Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4285
Peer-review started: January 15, 2021
First decision: February 11, 2021
Revised: February 22, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: June 16, 2021
Processing time: 131 Days and 4.8 Hours
Rosai-Dorfman disease (RDD) is a rare benign proliferative disease whose etiology is not clear and may be related to infection or unexplained immune dysfunction. The authors present a case of RDD with lung involvement in a 10-year-old patient.
A 10-year-old girl found that her left cervical lymph nodes were enlarged for more than 7 mo, and the largest range was about 6.5 cm × 5.9 cm × 8.1 cm. Cervical magnetic resonance imaging showed multiple masses in the left neck, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. A malignant tumor, with a high possibility of lymph node metastasis, was initially considered. At the same time, lung computed tomography showed multiple nodules of different sizes scattered on both sides of the lung, with uniform internal density. Thus, a possible metastatic tumor was considered. Finally, RDD was diagnosed by pathology and immunohistochemistry. According to the antibiogram, clindamycin was administered for 2 wk, and prednisone acetate was administered for 7 wk. Nine months later, the ulcer in the left neck was better than before, but the imaging showed that the lesion was not controlled.
The diagnosis of RDD cannot be made by a single tool and its treatment is a long-term exploratory process. Follow-up is necessary.
Core Tip: In this paper we report a 10-year-old girl characterized by enlarged left cervical lymph nodes. Imaging suggested a malignant tumor with pulmonary metastasis, and Rosai-Dorfman disease (RDD) was finally diagnosed by pathological and immunohistochemical methods. This case suggests that attention should be paid to RDD in the differential diagnosis of cervical masses. Second, the diagnosis of RDD should not rely solely on imaging examination, and different diagnostic methods should be used. Moreover, although rare, there is a situation that RDD involves the lungs. Finally, the treatment of RDD needs to be constantly explored and the treatment plan should be adjusted according to the follow-up results.
- Citation: Wu GJ, Li BB, Zhu RL, Yang CJ, Chen WY. Rosai-Dorfman disease with lung involvement in a 10-year-old patient: A case report. World J Clin Cases 2021; 9(17): 4285-4293
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4285.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4285
Rosai-Dorfman disease (RDD), also called sinus histiocytosis with massive lymphadenopathy, was first described by the French pathologist Paul Destombes in 1965 and recognized as a distinct clinicopathologic entity by Rosai and Dorfman in 1969. It is a rare, benign, and self-limited histiocytosis characterized by histiocytic proliferation with enlarged lymph nodes[1-5]. It usually affects children and young people, with more than 80% of cases occurring in children under the age of 20, and the disease has a slight male predilection, with a male/female ratio of 3:1, and more frequently affects males of African descent[6]. RDD, whose main manifestation is pain-free enlarged lymph nodes on both sides of the neck (lymph nodes being the most easily involved organs), is usually accompanied by fever, leukocytosis, anemia, polyclonal hyperglobulinemia, and increases in the erythrocyte sedimentation rate; multiple extranodal organs may also be involved[3,7]. The main treatments for RDD include observation, surgery, corticosteroids, sirolimus, chemotherapy, immunomodulatory therapy, targeted therapies, and radiotherapy, but no uniform approach has been delineated for RDD, and treatment is best tailored to the individual clinical circumstances[8]. We report a case of RDD with lung involvement characterized by left cervical lymph node enlargement.
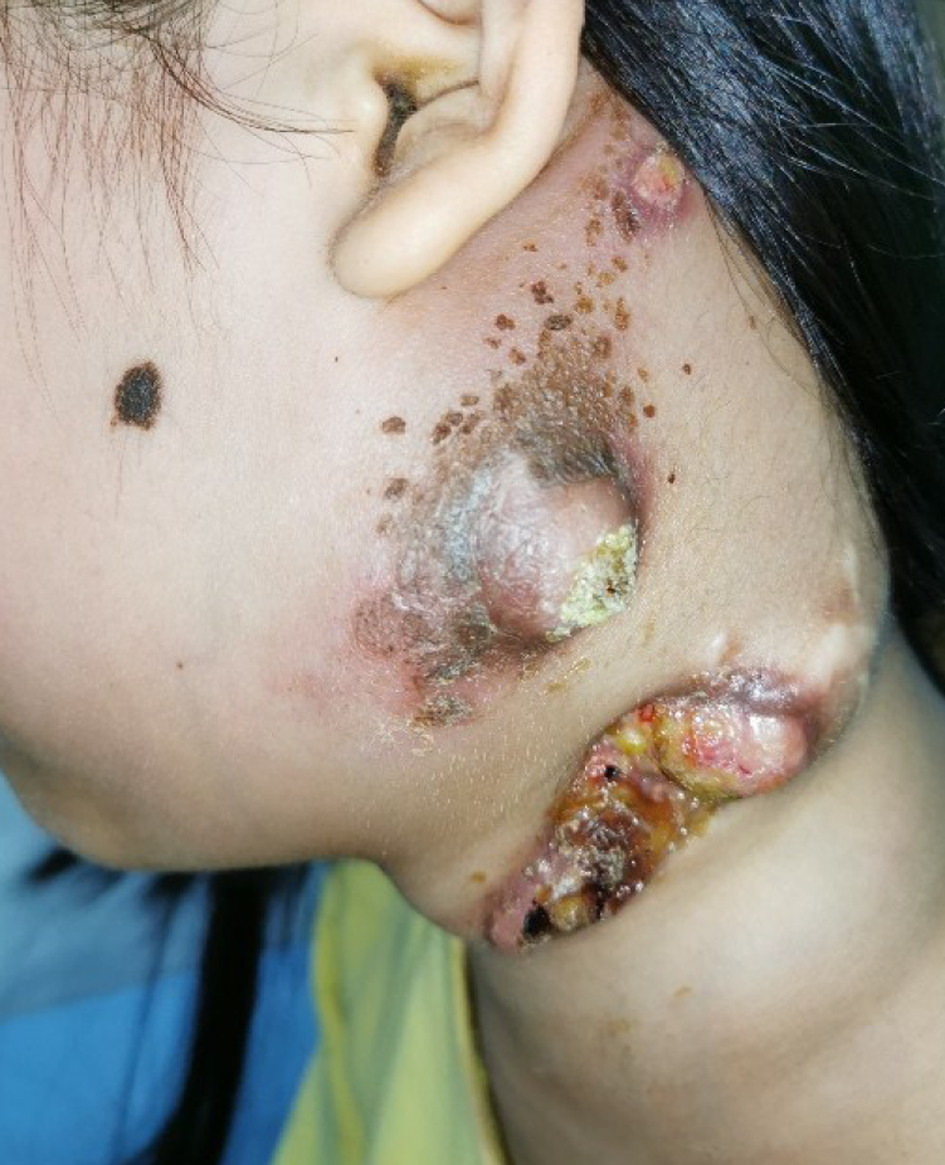
A 10-year-old girl was admitted to Guangdong Provincial Hospital of Traditional Chinese Medicine with enlargement of the left lymph node for more than 7 mo (Figure 1). Two months before this attack, the patient underwent surgery to remove the enlarged lymph node at another hospital.
The patient’s symptoms started 7 mo before and showed progressive development. There was no discomfort except for enlarged cervical lymph nodes.
The patient had a free previous medical history.
The patient had no special personal history or family history.
The patient had no fever since the onset of the disease. The left cervical lymph nodes were enlarged (the largest one was 5 cm × 4 cm and the smallest one was 3 cm × 1 cm) and ruptured, accompanied with purulent secretions, occasional bleeding, non-healing, being hard, and no tenderness, and the remaining superficial lymph nodes were not touched. Our clinical consideration was lymph node tuberculosis or a malignant tumor.
Leukocyte cell count (19.76 × 109/L), neutrophil count (16.95 × 109/L), platelet count (552 × 109/L), and erythrocyte sedimentation rate (> 120 mm/h) were all elevated; liver function test showed elevated globulin (47.3 g/L). The immune results showed that immunoglobulin IgA (5.42 g/L), immunoglobulin IgG (24.80 g/L), and complement C3 (1.97 g/L) were increased. Mycobacterium tuberculosis nucleic acid test was negative. In the culture of wound secretion, no acid-fast bacilli or fungi were found, but Gram-positive cocci were found and sensitive to clindamycin.
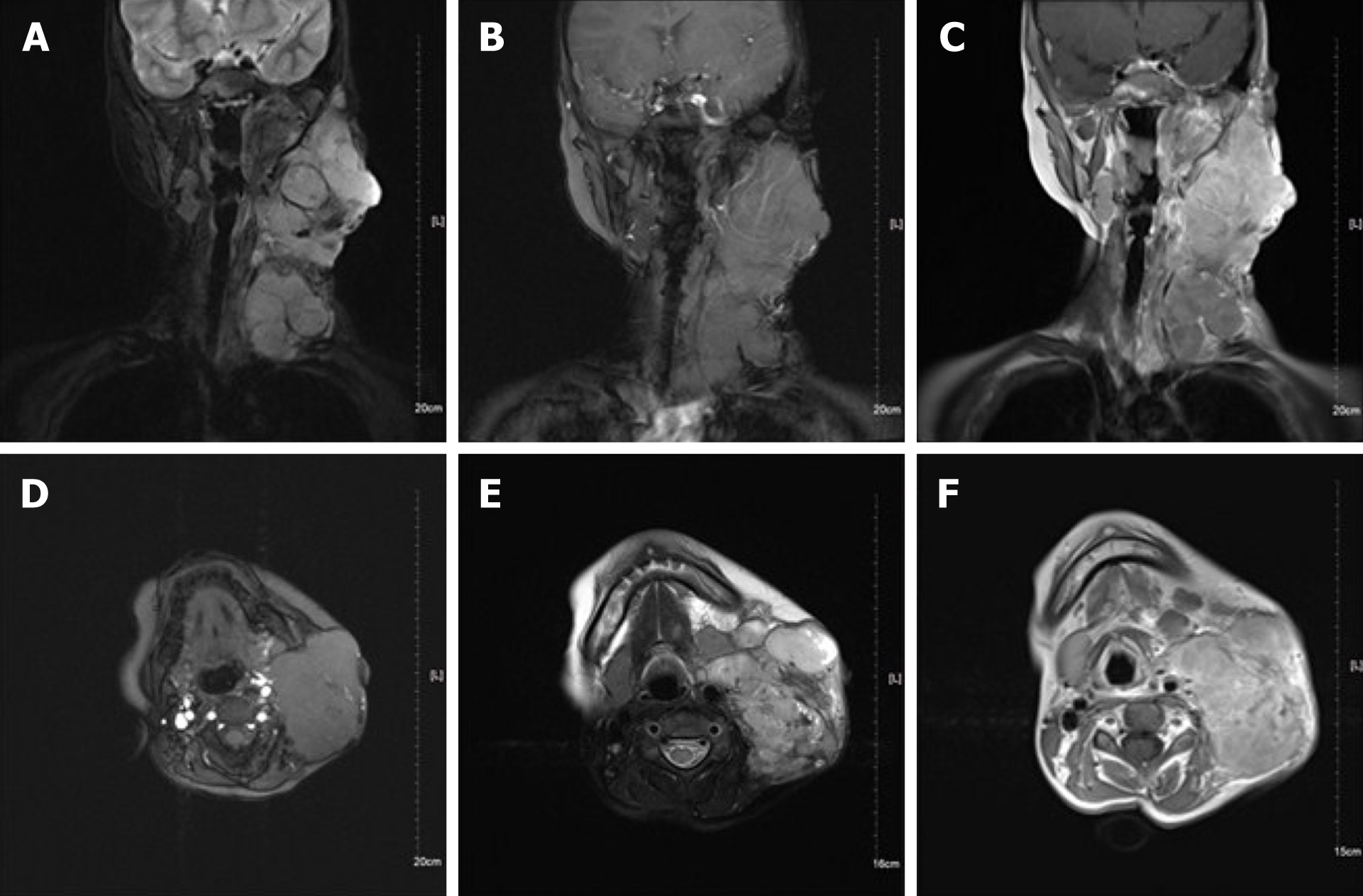
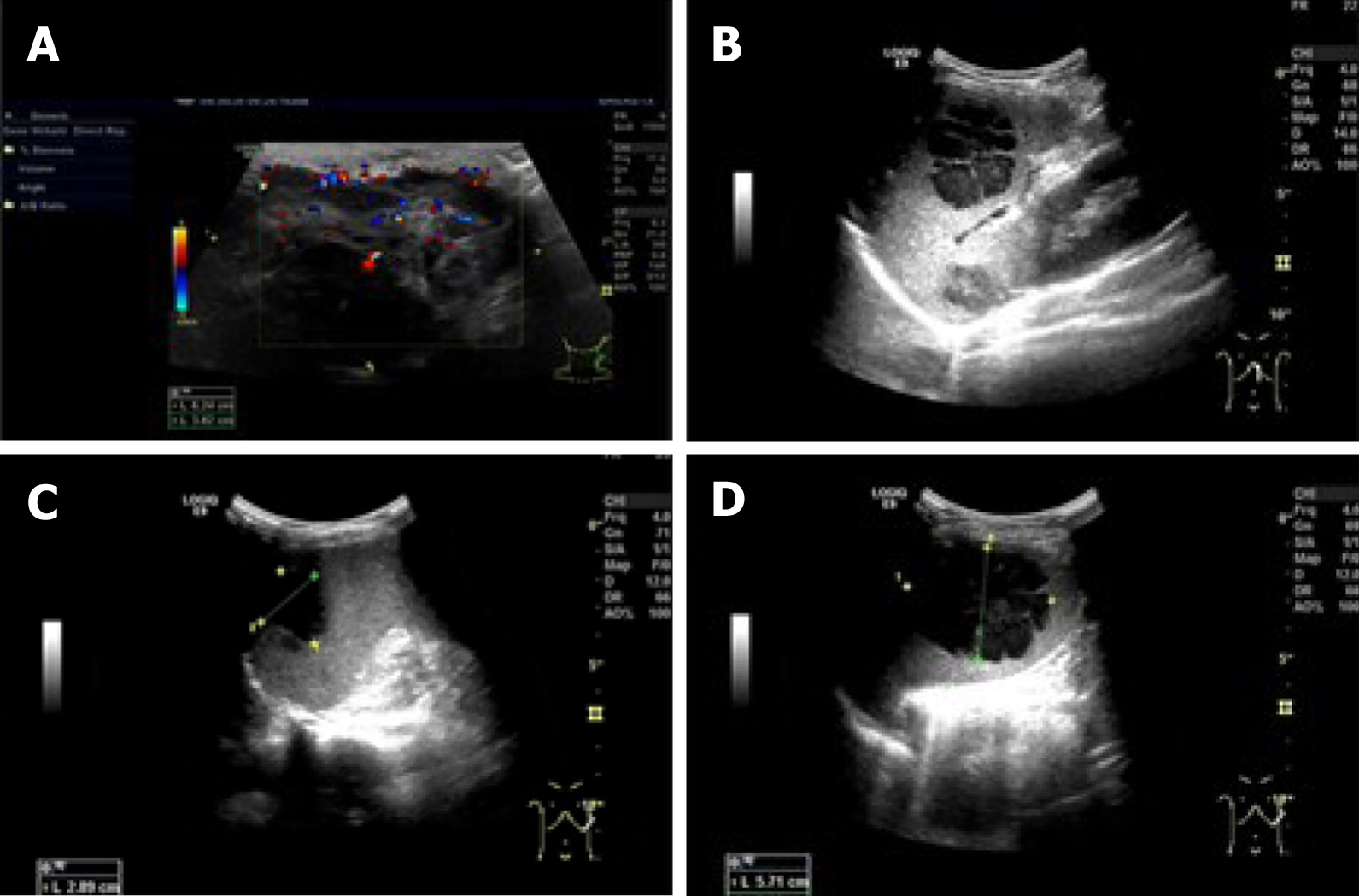
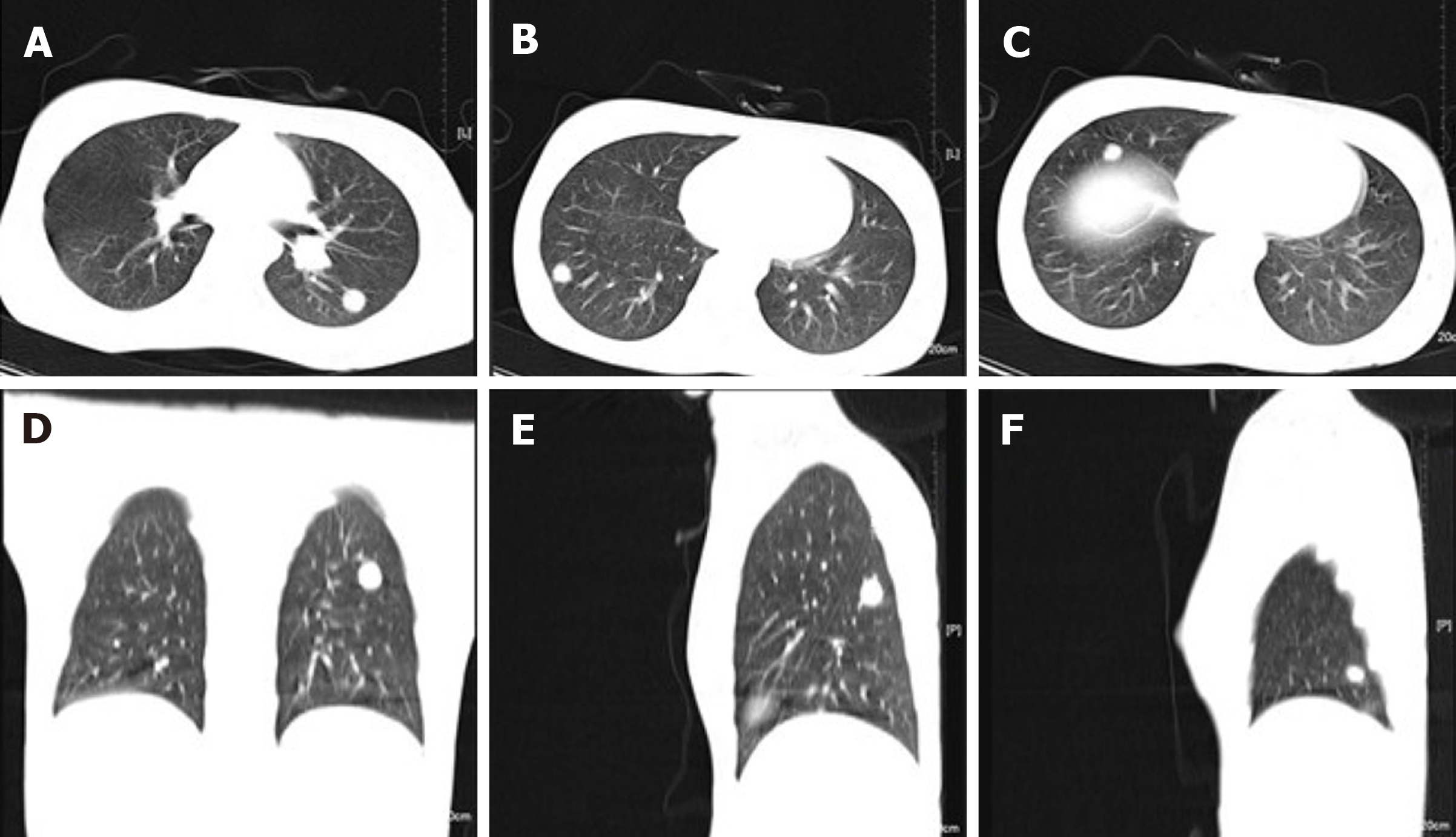
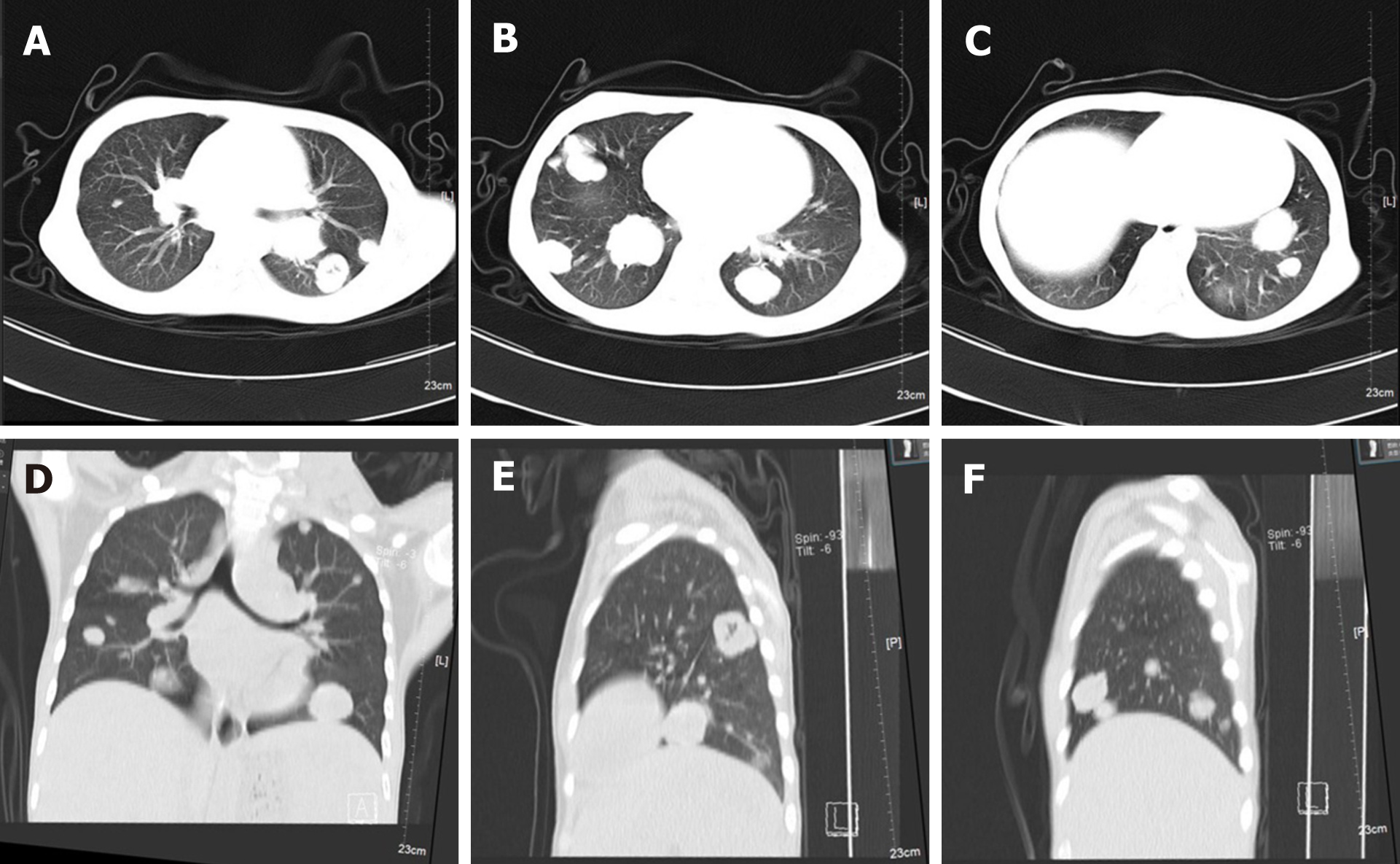
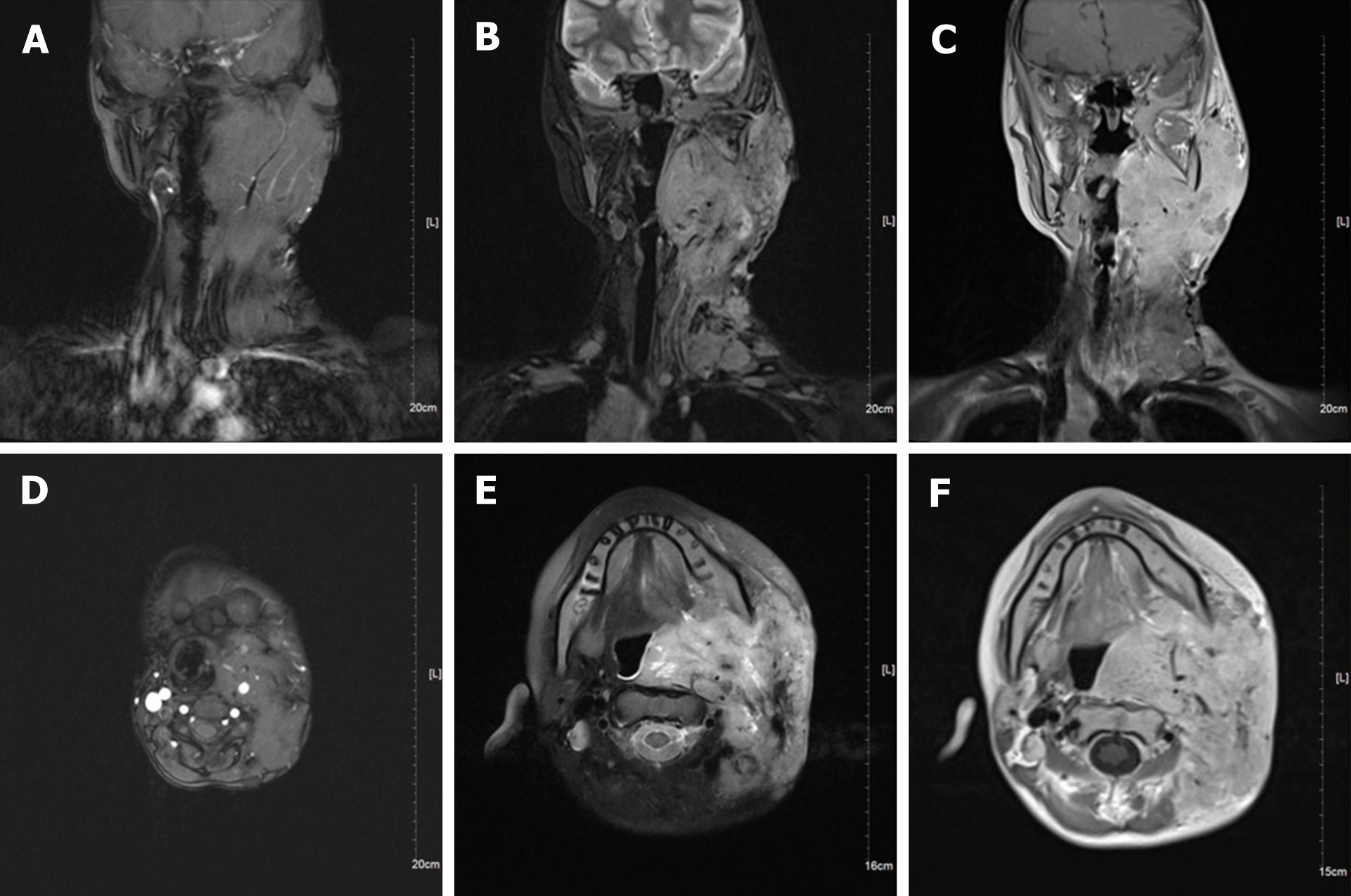
Neck magnetic resonance imaging (MRI) (1.5 T) (Figure 2) showed that the left parapharyngeal space, carotid sheath area, submandibular region, cervical root, supraclavicular fossa, and supraclavicular region presented with multiple masses (with the largest measuring 6.5 cm × 5.9 cm × 8.1 cm), with low signal intensity on T1 weighted imaging (WI) and high signal intensity on T2WI. The left muscle group adjacent to the neck, the left subclavian vein, and the common jugular vein were invaded. Abdominal color ultrasound (Figure 3) showed multiple hypoechoic masses in the spleen, the largest two of which measured approximately 59 mm × 42 mm and 28 mm × 26 mm, respectively. Multiple nodules were found in both lungs on a plain computed tomography (CT) scan. The largest nodule was located in the upper segment of the left lower lung with a diameter of approximately 1.2 cm (Figure 4).
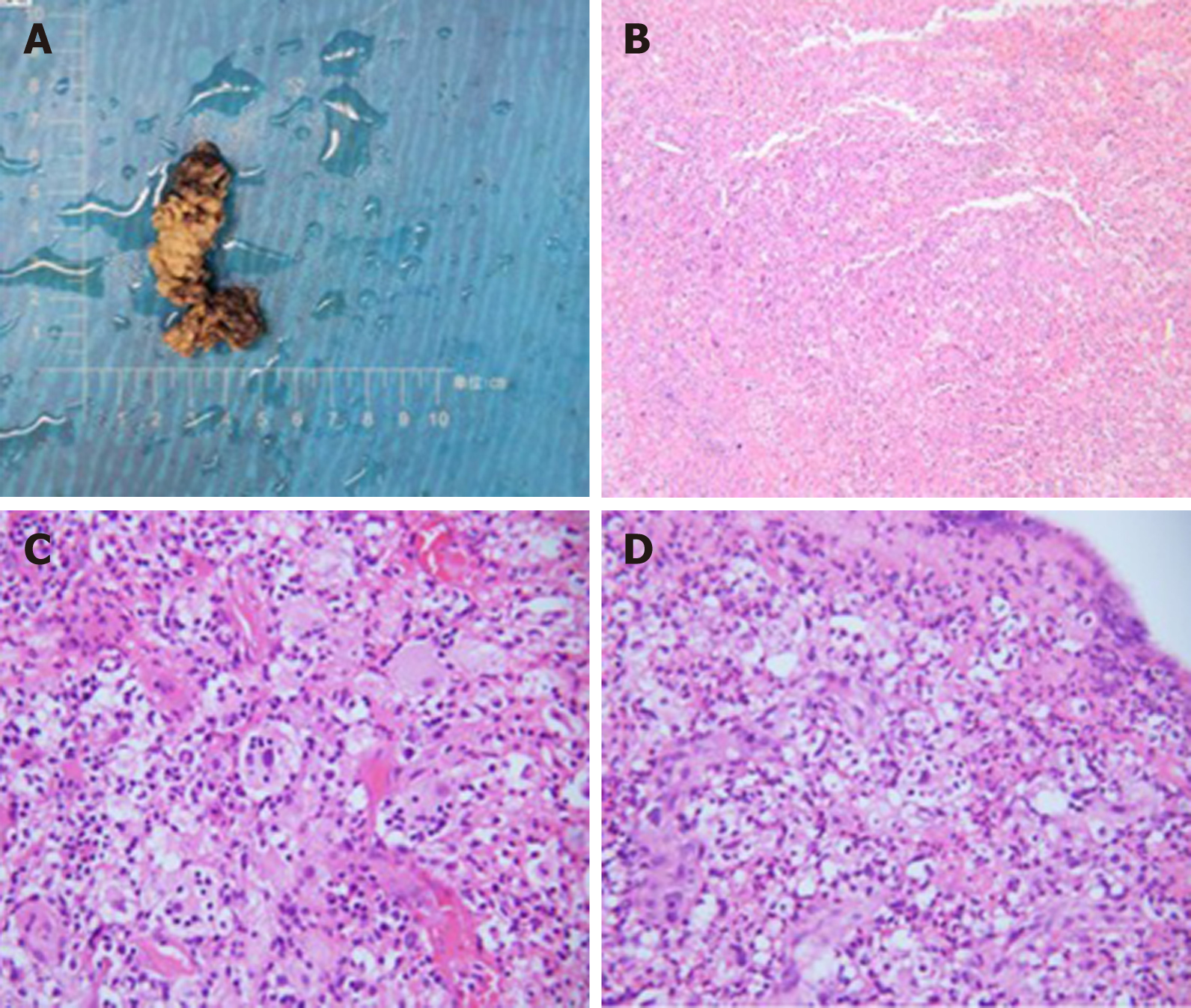
A pathological biopsy was performed to determine the diagnosis. The biopsy specimen of the lymph node in region V of the left neck showed histiocyte-like cells with rich and lightly stained cytoplasm distributed in patches, and complete neutrophils, lymphocytes, and plasma cells could be seen in some cells (Figure 5). Immunohistochemical staining showed that the specimen was positive for CD68, CD163, and S100 and partially positive for CD1a.
The final diagnosis of the presented case was RDD with lung involvement.
Since there were skin ulcers in the left neck, accompanied by purulent secretions, local infection was considered and empiric intravenous antibiotic therapy was administered for 9 d with cefmetazole sodium 1 g bid. After Gram-positive cocci were found in wound secretion culture and drug sensitivity test was performed, cefmetazole sodium was stopped and intravenous clindamycin hydrochloride injection 0.15 g bid was used for 7 d. According to the pathological and immunohistochemical results of lymph nodes, RDD was diagnosed. The patient was discharged from the hospital after she recovered well from the operation, and was treated with 75 mg of clindamycin palmitate dispersible tablets 3 times a day for 1 wk and prednisone acetate 15 mg once a day for 1 wk followed by 5 mg once a day for 6 wk.
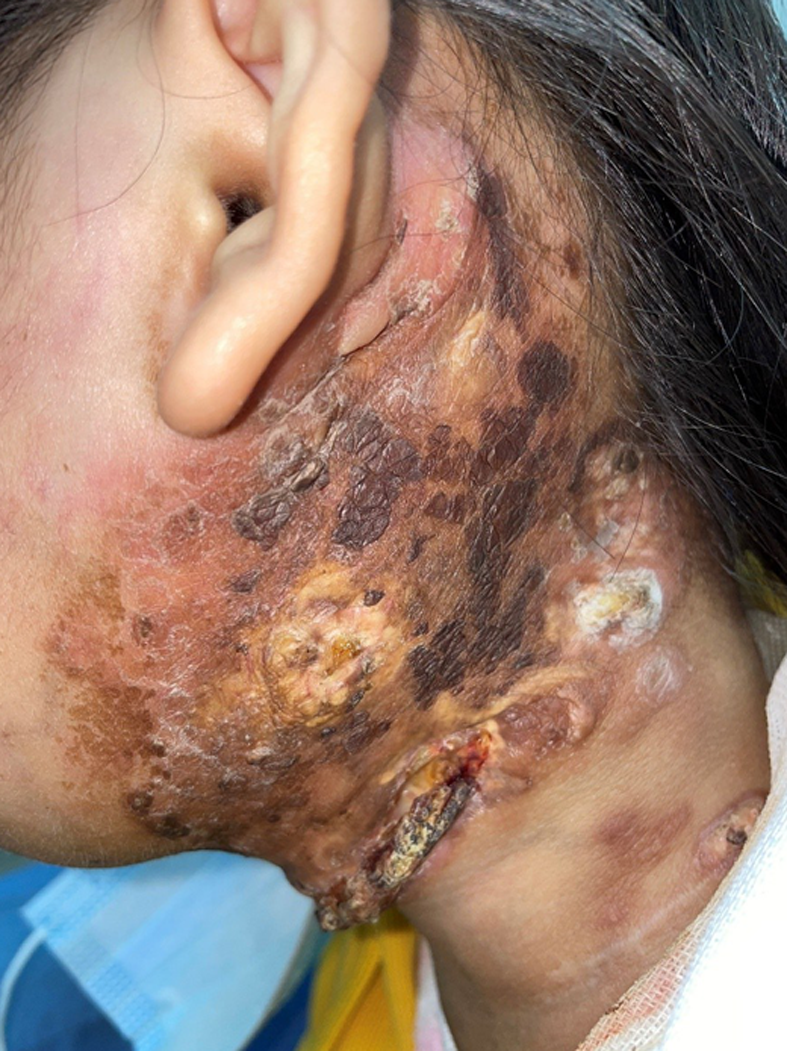
The patients were followed 9 mo later (February 2021). The ulcer in the left neck was better than before, and no purulent secretion was found (Figure 6). CT showed that the bilateral pulmonary nodules were significantly increased and enlarged. The largest nodule was located in the right lower lung with a diameter of about 3.3 cm (Figure 7). Neck MRI showed multiple round-like soft tissue masses of different sizes in the left parapharyngeal space, carotid sheath area, submandibular region, cervical root, supraclavicular fossa, and supraclavicular region. Most of the lesions were fused into masses (the largest was 6.9 cm × 9.0 cm × 12 cm), with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Figure 8). With the consideration of current poor control of the disease, the doctor advised the patient to be hospitalized again. The patient's family said that they needed to consider it.
RDD is a rare benign proliferative disease of unclear etiology, and it may be related to infection or unknown immune dysfunction[9,10]. Clinically, according to the extent of lesion involvement, it can be divided into three subtypes: Purely nodal, extranodal, and both nodal and extranodal[11]. RDD can demonstrate local or whole-body involvement. In terms of imaging, Raslan et al[12] reported that the affected lymph nodes were homogeneously enhanced and might show central hypodensity on CT. MRI characteristics of the involved areas are generally T1 isointensity, T2 isointensity, and intense enhancement with gadolinium agents. PET shows variable uptake[12]. At present, the diagnosis of RDD depends on histopathology[13]. This disease is characterized histologically by abnormal proliferation of histiocytes, typically with positive immunolabeling for S100 protein and CD68, with engulfment of lymphocytes, called emperipolesis. Immunohistological staining for CD1a is negative, excluding a dia
What is peculiar in this case is that the RDD lesions involved the lungs. The involvement of RDD in the lungs is very rare. RDD lesions in the lungs are similar to tracheal carcinoma or other rare tumors and can be fatal in severe cases[14]. Moyon et al[15] retrieved a total of 69 references published in English and French from 1978 to September 2018, from which 47 patients in 38 references were analyzed. The predominant feature of thoracic RDD was mediastinal involvement (27.57%), followed by lung disease (14.30%), and airway (13.28%) and pleural involvement (4.9%). RDD with lung involvement has no obvious specific clinical symptoms or imaging manifestations, so lesions are easily misdiagnosed as a malignant tumor, as in this case. Ultimately, a pathological diagnosis is needed. In this case, the patient had multiple pulmonary nodules, but no clinical symptoms such as cough, expectoration, hemoptysis, low fever, and chest pain. The CT image was inconsistent with the image of pulmonary tuberculosis. The pathological manifestation, in this case, was typical; immunohistochemistry revealed positivity for CD68, CD163, and S100, indicating the possibility of RDD with lung involvement. However, no further examination to exclude Mycobacterium tuberculosis infection was the limitation of this case.
Concerning skin ulcers, we have the following considerations: First, before the onset of this disease, the patient had undergone surgery at another hospital, and there was the possibility of poor local skin healing. Second, the skin of children is delicate, and the tumor continues to increase and oppress, affecting the local blood supply and leading to inflammation, which are all factors for skin damage. Third, improper nursing care for family members and the patient after skin damage led to infection and procrastination. Finally, the nature of the tumor had not been clear for a long time, and there was no effective treatment to control the disease. These causes led to ulceration of the skin, an unusual clinical presentation.
The follow-up results showed that the cervical lymph nodes and pulmonary nodules were enlarged at the same time, and we thus considered that both lesions were caused by RDD. At present, the cervical lymph nodes and pulmonary nodules were further enlarged and increased. Considering the poor treatment control and the need to change the treatment plan, we will continue to follow up on this case.
This case was characterized by enlarged cervical lymph nodes and lung involvement. Simple imaging examination can easily lead to a misdiagnosis and ultimately needs to be confirmed by pathology. It is necessary to comprehensively consider the results of various tests to make a correct diagnosis of RDD. Although RDD has not yet been currently classified as a neoplastic disorder, recent evidence demonstrating clonality in a subset of cases raises the possibility of a neoplastic process[16]. The treatment of RDD is a long-term exploratory process. RDD management requires timely adjustment of treatment plan according to curative effect evaluation during follow-up. Therefore, the patients need long-term follow-up.
Manuscript source: Unsolicited manuscript
Specialty type: Otorhinolaryngology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Buehler MM, Tahtabasi M S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Wan M, Ding A, Liu H, Ou J, Zhang J. Successful treatment of large cutaneous facial Rosai-Dorfman disease using combination of subtotal resection and ALA-PDT: A case report. Photodiagnosis Photodyn Ther. 2020;31:101879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Sun L, Shi J, Su Z, Zhang M, Lu Y. Successful treatment of Rosai-Dorfman disease using ALA-PDT. Photodiagnosis Photodyn Ther. 2018;21:128-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Lu CI, Kuo TT, Wong WR, Hong HS. Clinical and histopathologic spectrum of cutaneous Rosai-Dorfman disease in Taiwan. J Am Acad Dermatol. 2004;51:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Destombes P. [Adenitis with lipid excess, in children or young adults, seen in the Antilles and in Mali. (4 cases)]. Bull Soc Pathol Exot Filiales. 1965;58:1169-1175. [PubMed] |
| 5. | Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70. [PubMed] |
| 6. | Varrassi M, Corridore A, Tommasino E, Saltelli G, Bruno F, Di Sibio A, Splendiani A, Di Cesare E, Masciocchi C. MR imaging of cerebral involvement of Rosai-Dorfman disease: a single-centre experience with review of the literature. Radiol Med. 2021;126:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Mantilla JG, Goldberg-Stein S, Wang Y. Extranodal Rosai-Dorfman Disease: Clinicopathologic Series of 10 Patients With Radiologic Correlation and Review of the Literature. Am J Clin Pathol. 2016;145:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Abla O, Jacobsen E, Picarsic J, Krenova Z, Jaffe R, Emile JF, Durham BH, Braier J, Charlotte F, Donadieu J, Cohen-Aubart F, Rodriguez-Galindo C, Allen C, Whitlock JA, Weitzman S, McClain KL, Haroche J, Diamond EL. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131:2877-2890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 371] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 9. | Hsiao CH, Tsai TF, Yang TH, Liu CM. Clinicopathologic characteristics of Rosai-Dorfman disease in a medical center in northern taiwan. J Formos Med Assoc. 2006;105:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Nathan B, Roomallah B, Salisbury JR, Tenant-Flowers M, Kassam S. A rare cause of lymphadenopathy - Rosai-Dorfman disease in a HIV-positive Ugandan woman. Int J STD AIDS. 2016;27:1123-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Zhu F, Zhang JT, Xing XW, Wang DJ, Zhu RY, Zhang Q, Wang HT, Lang SY. Rosai-Dorfman disease: a retrospective analysis of 13 cases. Am J Med Sci. 2013;345:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Raslan OA, Schellingerhout D, Fuller GN, Ketonen LM. Rosai-Dorfman disease in neuroradiology: imaging findings in a series of 10 patients. AJR Am J Roentgenol. 2011;196:W187-W193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Wang F, Xu J, Chen J, Fu H, Wang Z, Cai D, Kang X, Zhang BB, Matz EL, Zhang Y, Wang W. A case report of Rosai-Dorfman disease with kidney involvement. J Xray Sci Technol. 2018;26:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Boissière L, Patey M, Toubas O, Vella-Boucaud J, Perotin-Collard JM, Deslée G, Lebargy F, Dury S. Tracheobronchial Involvement of Rosai-Dorfman Disease: Case Report and Review of the Literature. Medicine (Baltimore). 2016;95:e2821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Moyon Q, Boussouar S, Maksud P, Emile JF, Charlotte F, Aladjidi N, Prévot G, Donadieu J, Amoura Z, Grenier P, Haroche J, Cohen Aubart F. Lung Involvement in Destombes-Rosai-Dorfman Disease: Clinical and Radiological Features and Response to the MEK Inhibitor Cobimetinib. Chest. 2020;157:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |