Pinna AD, Bolondi L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 23. | Adhoute X, Pénaranda G, Sellier F, Raoul JL. Response to 'the flexible therapeutic approach to the BCLC B stage': Time for scoring systems? J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Miyayama S, Matsui O, Zen Y, Yamashiro M, Hattori Y, Orito N, Matsui K, Tsuji K, Yoshida M, Sudo Y. Portal blood supply to locally progressed hepatocellular carcinoma after transcatheter arterial chemoembolization: Observation on CT during arterial portography. Hepatol Res. 2011;41:853-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Jiang H, Meng Q, Tan H, Pan S, Sun B, Xu R, Sun X. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int J Cancer. 2007;121:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 27. | Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, Stubbs C, Stocken DD, Wall L, Watkinson A, Hacking N, Evans TRJ, Collins P, Hubner RA, Cunningham D, Primrose JN, Johnson PJ, Palmer DH. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 378] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 28. | ClinicalTrials.gov. Study of Pembrolizumab Following TACE in Primary Liver Carcinoma (PETAL). [accessed 2019 September 12]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03397654 ClinicalTrials.gov Identifier: NCT03397654. |
| 29. | Wang ZX, Wang EX, Bai W, Xia DD, Mu W, Li J, Yang QY, Huang M, Xu GH, Sun JH, Li HL, Zhao H, Wu JB, Yang SF, Li JP, Li ZX, Zhang CQ, Zhu XL, Zheng YB, Wang QH, Yuan J, Li XM, Niu J, Yin ZX, Xia JL, Fan DM, Han GH; On Behalf Of China Hcc-Tace Study Group. Validation and evaluation of clinical prediction systems for first and repeated transarterial chemoembolization in unresectable hepatocellular carcinoma: A Chinese multicenter retrospective study. World J Gastroenterol. 2020;26:657-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Hucke F, Pinter M, Graziadei I, Bota S, Vogel W, Müller C, Heinzl H, Waneck F, Trauner M, Peck-Radosavljevic M, Sieghart W. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J Hepatol. 2014;61:1287-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |