Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.3895
Peer-review started: November 6, 2020
First decision: January 23, 2021
Revised: January 31, 2021
Accepted: February 25, 2021
Article in press: February 25, 2021
Published online: June 6, 2021
Processing time: 189 Days and 3.8 Hours
Gastric intestinal metaplasia (GIM) is a precancerous lesion of the stomach, which severely affects human life and health. Currently, a variety of endoscopic techni
To directly compare the diagnostic value of WLE, ME-AAC, and ME-OE for detection of GIM.
A total of 156 patients were subjected to consecutive upper gastrointestinal endoscopy examinations using WLE, ME-AAC, and ME-OE. Histopathological findings were utilized as the reference standard. Accuracy, sensitivity, specificity, and positive and negative predictive values of the three endoscopy methods in the diagnosis of GIM were evaluated. Moreover, the time to diagnosis with ME-AAC and ME-OE was analyzed. Two experts and two non-experts evaluated the GIM images diagnosed using ME-OE, and diagnostic accuracy and intra- and inter-observer agreement were analyzed.
GIM was detected in 68 of 156 patients (43.6%). The accuracy of ME-OE was highest (91.7%), followed by ME-AAC (86.5%), while that of WLE (51.9%) was lowest. Per-site analysis showed that the overall diagnostic accuracy of ME-OE was higher than that of ME-AAC (P = 0.011) and WLE (P < 0.001). The average diagnosis time was lower in ME-OE than in ME-AAC (64 ± 7 s vs 151 ± 30 s, P < 0.001). Finally, the inter-observer agreement was strong for both experts (k = 0.862) and non-experts (k = 0.800). The internal consistency was strong for experts (k = 0.713, k = 0.724) and moderate for non-experts (k = 0.667, k = 0.598).
For endoscopists, especially experienced endoscopists, ME-OE is an efficient, convenient, and time-saving endoscopic technique that should be used for the diagnosis of GIM.
Core Tip: This study evaluated the diagnostic value of optical-enhanced magnifying endoscopy (ME-OE) for gastric intestinal metaplasia. By comparing ME-OE with existing magnifying endoscopy techniques, the study evaluated their diagnostic ability, operating time, and inter- and intra-observer agreements. Our study showed that ME-OE is an efficient, convenient, and time-saving endoscopic technique for endoscopists, especially experienced endoscopists. We recommend its wide clinical application in diagnosing gastric intestinal metaplasia.
- Citation: Song YH, Xu LD, Xing MX, Li KK, Xiao XG, Zhang Y, Li L, Xiao YJ, Qu YL, Wu HL. Comparison of white-light endoscopy, optical-enhanced and acetic-acid magnifying endoscopy for detecting gastric intestinal metaplasia: A randomized trial. World J Clin Cases 2021; 9(16): 3895-3907
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/3895.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.3895
Gastric cancer poses a tremendous threat to human health due to its high morbidity and mortality worldwide. It is the third leading cause of mortality among all cancers[1]. Correa[2] proposed a cascade (superficial gastritis, atrophic gastritis, gastric intestinal metaplasia (GIM), gastric intraepithelial neoplasia, and intestinal-type gastric cancer), of which GIM is of high clinical significance.
In most cases GIM develops from atrophic gastritis. Early diagnosis and prompt treatment may prevent GIM from progressing to intestinal-type gastric cancer. If left untreated, GIM will further evolve into intraepithelial neoplasia and eventually progress to intestinal-type gastric cancer. Patients with GIM are prone to gastric cancer, and those with high grade dysplasia have a 25% chance of developing gastric cancer within one year[3], which is approximately 10 to 20 times higher in terms of relative risk of gastric cancer development compared with the general population[4]. Therefore, early and accurate assessment is critical for the treatment and prognosis of gastric cancer[5]. Annual endoscopic examinations for these high-risk patients increase the chance of detecting early gastric cancer, and thus improving the survival rate[6]. In addition, they are a cost-effective way of detecting early gastric cancer and precan
However, a previous study showed the limitations of white-light endoscopy (WLE) for the detection of GIM[5]; the major factors responsible for these limitations include the lack of specific manifestations of GIM under white light and lack of effective diagnostic indicators for GIM under WLE. With the development of endoscopic techniques, the application of acetic-acid chromoendoscopy (AAC) has gradually increased. Acetic acid reversibly denatures cytoplasmic proteins by destroying disulfide bonds of glycoproteins, thus enhancing the mucosal architecture and pit-pattern of the columnar epithelium[8]. Previous studies have shown that AAC can be used effectively to detect GIM[8,9]. Optical-enhanced endoscopy (OE) is a novel technique of electronic chromoendoscopy[10]. The innovative optical filters may achieve higher overall transmittance by connecting the peaks of hemoglobin absorption spectrum (415 nm, 540 nm and 570 nm), generating a continuous wavelength spectrum. There are two modes with different OE filters (Modes 1 and 2). Mode 1 is designed mainly to improve visualization of microvessels with a sufficient amount of light, and Mode 2 is designed to improve contrast of white-light observation by bringing the color tone of the overall image closer to that of natural color (white-color tone) with much more light than with Mode 1 filter.
Since the gastric epithelium requires careful observation and the light blue crest (LBC) sign is important under narrow band imaging, Mode 1 enables better diagnosis of GIM through the use of a high-resolution magnifying endoscope system. However, to the best of our knowledge, no study has been conducted to evaluate the efficacy of OE for GIM detection. In addition, there is a scarcity of data regarding the diagnosis of GIM using only acetic-acid chromoendoscopy combined with magnifying endoscopy (ME-AAC) or optical-enhanced magnifying endoscopy (ME-OE). Therefore, in this study, we evaluated and compared the efficacies of ME-OE and ME-AAC for the detection of GIM.
This was a prospective, randomized, and single-center study conducted at a teaching hospital in Zhengzhou, China. All participating patients provided written informed consent. The research protocol was approved by the Ethics Committee of Zhengzhou Central Hospital Affiliated to Zhengzhou University (202031) and registered in the China Clinical Trial Registry (ChiCTR2000032072).
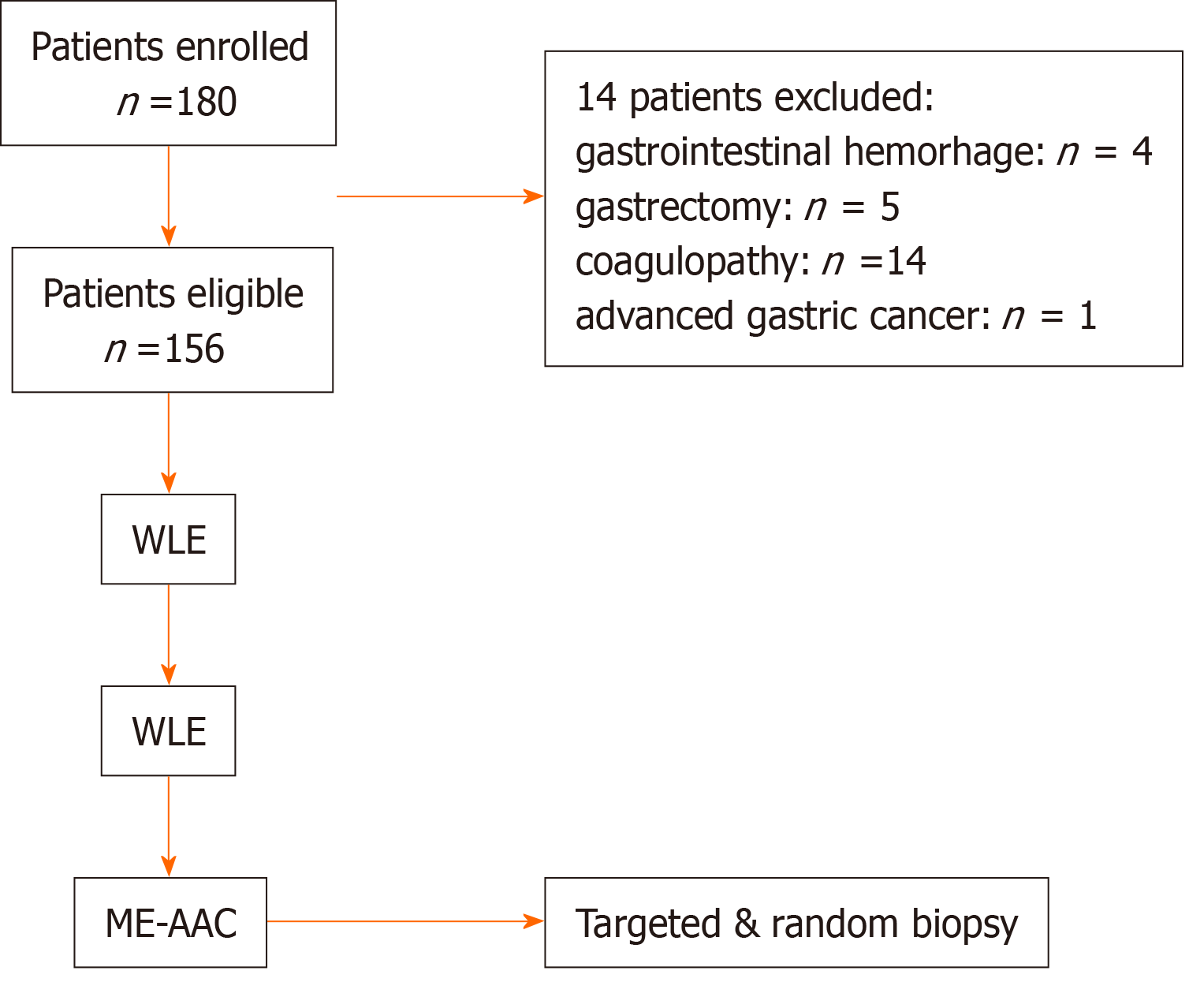
All consecutive patients who were undergoing gastroscopy examinations were routinely evaluated for potential symptoms including upper abdominal discomfort/ pain, anemia, acid reflux/heartburn, suspected peptic ulcer, and dyspepsia. Other relevant data such as the findings on physical examination and medical history (smoking habit, alcohol consumption, Helicobacter pylori infection, and gastric cancer family history) were also included. The exclusion criteria were as follows: (1) age < 18 years or > 80 years; (2) gastrointestinal hemorrhage, peptic ulcer, history of gastrectomy, or advanced gastric cancer; (3) dysfunction of the coagulation system; (4) allergy to acetic acid or narcotic drugs; and (5) inability to provide written informed consent.
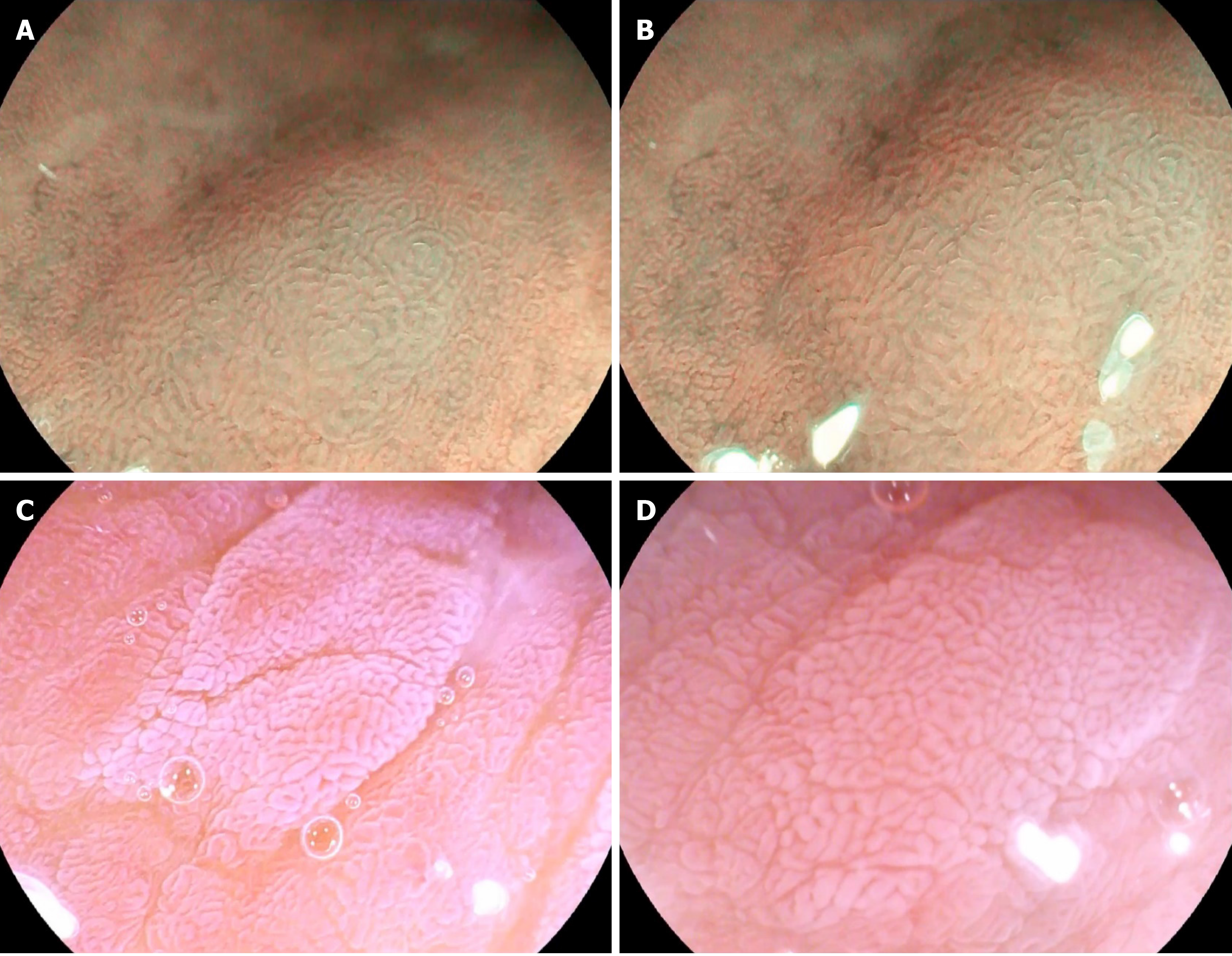
As no diagnostic criteria are currently available for GIM by ME-OE, we considered the LBC (Figure 1A and B)[11] identified on the surface of gastric mucosa by magnifying narrow-band imaging system endoscopy (ME-NBI) as a manifestation of GIM. Tanaka, Toyoda[12] divided the enhanced microstructure of the gastric mucosa surface after spraying acetic acid solution into five types, and classified type III (gastric pits demonstrating cerebral gyrus- or villi-like changes, different from oval, patchy, or irregular white nodules of the surrounding mucosa) (Figure 1C and D) as GIM manifestations.
As the effect of acetic acid on the gastric mucosa usually lasts from a few seconds to a few minutes[8,13], we were unable to observe the entire stomach in such a short period of time. Therefore, the gastric antrum and angular notch, the most common localizations of GIM[13], were chosen as the main examination areas[14].
The two endoscopists (Wu, Li), who had more than five years of endoscopic operation experience, were trained for two weeks on endoscopic images containing LBC and cerebral gyrus-like or villi-like changes and were subsequently evaluated. Fifty images containing LBC changes, 50 images containing cerebral gyrus or villi-like changes, and 20 images of normal gastric mucosa were selected. Eighty images were randomly selected each time for evaluation. In total, three evaluations were performed, and an accuracy rate greater than 80% in each evaluation was required from the endoscopists for their qualification. Moreover, each endoscopic technique was performed for at least 50 pre-study trainings using the same equipment.
Approximately 15-20 min before the examination, patients were given oral streptomycin particles and simethicone emulsion (method: 20000 units of dew protease particles, sodium bicarbonate 1 g, and simethicone emulsion 5 mL dissolved in 60 mL lukewarm water). The endoscopy system used in this study was EK-i7000 from Pentax (Pentax, Tokyo, Japan), and the magnifying endoscope model was MagniView EG-2990Zi, with a focal range of 4–100 mm, and the maximum 136 × optical zoom capability. All gastroscopic examinations were performed with a black silicone elastomer cap on the distal end of the gastroscope (Pentax DiStal Rubber Hood OE-A59, Pentax, Tokyo, Japan). All enrolled patients received intravenous anesthesia. The anesthetic drugs (propofol 10 mL or midazolam 10 mL) were administered intravenously 5 min before the endoscopic examinations. All patients were given nasal cannula oxygen during the anesthesia process, the entire anesthesia process was performed by a certified anesthetist, and vital signs were monitored throughout the entire procedure.
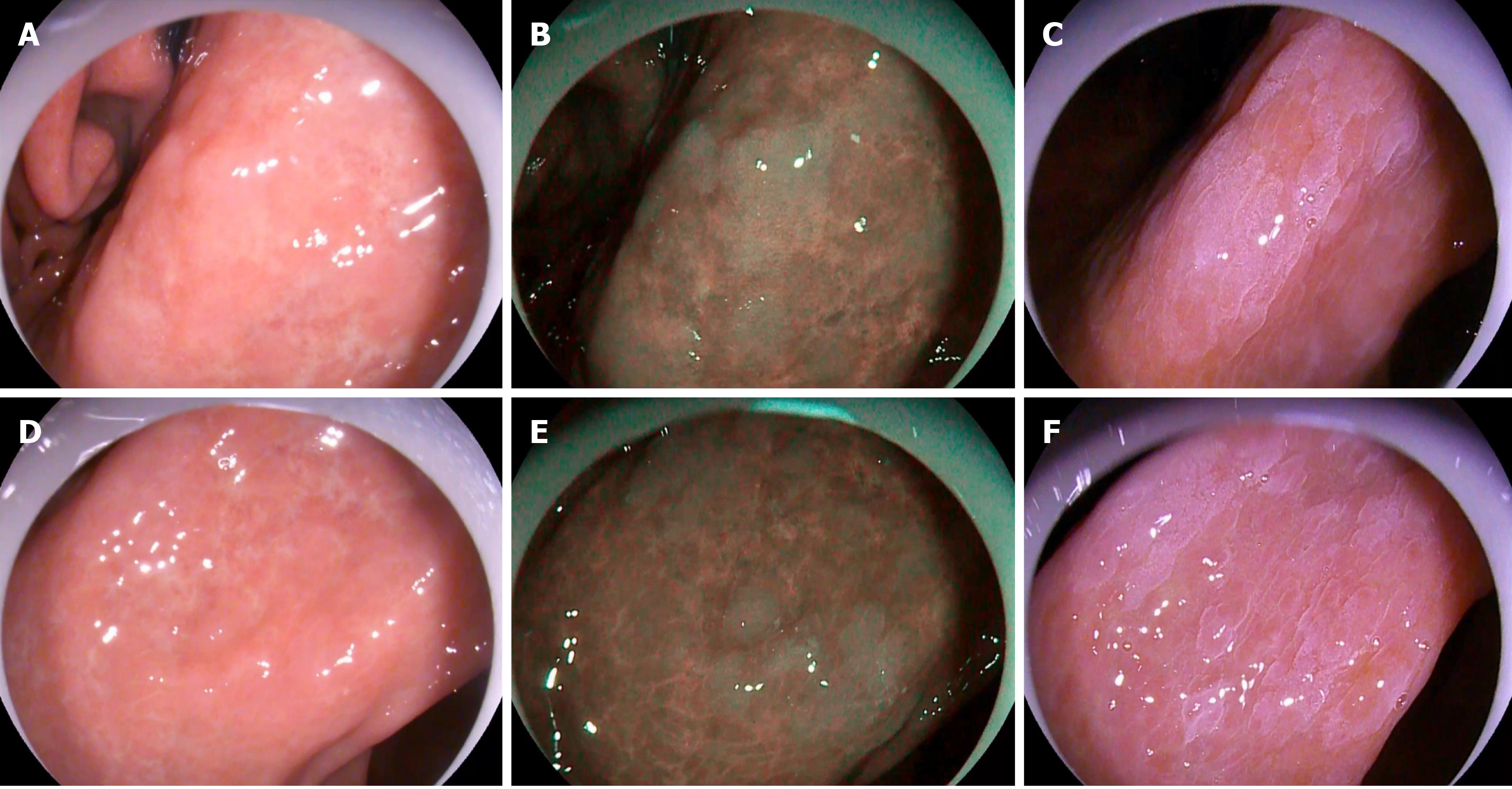
The study flowchart is shown in Figure 2. First, a random endoscopist entered the room. The first one to enter the room was referred to as the ME-OE endoscopist, and the second one to enter the room was referred to as the ME-AAC endoscopist. The examination was performed with the routine WLE procedure. Any mucosal abnormalities, such as rough mucosal surface and localized discoloration, were carefully examined and evaluated. Among the abnormalities, gray-white nodule changes (Figure 3A and D) were considered indicators of GIM lesions in WLE. The locations of the abnormalities were recorded and potential GIM lesions were reported to a research assistant. The areas of the abnormalities were then rinsed. For areas difficult to rinse, pronase and simethicone were added. Subsequently, OE Mode 1 was turned on, and it was observed whether there were bluish-whitish areas (Figure 3B and E). After the area was zoomed in to observe whether there was LBC on the mucosal surface (Figure 1A and B), the area was photographed and the findings reported to a research assistant. The assistant recorded the time from turning on Mode 1 to reaching the diagnosis. After turning off the OE Mode 1, the area was rinsed, and fluid in the stomach was completely aspirated. In order to avoid damaging the mucous membrane and affecting the observation, we did not perform the biopsy until after the ME-AAC examination. The ME-OE endoscopist left the room after the procedure, and then the ME-AAC endoscopist entered the room. During the whole process, any form of communication between the endoscopists was not allowed. A total of 20 mL of 2% acetic acid solution was evenly sprayed on the gastric antrum and angular notch using a spray tube, and excess acetic acid solution was aspirated after spraying. After about 5–10 s, when the reaction appeared (Figure 3C and F), the presence of gyrus- or villi-like changes (Figure 1C and D) was observed in magnification mode, photographed, and reported to the research assistant. The research assistant recorded the location of the lesion in detail, as well as the time from inserting the spray tube to reaching the diagnosis and withdrawing the spray tube. Targeted biopsies were performed whenever lesions were suspected by the ME-AAC endoscopist. The ME-AAC endoscopist then performed targeted biopsies of the sites that had been identified by the ME-OE endoscopist if they had not already been sampled during the ME-AAC procedure. The research assistant, who was present throughout the process, confirmed that the ME-AAC endoscopist had taken one biopsy from each identified site. In addition, three random biopsies were collected by the ME-AAC endoscopist from the gastric antrum and angular notch of each patient. The research assistant recorded the biopsy locations. The specimens were subjected to histopathological examination.
The tissue specimens collected during endoscopies were placed into formalin solution for 24 h, subjected to conventional dehydration, paraffin embedding, and sectioning, and then stained using the hematoxylin and eosin (H/E) staining method. Two experienced pathologists reviewed the slides independently and reached histological conclusions without knowing the endoscopic findings. If the conclusions of the two pathologists were notably different, a third pathologist was consulted. The diagnostic histopathological criteria were based on the Vienna classification.
With histopathological evaluation as a reference standard, we evaluated the accuracy, sensitivity, specificity, and positive and negative predictive values of WLE, ME-AAC, and ME-OE endoscopy for detection of GIM, as primary outcomes.
The secondary outcome measures included the comparison of the time needed to reach a diagnosis of GIM by ME-AAC and ME-OE, and the intra- and inter-observer agreements when ME-OE was used.
In this study, histopathological diagnosis from tissue biopsies was used as the gold standard. WLE, ME-AAC, and ME-OE endoscopic examinations were performed in enrolled patients to evaluate the diagnostic value of the three examination methods. The sensitivity of ME-AAC for the diagnosis of GIM was estimated to be 77.6%[9] with an acceptable error of sensitivity of 10%; the specificity was 94.4%[9] with an acceptable error of 10%, and the confidence was specified as 0.95. The same number of samples was used to calculate both parameters using PASS11 software. The required sample size was 152 patients.
Statistical analysis was performed using SPSS 26.00 statistical software. Frequencies with percentages were used to represent qualitative variables, whereas means and standard deviations were used to represent quantitative variables. Comparisons of qualitative variables were conducted using Fisher's exact probability test or the chi-square test, while for comparisons of continuous variables we used the t test. Intra-observer agreement and inter-observer agreement were determined by the kappa test (higher values of kappa coefficient denote higher intra- and inter-observer agreements). A value of P < 0.05 was considered statistically significant.
From April 2020 to October 2020, 180 patients were screened. After excluding 24 patients, 156 patients were included in the study. The detailed clinical characteristics are presented in Table 1. There were no differences in clinical characteristics.
| Parameter | Value | |
| Number | 156 | |
| Gender (M/F) | 83/73 | |
| Age (mean ± SD), yr | M | 58.9 ± 8.9 |
| F | 58.5 ± 7.9 | |
| Physical examination | 22 | |
| Symptoms | ||
| Upper abdominal discomfort/pain | 30 | |
| Anemia | 13 | |
| Acid reflux/heartburn | 37 | |
| Suspected peptic ulcer | 9 | |
| Dyspepsia | 45 | |
| Medical history | ||
| Smoking habit | 52 | |
| Alcohol consumption | 57 | |
| Helicobacter pylori infection | 31 | |
| Family history of gastric cancer | 12 | |
Per-participant analysis: Among 156 patients, 68 had GIM. There were 85 patients with suspected GIM based on WLE, of which 27 were confirmed by histopathology. Seventy-one patients with suspected GIM were found using ME-OE, of which 63 were confirmed by histopathology. Sixty-nine patients with suspected GIM were found by ME-AAC, of which 58 were confirmed by histopathology. With histopathology as a reference method, the specificity, sensitivity, and accuracy of the three endoscopic methods were determined (Table 2). The accuracy of ME-OE was the highest (91.7%), followed by ME-AAC (86.5%), and WLE (51.9%). The overall diagnostic accuracy of ME-OE was higher than that of ME-AAC (P < 0.001) and WLE (P < 0.001).
| Sensitivity | Specificity | PPV | NPV | Accuracy | P value | ||
| WLE | 39.7% | 61.4% | 44.3% | 56.8% | 51.9% | 0.001 | |
| ME-OE | 92.6% | 90.9% | 88.7% | 94.1% | 91.7% | 0.001 | |
| ME-AAC | 85.3% | 87.5% | 84.1% | 88.5% | 86.5% | ||
Per-site analysis: A total of 361 targeted biopsies were taken, and 248 of them were diagnosed as GIM by histopathology. WLE assessment indicated suspected GIM in 106 sites, of which 85 were confirmed by histopathology. ME-OE suspected GIM in 275 sites, of which 239 were confirmed by histopathology. ME-AAC found suspected GIM in 291 sites, of which 230 were confirmed by histopathology. In addition, a total of 468 random biopsies were analyzed, of which 40 were histopathologically diagnosed as GIM. The accuracy of ME-OE (89.7%) was higher than that of ME-AAC (85.6%) and WLE (73%). The overall diagnostic accuracy of ME-OE was higher than that of ME-AAC (P = 0.011) and WLE (P < 0.001) as shown in Table 3.
| Sensitivity | Specificity | PPV | NPV | Accuracy | P value | ||
| WLE | 29.5% | 96.1% | 80.2% | 71.9% | 73% | 0.001 | |
| ME-OE | 82.9% | 93.3% | 86.9% | 91.2% | 89.7% | 0.011 | |
| ME-AAC | 79.9% | 88.7% | 79.0% | 89.2% | 85.6% | ||
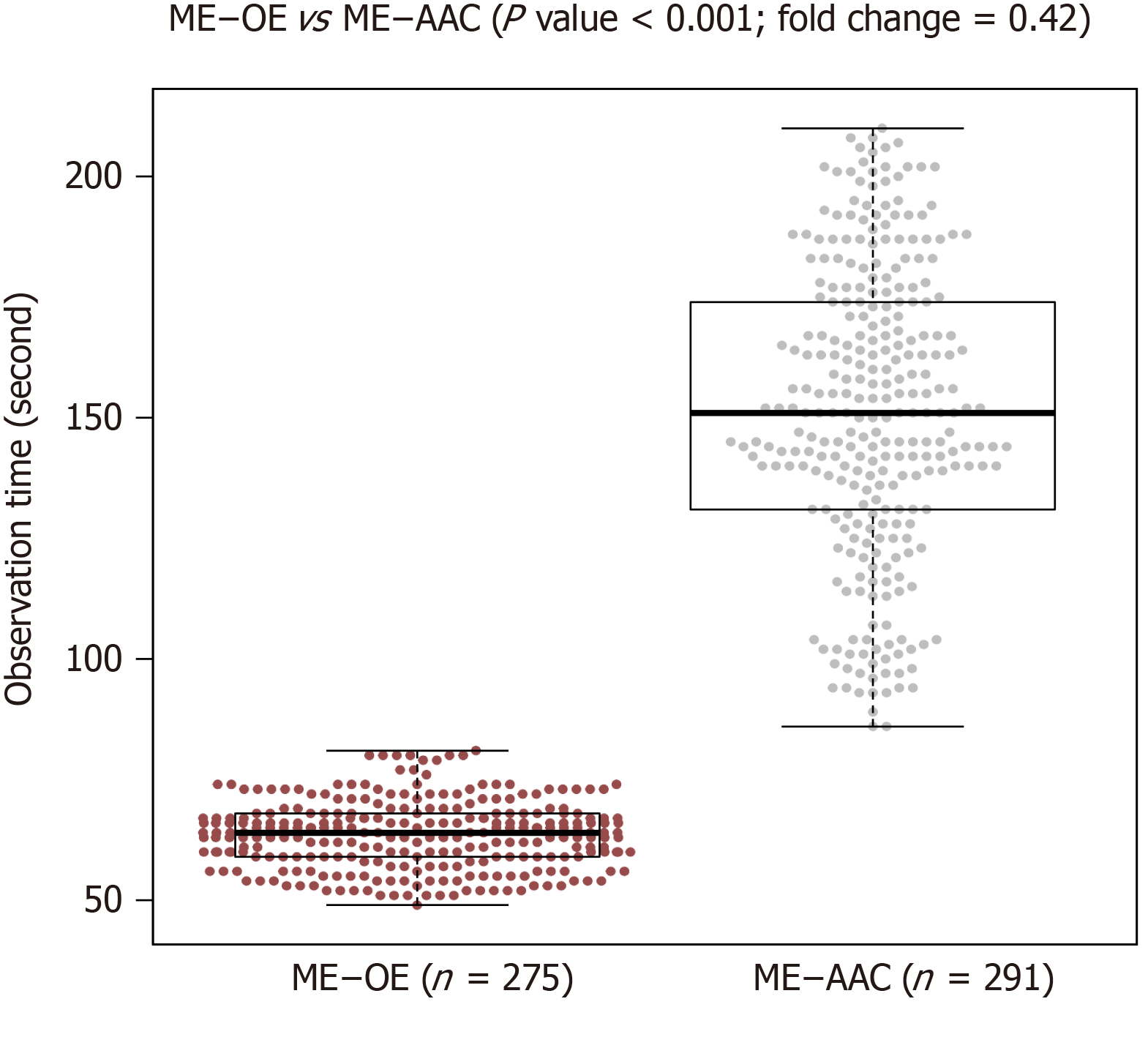
Average diagnosis time was 64 ± 7 s for ME-OE and 151 ± 30 s for ME-AAC (P < 0.001), indicating that ME-OE took only 40% of the time taken by ME-AAC (Figure 4). White light endoscopy is only a preliminary identification of abnormal areas. Thus, the diagnosis time was not evaluated. After completion of all experiments, we conducted a retrospective analysis. From all the images obtained in this study, 20 ME-OE images diagnosed with GIM and 10 ME-OE images without GIM were selected by the research assistant. Two experts (Liang and Xu) with more than 5 years of endoscopic experience and two non-experts (Chang and Qu) with less than 2 years of endoscopic experience, who did not participate in the initial analysis, judged these images immediately after 30 min of training and again after 2 wk. The overall accuracy of the four physicians was 79.2%, the overall accuracy of the two experts was 83.3% (83.3%, 83.3%), and the overall accuracy of the two non-expert doctors was 75% (76.7%, 80%). The inter-observer agreement was almost perfect (k = 0.862) for experts and substantial (k = 0.800) for non-experts. The intra-observer agreement was substantial for the experts (k = 0.713, k = 0.724) and substantial (k = 0.667) and moderate (k = 0.598) for the non-experts (Tables 4-6).
| Endoscopist | Sensitivity | Specificity | PPV | NPV | Accuracy |
| Expert Liang | 85% | 80% | 89.5% | 72.7% | 83.3% |
| Expert Xu | 80% | 90% | 94.1% | 69.2% | 83.3% |
| Non-expert Chang | 65% | 80% | 86.7% | 53.3% | 76.7% |
| Non-expert Qu | 75% | 90% | 93.8% | 64.3% | 80% |
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Experts (n = 2) | 82.5% | 85% | 91.7% | 70.8% | 83.3% |
| Non-experts (n = 2) | 70% | 85% | 90.3% | 58.6% | 75% |
| All observers (n = 4) | 76.3% | 85% | 91.0% | 64.1% | 79.2% |
| Inter-observer agreement | Intra-observer agreement | ||
| Experts (n = 2) | 0.862 | Expert Liang | 0.713 |
| Expert Xu | 0.724 | ||
| Non-experts (n = 2) | 0.800 | Non-expert Chang | 0.667 |
| Non-expert Qu | 0.598 | ||
Our study shows that ME-OE is more advantageous than WLE and ME-AAC in the diagnosis of GIM. First, more patients with GIM and more GIM sites were detected by ME-OE than by the other two methods. Second, ME-OE is a more reliable GIM diagnostic tool than ME-AAC for an experienced endoscopist. Third, compared with ME-AAC, ME-OE significantly reduces the procedure time.
WLE is used as a standard endoscopy examination and is the most commonly used endoscopic technique for digestive tract diseases. Kaminishi et al[15] evaluated the accuracy of endoscopy in the diagnosis of chronic gastritis, and found that the gray-white nodule changes showed high specificity, but very low sensitivity. In our study, the sensitivity of WLE for diagnosis of GIM was 39.7%, specificity was 61.4%, and accuracy was 51.9%. Similar observations were reported in other studies[5,16]. As WLE cannot adequately predict the presence of GIM[17],which may lead to missed diagnosis of some early cancers and precancerous lesions. Another study suggested a very poor correlation between WLE endoscopic manifestations and histopathological diagnosis[18], consistent with our WLE results of correct diagnosis in 27 along with misjudgment in 58 patients. This may reflect (1) the high requirements for the operation ability and professional knowledge of endoscopists[19,20]; and (2) the fact that WLE can only observe the morphology of mucous membrane macroscopically, while missing some microstructural mucosal lesions. These results demonstrate that WLE has a low diagnosis rate for GIM, and may not be suitable for the diagnosis of GIM.
Acetic-acid staining is a classic staining method first applied in colposcopy by Hinselmann[21] in 1938. Then, acetic-acid chromoendoscopy was mainly used for staining the gastric and esophageal mucosa. Currently, AAC is the most commonly used method for gastric mucosa staining as it is a practical and economical method for evaluating the size and degree of mucosal lesions. After spraying acetic acid, optical properties of the epithelial cells are changed by slightly changing the pH or by reversibly changing their protein architecture, highlighting the columnar epithelial cells and reflecting white light from the mucosal surface[22]. In a previous study of acetic-acid chromoendoscopy without magnification, Song et al[9] showed that the overall diagnostic accuracy of acetic-acid gastroscopy could reach 89%, while the sensitivity and specificity were 77.6% and 94.4%, respectively. AAC is a valid and reproducible approach to determine the extent of GIM and may become a practical method for identifying individuals at high risk of gastric cancer. According to the available data, there seems to be no significant difference between the use of AAC without magnification and the use of ME-AAC in the diagnosis of GIM. In our study, we did not use AAC without magnification to diagnose GIM, and we will confirm our speculation in future studies.
The principle of ME-OE is similar to that of ME-NBI. Uedo et al[11] reported that the detection of LBC on the surface of gastric mucosa epithelium in ME-NBI may be a unique endoscopic manifestation related to intestinal metaplasia, and other ME-NBIs studies[11,23,24], confirmed LBC as a highly accurate sign of the histological presence of intestinal metaplasia. In addition, LBC is associated with the progression to severe GIM[25]. Due to the nature of the NBI filter, the resulting light is very weak, so the image is very dark, However, this drawback is compensated by OE. This may bring some benefits to diagnosing GIM.
Per-participant detection rates of GIM differed significantly among the three endoscopic methods. The diagnostic accuracy of ME-OE for GIM was significantly higher than that of ME-AAC (91.7% vs 86.5%, P < 0.001) and WLE (91.7% vs 51.9%, P < 0.001). Per-site diagnostic accuracy of ME-OE for GIM was also significantly higher than that of ME-AAC (89.7% vs 85.6%, P = 0.011) and WLE (89.7% vs 73%, P < 0.001), and more GIM patients and GIM sites were diagnosed. This finding is relevant, because, at present, sufficient evidence on the usefulness of ME-OE for GIM is lacking. Our study shows higher diagnostic capability of ME-OE compared with the other two techniques, proving that LBC diagnosis of GIM is also applicable to ME-OE. The possible explanations for this are as follows: (1) WLE can only detect lesions visible to the naked eye, causing misdiagnosis of some mucosal microstructural lesions; (2) ME-AAC spraying skills determine how much the lesion site is exposed (inability to master the spraying technique will lead to missed diagnosis of the lesion site); (3) ME-OE can be switched to the correct mode by simply pressing a button. The operation is simple, no additional skills are needed, and changes in mucosal microstructure can be observed, which greatly enhances the diagnosis; and (4) ME-OE overcomes the disadvantage of the dim NBI light source.
In terms of diagnosis time, ME-OE was also faster and convenient than ME-AAC (64 ± 7 s vs 151 ± 30 s, P < 0.001). After the study, we communicated with the operating physicians and summarized the possible reasons for longer diagnosis time with ME-AAC as follows: (1) it takes a certain time to insert and withdraw the spray tube; (2) it is difficult to accurately and successfully spray the lesion in one attempt; (3) the physician needs to wait for the whitening reaction of acetic acid; and (4) inaccurate spraying or insignificant whitening reaction results in a longer judgment time. Of these, the difficulty to accurately spray in one attempt is likely the major factor affecting the operation time. Furthermore, we also analyzed the operation time of ME-OE and showed that the time was mainly spent on flushing the mucous membrane surface and magnifying the view to find LBC. Our study found that most of the subtle lesions were blocked by mucus or other substances; thus, it is necessary to rinse the mucosal surface until it is clean, as otherwise it may affect the observation and judgment. However, improper irrigation can also cause minor mucosal bleeding, which may affect the observation. Hence, this requires the physicians to operate gently and observe carefully.
This is also the first study to investigate the inter- and intra-observer agreement for GIM judgment using ME-OE, and to further compare the diagnostic accuracy of experienced and inexperienced endoscopists to understand whether the degree of experience affects the accuracy of diagnosis. Here we showed that the consistency in both the experts and non-experts was strong. The diagnostic accuracy of experienced endoscopists was better than that of inexperienced endoscopists (83.3% vs 75%). The internal consistency of the expert observers was strong, while that of non-expert observers was moderate. Nevertheless, the diagnostic consistency of ME-OE was acceptable even for non-experts. The European Society of Gastrointestinal Endos
This study also had some limitations. Namely, this was a single-center study with a small sample size, and thus, further in-depth, large-scale, and multi-center studies are required in the future. In addition, no specific GIM optical diagnosis criteria for ME-OE are available thus far, and we hope that in the near future an ME-OE diagnostic standard for GIM can be established. In this study, we selected the regions with the highest prevalence of intestinal metaplasia, the gastric antrum and angular notch. Future studies should examine the whole stomach to reduce the risk of misdiagnosis. The participants in this study were all from the same hospital, which may also have affected the results. The repeatability of ME-OE diagnosis of GIM needs to be further verified by more cases and observations.
In spite of its limitations, the strengths of the study included the in-depth analysis of the reasons for the differences in accuracy and operation time of various endoscopic techniques on the basis of previous similar studies. Despite the established association with increased gastric cancer risk, the diagnosis of GIM presents a dilemma for many gastroenterologists. WLE in the diagnosis of GIM seems to impracticable, the ability to diagnose ME-AAC is modest and a significant amount of time is necessary[27]. The study adds to our understanding of the diagnosis of GIM by ME-OE. In addition, the ability of ME-OE to diagnose GIM was significantly better than that of ME-AAC and WLE, the method not only has high accuracy but is also simple to perform with ME-AAC and saves time. Furthermore, endoscopic experience and education are needed to raise the diagnostic accuracy of GIM.
ME-OE is an efficient, convenient, and time-saving endoscopic technique for diagnosing GIM, and the results of this study have some important implications for future practice. Further research is required to establish its diagnostic efficiency for GIM.
Optical-enhanced endoscopy is a newly developed endoscopic technology and is currently commercially available. However, there is a scarcity of data regarding the diagnosis of gastric intestinal metaplasia (GIM) using only acetic-acid chromoen
To provide a theoretical basis and practical guidance for new endoscopic techniques to diagnose GIM.
To directly compare the diagnostic value of white-light endoscopy (WLE), ME-AAC, and ME-OE for the detection of GIM.
A total of 156 patients were subjected to consecutive upper gastrointestinal endoscopy examinations using WLE, ME-AAC, and ME-OE. The accuracy of these three endoscopic methods in the diagnosis of GIM were evaluated. Moreover, the time to diagnosis with ME-AAC and ME-OE was analyzed. Two experts and two non-experts evaluated the GIM images diagnosed using ME-OE, and diagnostic accuracy and intra- and inter-observer agreement were analyzed.
The ability of ME-OE to diagnose GIM was significantly better than that of ME-AAC and WLE, the method not only has high accuracy but is also simple to perform with ME-AAC and saves time. Furthermore, endoscopic experience and education are needed to raise the diagnostic accuracy of GIM.
For endoscopists, especially experienced endoscopists, ME-OE is an efficient, convenient, and time-saving endoscopic technique for the diagnosis of GIM.
The results of this study have some important implications for future practice. Further research is required to establish the diagnostic efficiency of ME-OE for GIM.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Eleftheriadis N S-Editor: Fan JR L-Editor: Webster JR P-Editor: Xing YX
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554-3560. [PubMed] |
| 3. | Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, Lane C, Dias-Silva D, Sahakian A, Jayaram P, Pimentel-Nunes P, Shue D, Pepper M, Cho D, Laine L. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc. 2017;86:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Kim N, Park RY, Cho SI, Lim SH, Lee KH, Lee W, Kang HM, Lee HS, Jung HC, Song IS. Helicobacter pylori infection and development of gastric cancer in Korea: long-term follow-up. J Clin Gastroenterol. 2008;42:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Xirouchakis E, Laoudi F, Tsartsali L, Spiliadi C, Georgopoulos SD. Screening for gastric premalignant lesions with narrow band imaging, white light and updated Sydney protocol or both? Dig Dis Sci. 2013;58:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50:378-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Lim LG, Yeoh KG, Srivastava S, Chan YH, Teh M, Ho KY. Comparison of probe-based confocal endomicroscopy with virtual chromoendoscopy and white-light endoscopy for diagnosis of gastric intestinal metaplasia. Surg Endosc. 2013;27:4649-4655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Chen H, Wu X, Liu Y, Wu Q, Lu Y, Li C. Blue laser imaging with acetic acid enhancement improved the detection rate of gastric intestinal metaplasia. Lasers Med Sci. 2019;34:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Song KH, Hwang JA, Kim SM, Ko HS, Kang MK, Ryu KH, Koo HS, Lee TH, Huh KC, Choi YW, Kang YW. Acetic acid chromoendoscopy for determining the extent of gastric intestinal metaplasia. Gastrointest Endosc. 2017;85:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Neumann H, Fujishiro M, Wilcox CM, Mönkemüller K. Present and future perspectives of virtual chromoendoscopy with i-scan and optical enhancement technology. Dig Endosc. 2014;26 Suppl 1:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S, Tatsuta M. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Tanaka K, Toyoda H, Kadowaki S, Kosaka R, Shiraishi T, Imoto I, Shiku H, Adachi Y. Features of early gastric cancer and gastric adenoma by enhanced-magnification endoscopy. J Gastroenterol. 2006;41:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Sha J, Wang P, Zhu B, Zhu M, Li X, Gao F. Acetic Acid Enhanced Narrow Band Imaging for the Diagnosis of Gastric Intestinal Metaplasia. PLoS One. 2017;12:e0170957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Mastracci L, Bruno S, Spaggiari P, Ceppa P, Fiocca R. The impact of biopsy number and site on the accuracy of intestinal metaplasia detection in the stomach A morphometric study based on virtual biopsies. Dig Liver Dis. 2008;40:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kaminishi M, Yamaguchi H, Nomura S, Oohara T, Sakai S, Fukutomi H, Nakahara A, Kashimura H, Oda M, Kitahora T, Ichikawa H, Yabana T, Yagawa Y, Sugiyama T, Itabashi M, Unakami M, Oguro Y, Sakita T. Endoscopic classification of chronic gastritis based on a pilot study by the research society for gastritis. Dig Endosc. 2002;14:138-151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Capelle LG, Haringsma J, de Vries AC, Steyerberg EW, Biermann K, van Dekken H, Kuipers EJ. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig Dis Sci. 2010;55:3442-3448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Castro R, Rodriguez M, Libânio D, Esposito G, Pita I, Patita M, Santos C, Pimentel-Nunes P, Dinis-Ribeiro M. Reliability and accuracy of blue light imaging for staging of intestinal metaplasia in the stomach. Scand J Gastroenterol. 2019;54:1301-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Atkins L, Benedict EB. Correlation of gross gastroscopic findings with gastroscopic biopsy in gastritis. N Engl J Med. 1956;254:641-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Laine L, Cohen H, Sloane R, Marin-Sorensen M, Weinstein WM. Interobserver agreement and predictive value of endoscopic findings for H. pylori and gastritis in normal volunteers. Gastrointest Endosc. 1995;42:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, Lara-Santos L, Guilherme M, Moreira-Dias L, Lomba-Viana H, Ribeiro A, Santos C, Soares J, Mesquita N, Silva R, Lomba-Viana R. Magnification chromoendoscopy for the diagnosis of gastric intestinal metaplasia and dysplasia. Gastrointest Endosc. 2003;57:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Hinselmann H. Die Essigsäureprobe ein Bestandteil der erweiterten Kolposkopie. Dtsch Med Wochenschr. 1938;64:40-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Ji R, Liu J, Zhang MM, Li YY, Zuo XL, Wang X, Li YQ. Optical enhancement imaging versus acetic acid for detecting gastric intestinal metaplasia: A randomized, comparative trial. Dig Liver Dis. 2020;52:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Rerknimitr R, Imraporn B, Klaikeaw N, Ridtitid W, Jutaghokiat S, Ponauthai Y, Kongkam P, Kullavanijaya P. Non-sequential narrow band imaging for targeted biopsy and monitoring of gastric intestinal metaplasia. World J Gastroenterol. 2011;17:1336-1342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Wei N, Mulmi Shrestha S, Shi RH. Markers of gastric intestinal metaplasia under digital chromoendoscopy: systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | An JK, Song GA, Kim GH, Park DY, Shin NR, Lee BE, Woo HY, Ryu DY, Kim DU, Heo J. Marginal turbid band and light blue crest, signs observed in magnifying narrow-band imaging endoscopy, are indicative of gastric intestinal metaplasia. BMC Gastroenterol. 2012;12:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Dekker E, Houwen BBSL, Puig I, Bustamante-Balén M, Coron E, Dobru DE, Kuvaev R, Neumann H, Johnson G, Pimentel-Nunes P, Sanders DS, Dinis-Ribeiro M, Arvanitakis M, Ponchon T, East JE, Bisschops R. Curriculum for optical diagnosis training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2020;52:899-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 27. | Huang RJ, Choi AY, Truong CD, Yeh MM, Hwang JH. Diagnosis and Management of Gastric Intestinal Metaplasia: Current Status and Future Directions. Gut Liver. 2019;13:596-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |