Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.4016
Peer-review started: January 9, 2021
First decision: February 11, 2021
Revised: February 22, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: June 6, 2021
Processing time: 116 Days and 0.3 Hours
Primary anaplastic large cell lymphoma of the lung represents a diagnostic challenge due to diverse manifestations and non-specific radiological findings, particularly in cases that lack extra-pulmonary manifestations and lung biopsy.
A 40-year-old woman presented with a 6-d history of fever, dry coughing, and dyspnea. Her white blood cell count was 20100/mm3 with 90% neutrophils. PaO2 was 60 mmHg and SaO2 was 90% when breathing ambient air. Chest computed tomography (CT) identified a solid nodule, 15 mm in diameter, with a poorly defined boundary in the upper right lung, and several smaller solid nodules throughout both lungs. Pulmonary artery CT and subsequent bedside X-ray showed diffuse patchy shadows throughout both lungs. Repeated cultures of blood samples and alveolar lavage failed to identify any pathogen. Due to the mismatch between clinical and imaging features, we conducted a bone marrow biopsy, and the results showed proliferation along all three lineages but no atypical or malignant cells. The patient received empirical antibacterial, antiviral, and antifungal treatments, as well as corticosteroids. The patient’s condition deteriorated rapidly despite treatment. The patient died 6 d after hospitalization due to respiratory failure. Post-mortem lung biopsy failed to show inflammation but identified widespread infiltration of alveolar septum by anaplastic lymphoma kinase (ALK)-positive anaplastic cells.
ALK-positive anaplastic large cell lymphoma could present as a primary pulmonary disease without extra-pulmonary manifestations.
Core Tip: Primary anaplastic large cell lymphoma of the lung represents a diagnostic challenge due to diverse manifestations and non-specific radiological findings. We report a case of rapidly progressing anaplastic lymphoma kinase (ALK)-positive primary pulmonary anaplastic large cell lymphoma (ALCL) with bilateral multiple pulmonary consolidations, presenting initially as an acute lung infectious disease. ALK-positive ALCL could present as a primary pulmonary disease without extra-pulmonary manifestations.
- Citation: Jiang JH, Zhang CL, Wu QL, Liu YH, Wang XQ, Wang XL, Fang BM. Rapidly progressing primary pulmonary lymphoma masquerading as lung infectious disease: A case report and review of the literature. World J Clin Cases 2021; 9(16): 4016-4023
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/4016.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.4016
Anaplastic large cell lymphoma (ALCL) is a CD30 positive mature T-cell non-Hodgkin lymphoma (NHL) and in rare cases involves the lungs[1,2]. There are two variants of ALCL according to the World Health Organization classification: Anaplastic lymphoma kinase (ALK)-positive and negative ALCL[3]. The former has a characteristic t(2;5)(p23;35) chromosomal translocation, which fuses ALK on chromosome 2 with the nucleophosmin gene on chromosome 5, leading to ALK overexpression and constitutive tyrosine kinase activity[4,5]. ALK-positive ALCL, including ALK-positive primary pulmonary ALCL, tends to respond well to standard chemotherapy and is associated with a benign prognosis[6-8]. ALCL is one of the most curable cancers, with a cure rate of 65%-90% in children and over 70% in adults[9].
Because of its rarity, extranodal ALCL may present a diagnostic challenge, leading to delays in diagnosis and treatment and ultimately resulting in disease progression[10-12]. Herein, we report a case of rapidly progressing ALK-positive primary pulmonary ALCL with bilateral multiple pulmonary consolidations, presenting initially as an acute lung infectious disease. This case highlights the need to consider alterative diagnoses in cases of suspected lung infections that fail to respond to antibiotics treatment.
A 40-year-old woman presented on May 10, 2017 with a 6-d history of fever (up to 38.0 °C), non-productive cough, and exertional dyspnea.
The patient received empiric amoxycillin (1.5 g/d) at a community clinic 3 d ago, but symptoms persisted. The patient denied hemoptysis, chest pain, and weight loss.
The patient was a nonsmoker. She had no family history of hematologic or lung malignancies.
Physical examination at admission revealed a body temperature of 39.0 °C, heart rate of 103 beats/min, blood pressure of 120/70 mmHg, and respiratory rate of 25 breaths/min. Wheezes and dry rales were not heard in bilateral lungs on auscultation. No other remarkable abnormalities were found.
Laboratory test revealed hemoglobin 115 g/L, red blood cell count 3.98 × 1012/L, and increased leucocyte count (20100/mm3) with 90% neutrophils, 4% lymphocytes, and 1% eosinophils. No atypical lymphocytes were present in the peripheral blood. Laboratory investigations showed elevated C-reactive protein (230.1 mg/mL; normal reference range: < 10.0 mg/mL), procalcitonin (0.88 ng/mL; normal: < 0.10 ng/mL), and erythrocyte sedimentation rate (10 mm/h; normal: < 20 mm/h). Biochemical studies showed hypoalbuminemia (33.2 g/L; normal: 40.0-55.0 g/L), elevated alanine aminotransferase (65 U/L; normal: 7-40 U/L), aspartate transaminase (55 U/L; normal: 13-35 U/L), alkaline phosphatase (129 U/L; normal: 35-100 U/L), and lactate dehydrogenase (LDH) (549 U/L; normal: 114-240 U/L). Arterial blood gas analysis revealed PaO2 at 60 mmHg (normal: 80-100 mmHg) and SaO2 at 90% when breathing ambient air. Tumor biomarkers (e.g., CEA125, CA153, and CA199) were negative. Plasma virus tests for Epstein-Barr virus, cytomegalovirus, and respiratory syncytial virus were negative.
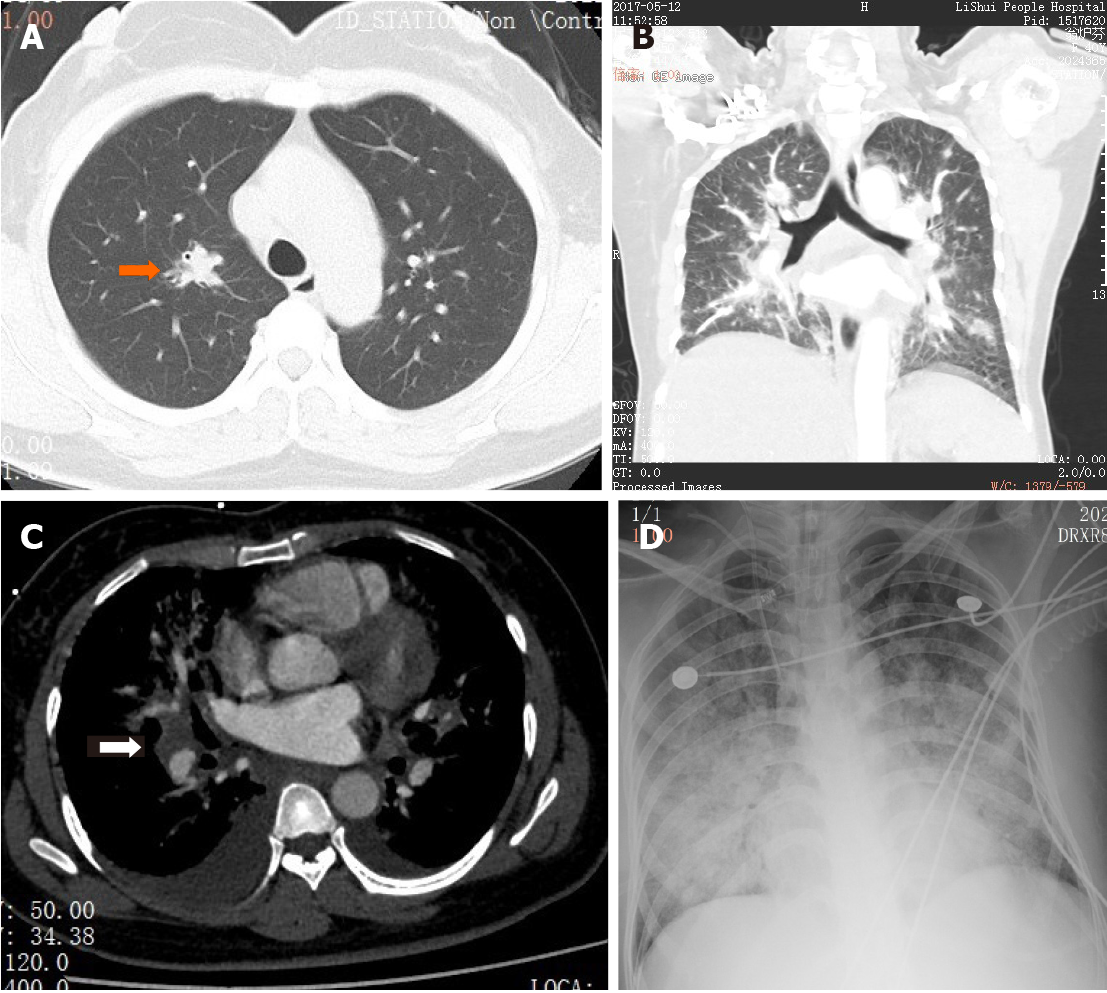
Contrast-enhanced computed tomography of the chest demonstrated multiple solid nodules throughout both lungs with the largest measuring 15 mm in diameter, with poor defined boundaries in the right upper lung (Figure 1A). No mediastinal lymphadenopathy was demonstrated. A blood culture was ordered.
A preliminary diagnosis of lung infection with atypical radiologic features was made. Differential diagnosis included malignancy and atypical infections such as a fungal, viral, or atypical bacterial infection.
Empiric cefoperazone sulbactam (1.0 g/8 h intravenously) and azithromycin (0.5 g once daily intravenously) were started. Oxygen supplementation was conducted via a nasal cannula.
The patient’s symptoms deteriorated rapidly and she was transferred to intensive care unit 3 d after hospital admission. Arterial blood gas analysis showed PaO2 at 48.6 mmHg and SaO2 at 84.2% when breathing oxygen at a rate of 5 L/min through a mask. Routine blood tests revealed a leucocyte count of 40800/mm3 with 96% neutrophils (39168/mm3) and again, no atypical lymphocytes. Flow cytometry showed no abnormal clones. A bone marrow smear suggested active proliferation along all three lineages, but no abnormal cells were found. A pulmonary artery computed tomography (CT) scan revealed normal pulmonary vasculature. However, diffuse patchy shadows with poorly defined boundaries were present in both lungs, with bilateral pleural effusion and an enlarged lymph node (25 mm × 21 mm) in the right hilum (Figure 1B). Fiber bronchoscopy and bronchoalveolar lavage were unremarkable. Antibiotics treatment was switched to meropenem (1.0 g/8 h) and vancomycin (1.0 g/12 h). A lung biopsy was recommended but the family members of the patient declined. Despite aggressive antimicrobial therapy, her respiratory failure continued to worsen and the patient eventually required 100% oxygen via the ventilator. Bedside X-ray revealed diffuse patch shadows in bilateral lungs (Figure 1C). Methylprednisolone (80 mg/8 h), ganciclovir (0.3 g/12 h intravenously), and caspofungin (70 mg for the first 24 h intravenously and 50 mg/d thereafter) were empirically initiated in order to broadly cover any atypical infection or inflammatory condition. Despite all aggressive measures, the patient’s condition deteriorated and on hospitalization day 6, she passed away due to refractory hypoxemic respiratory failure.
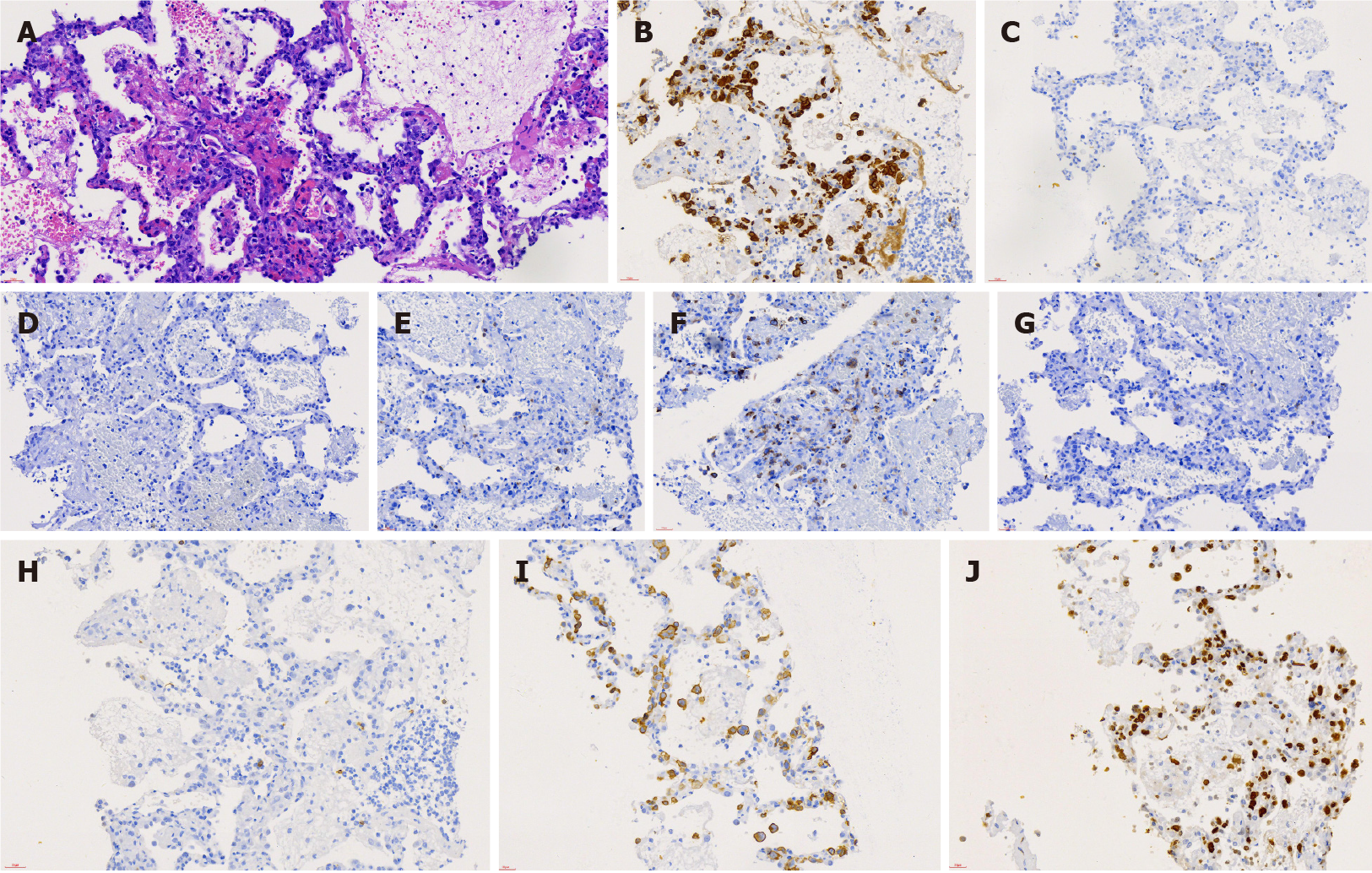
A post-mortem lung biopsy revealed an intact alveolar structure and no apparent inflammation. The alveolar septum was infiltrated with massive anaplastic lymphocytes and occasional neutrophils. The anaplastic lymphocytes were strongly positive for ALK and CD30, partially positive for CD7, and negative for CD20, CD3, CD5, CD4, CD8, and EBER. Most anaplastic lymphocytes were Ki-67 positive (Figure 2). Bone marrow tissue examination revealed normal karyotype and no anaplastic lymphocytes. Flow cytometry showed no abnormal clones.
ALK-positive primary pulmonary ALCL in the current case initially presented as an acute lung infectious disease. The patient exhibited signs mimicking a lung infection (fever, marked neutrophilia, and pulmonary consolidations). Despite a thorough work-up for infectious and non-infectious causes and aggressive antimicrobial regimen, the patient deteriorated rapidly, eventually leading to death within a week of presentation.
The case highlights the importance of promptly reassessing patients with suspected lung infectious diseases who fail to respond to antibiotics or antiviral therapy and considering alternative diagnoses including primary pulmonary ALCL.
Most clinicians are not familiar with ALK-positive primary pulmonary ALCL due to its very low incidence rate and atypical clinical features, leading to delays in diagnosis and treatment. Primary pulmonary lymphoma is defined as clonal lymphoid proliferation involving the lung parenchyma and/or bronchi without detectable extra-pulmonary lymphoma at primary diagnosis or the subsequent 3 mo[13]. Fiche et al[14] retrospectively analyzed the pathological features of 69 primary pulmonary NHL and there was no case of T cell lymphoma. Yao et al[15] only collected one case of ALK-positive primary pulmonary lymphoma in 6 years. Zhao et al[16] searched the PubMed for the literature on primary pulmonary ALCL and identified 16 cases (11 men, age range: 17-68 years) between 1990 and 2015. Six (6/15) of the cases were ALK-positive. In addition, 6/13 patients died from 21 d to 6 mo while our case died within 12 d of diagnosis. Although primary pulmonary ALCL may have a rapidly progressing course, a protracted course may be seen in heavily treated cases[17]. Padhi et al[18] have recently reviewed 39 cases of pulmonary ALCL reported over the preceding 30 decades and reported that 13/39 cases were ALK-positive.
Age, high β2 microglobulin and neutrophilia, and primary lung lesions may be closely associated with a poor prognosis of ALK-positive ALCL[19]. Other adverse prognostic events include fever, progressive respiratory failure, performance status, and high serum LDH. Leukemic phase ALCL is rare, which can occur upon initial presentation and during the course of the disease and may be associated with an aggressive course of ALCL. Several reports described three adult cases of ALK-positive ALCL with peripheral blood leukocytosis[20-22]. Two patients died and one relapsed, suggesting a poor prognosis in patients with peripheral leukocytosis in ALK-positive ALCL. Grewal et al[23] reported three cases of highly aggressive ALK-positive ALCL with a leukemic phase and multi-organ involvement; all three patients died within months of diagnosis. Similarly, our patient had persistent leukocytosis with neutrophils at 90%-96%, but no atypical lymphocytes in peripheral blood smears and no abnormal clones on flow cytometry. Also, bilateral lung involvement, marked respiratory distress, and significant hepatic injuries in the current cases are suggestive of high-risk malignant neoplasm[24]. In addition, lung tissues biopsy of our patient revealed a large number of infiltrating large anaplastic lymphocytes in the alveolar septum with scattered neutrophils. Without other feasible explanations, we suspected that lymphoma progression may lead to neutrophil-reactive hyperplasia but such a possibility requires more clinical observation. A report suggests a particularly poor prognosis in patients with tumor invasion into the central nervous system and the lungs[25].
Dyspnea with rapid changes in various lung imaging features may be a characteristic marker for ALK-positive primary pulmonary ALCL. Investigators have summarized the features of nodules, mass-like consolidation, alveolar or interstitial infiltrates, non-segmental infiltration with a hazy margin, a clear air bronchogram, ground-glass opacity, masses of pleural origin, pleural effusion, and peribronchial or perivascular thickening on chest CT in lung NHL patients[26-30]. However, the pace of radiological changes of ALK-positive primary pulmonary ALCL has been rarely reported. In the current case, chest CT and radiographs showed rapid radiological changes synchronous with disease progression. In recent years, 18F-2-deoxy-2-fluoro-D-glucose-positron emission tomography/CT has developed rapidly in the field of staging of lymphoma and efficacy evaluation of treatment due to its more sensitive display than conventional CT. Lee et al[31] showed higher standardized uptake values (SUVs) in systemic ALCL than in other types of aggressive T-cell lymphoma, as well as higher SUVs in ALK-positive ALCL patients than in ALK-negative ones. It is necessary for definite diagnosis by CT-guided percutaneous needle lung biopsies as soon as possible[32].
ALK-positive primary pulmonary ALCL is a rare disease with diverse clinical and radiological manifestations. Our case highlights the importance of considering alternative diagnoses including ALCL in cases suspected of lung infections but failing to respond to antimicrobial treatment.
We thank Professor Li XH from Department of Pathology, Beijing University Cancer Hospital for pathologic analysis of the lung tissues.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Deshwal H S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wu YXJ
| 1. | Borie R, Wislez M, Thabut G, Antoine M, Rabbat A, Couderc LJ, Monnet I, Nunes H, Blanc FX, Mal H, Bergeron A, Dusser D, Israël-Biet D, Crestani B, Cadranel J. Clinical characteristics and prognostic factors of pulmonary MALT lymphoma. Eur Respir J. 2009;34:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Delsol G, Jaffe ES, Falini B, Gascoyne RD, Müller-Hermelink HK, Stein H. Anaplastic large cell lymphoma (ALCL), ALK-positive. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon: IARC Press, 2008: 312-316. |
| 4. | Morris SW, Kirstein MN, Valentine MB, Dittmer K, Shapiro DN, Look AT, Saltman DL. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1995;267:316-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Werner MT, Zhao C, Zhang Q, Wasik MA. Nucleophosmin-anaplastic lymphoma kinase: the ultimate oncogene and therapeutic target. Blood. 2017;129:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne RD, Armitage JO, Weisenburger DD; International Peripheral T-Cell Lymphoma Project. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496-5504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 617] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 7. | Piccaluga PP, Fuligni F, De Leo A, Bertuzzi C, Rossi M, Bacci F, Sabattini E, Agostinelli C, Gazzola A, Laginestra MA, Mannu C, Sapienza MR, Hartmann S, Hansmann ML, Piva R, Iqbal J, Chan JC, Weisenburger D, Vose JM, Bellei M, Federico M, Inghirami G, Zinzani PL, Pileri SA. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study. J Clin Oncol. 2013;31:3019-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Agnelli L, Mereu E, Pellegrino E, Limongi T, Kwee I, Bergaggio E, Ponzoni M, Zamò A, Iqbal J, Piccaluga PP, Neri A, Chan WC, Pileri S, Bertoni F, Inghirami G, Piva R; European T-Cell Lymphoma Study Group. Identification of a 3-gene model as a powerful diagnostic tool for the recognition of ALK-negative anaplastic large-cell lymphoma. Blood. 2012;120:1274-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Prokoph N, Larose H, Lim MS, Burke GAA, Turner SD. Treatment Options for Paediatric Anaplastic Large Cell Lymphoma (ALCL): Current Standard and beyond. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Wang LJ, Wu HB, Zhang Y, Zhou WL, Wang QS. A Rare Case of Neutrophil-Rich, ALK-Negative Anaplastic Large Cell Lymphoma in the Lung Mimicking a Pulmonary Abscess on 18F-FDG PET/CT. Clin Nucl Med. 2019;44:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Luo J, Jiang YH, Lei Z, Miao YL. Anaplastic lymphoma kinase-negative anaplastic large cell lymphoma masquerading as Behcet's disease: A case report and review of literature. World J Clin Cases. 2019;7:3377-3383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Khor LK, Wang S, Lu SJ. Anaplastic large cell lymphoma of the vertebra masquerading as tuberculous spondylitis: potential pitfalls of conventional imaging. Intern Emerg Med. 2012;7:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Cadranel J, Wislez M, Antoine M. Primary pulmonary lymphoma. Eur Respir J. 2002;20:750-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 254] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Fiche M, Caprons F, Berger F, Galateau F, Cordier JF, Loire R, Diebold J. Primary pulmonary non-Hodgkin's lymphomas. Histopathology. 1995;26:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Yao D, Zhang L, Wu PL, Gu XL, Chen YF, Wang LX, Huang XY. Clinical and misdiagnosed analysis of primary pulmonary lymphoma: a retrospective study. BMC Cancer. 2018;18:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Zhao Q, Liu Y, Chen H, Zhang Y, Du Z, Wang J, Wang Y. Successful Chemo-Radiotherapy for Primary Anaplastic Large Cell Lymphoma of the Lung: A Case Report and Literature Review. Am J Case Rep. 2016;17:70-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Mahuad CV, Repáraz Mde L, Zerga ME, Aizpurua MF, Casali C, Garate G. Three Years Sustained Complete Remission Achieved in a Primary Refractory ALK-Positive Anaplastic T Large Cell Lymphoma Treated with Crizotinib. Rare Tumors. 2016;8:6266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Padhi S, Panigrahi MK, Mohapatra S, Mishra P, Patra S, Sable M, Thakur B, Nayak M, Panigrahi A. Pulmonary anaplastic large-cell lymphoma: a case-based systematic review of world literature. J Cancer Res Ther. 2020;. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Sibon D, Fournier M, Brière J, Lamant L, Haioun C, Coiffier B, Bologna S, Morel P, Gabarre J, Hermine O, Sonet A, Gisselbrecht C, Delsol G, Gaulard P, Tilly H. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol. 2012;30:3939-3946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Anderson MM, Ross CW, Singleton TP, Sheldon S, Schnitzer B. Ki-1 anaplastic large cell lymphoma with a prominent leukemic phase. Hum Pathol. 1996;27:1093-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Villamor N, Rozman M, Esteve J, Aymerich M, Colomer D, Aguilar JL, Campo E, Montserrat E. Anaplastic large-cell lymphoma with rapid evolution to leukemic phase. Ann Hematol. 1999;78:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Awaya N, Mori S, Takeuchi H, Sugano Y, Kamata T, Takeuchi T, Abe T. CD30 and the NPM-ALK fusion protein (p80) are differentially expressed between peripheral blood and bone marrow in primary small cell variant of anaplastic large cell lymphoma. Am J Hematol. 2002;69:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Grewal JS, Smith LB, Winegarden JD 3rd, Krauss JC, Tworek JA, Schnitzer B. Highly aggressive ALK-positive anaplastic large cell lymphoma with a leukemic phase and multi-organ involvement: a report of three cases and a review of the literature. Ann Hematol. 2007;86:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Onciu M, Behm FG, Raimondi SC, Moore S, Harwood EL, Pui CH, Sandlund JT. ALK-positive anaplastic large cell lymphoma with leukemic peripheral blood involvement is a clinicopathologic entity with an unfavorable prognosis. Report of three cases and review of the literature. Am J Clin Pathol. 2003;120:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Nguyen KA, Su C, Bai HX, Zhang Z, Xiao R, Karakousis G, Zhang PJ, Zhang G. Disease site as a determinant of survival outcome in patients with systemic anaplastic lymphoma kinase positive anaplastic large cell lymphoma with extranodal involvement: an analysis of 1306 cases from the US National Cancer Database. Br J Haematol. 2018;181:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Tokuyasu H, Harada T, Watanabe E, Touge H, Kawasaki Y, Endo A, Maeda R, Isowa N, Ohnuma H, Miura H, Shimizu E. Non-Hodgkin's lymphoma accompanied by pulmonary involvement with diffuse ground-glass opacity on chest CT: a report of 2 cases. Intern Med. 2009;48:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Chen Y, Chen A, Jiang H, Zhang Y, Zhu L, Xia C, Yu H. HRCT in primary pulmonary lymphoma: can CT imaging phenotypes differentiate histological subtypes between mucosa-associated lymphoid tissue (MALT) lymphoma and non-MALT lymphoma? J Thorac Dis. 2018;10:6040-6049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Zhang J, Wang M, Yang X, Liu C, He X. Primary pulmonary extranodal NK/T-cell lymphoma of nasal type misdiagnosed as pneumonia: A case report and literature review. Medicine (Baltimore). 2017;96:e8914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Okada F, Sato H, Omeri AK, Ono A, Tokuyama K, Ando Y, Matsumoto A, Ogata M, Kohno K, Takano K, Mori H. Chest HRCT findings in acute transformation of adult T-cell lymphoma/Leukemia. Eur Radiol. 2015;25:1607-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Okada F, Ando Y, Kondo Y, Matsumoto S, Maeda T, Mori H. Thoracic CT findings of adult T-cell leukemia or lymphoma. AJR Am J Roentgenol. 2004;182:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Lee DY, Lee JJ, Kim JY, Park SH, Chae SY, Kim S, Yoon DH, Suh C, Huh J, Ryu JS. (18)F-FDG PET in Patients with Primary Systemic Anaplastic Large Cell Lymphoma: Differential Features According to Expression of Anaplastic Lymphoma Kinase. Nucl Med Mol Imaging. 2013;47:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Qian J, Luo DL, Zhang JE, Li WY, Gao XL, Fang XF, An H, Deng JL, Li Q, Wu J. Diagnostic and prognostic factors for patients with primary pulmonary non-Hodgkin's lymphoma: A 16-year single-center retrospective study. Oncol Lett. 2019;18:2082-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |