Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3765
Peer-review started: January 9, 2021
First decision: February 12, 2021
Revised: February 15, 2021
Accepted: March 8, 2021
Article in press: March 8, 2021
Published online: May 26, 2021
Processing time: 121 Days and 23.7 Hours
Liver transplantation (LT) is the most effective treatment strategy for advanced liver diseases. With the increasing survival rate and prolonged survival time, the postoperative long-term complications of LT recipients are becoming an important concern. Among them, the newly developed cancer after LT is the second complication and cause of LT-related death after cardiovascular disease. At present, few papers have reported multiple primary carcinomas (MPCs) after LT. Herein, we retrospectively analyzed an MPC case with gastric cancer and lung cancer after LT.
Herein, we retrospectively analyzed an MPC case with de novo gastric cancer and lung cancer after LT with no obvious complaints. Forty-one months after LT, the patient underwent radical distal gastrectomy (Billroth II) for intramucosal signet ring cell carcinoma, and then thoracoscopic wedge resection of the right lower lobe of the right lung and localized lymph node dissection 2 mo later. Therefore, paying attention to follow-up in LT recipients with early detection and intervention of de novo MPCs is the key to improving the survival rate and quality of life of LT recipients.
De novo MPCs after LT are rare, and the prognosis is poorer. However, early detection and related intervention can significantly improve the prognosis of patients. Therefore, we recommend that liver transplant recipients should be followed and screened for newly developed malignant tumors to improve the survival rate and quality of life.
Core Tip: Few papers have reported de novo multiple primary carcinomas (MPCs) after liver transplantation (LT) yet. Herein, we retrospectively analyzed an MPC case with gastric cancer and lung cancer after LT. Forty-one months after LT, the patient underwent radical distal gastrectomy for a de novo intramucosal signet ring cell carcinoma in the stomach. Two months later, the patient underwent thoracoscopic wedge resection of the right lower lobe of the right lung for the de novo lung cancer. The patient has survived till now with stable function of the graft. Early diagnosis and early treatment of de novo MPCs are the keys to improving the survival rate and quality of life of LT recipients with de novo MPCs.
- Citation: Rao W, Liu FG, Jiang YP, Xie M. De novo multiple primary carcinomas in a patient after liver transplantation: A case report. World J Clin Cases 2021; 9(15): 3765-3772
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3765.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3765
Liver transplantation (LT) is the most effective treatment strategy for advanced liver diseases. With the increasing survival rate and prolonged survival time, the postoperative long-term complications of LT recipients are becoming an important concern. Among them, newly developed cancer after LT is the second complication and cause of LT-related death after cardiovascular disease[1]. At present, few papers have reported multiple primary carcinomas (MPCs) after LT[2-4] (Table 1). Herein, we retrospectively analyzed an MPC case with gastric cancer and lung cancer after LT. The diagnosis and treatment were performed in the Affiliated Hospital of Qingdao University. Our aim was to explore the clinical characteristics, early diagnosis, and early treatment of MPC after LT.
| Ref. | Year of report | Gender/age | Time of LT | Indication of LT | IS after LT | Characteristic of MPC | Prognosis | ||||
| Time of diagnosis | Duration of MPC and LT (mo) | Symptom | Pathological diagnosis | Treatment | |||||||
| Wang et al[2] | 2009 | Male/56 | September, 2006 | Liver cirrhosis related to HBV, HCC | Tac + MMF + Pred, Tac monotherapy 3 mons after LT | June, 2007 | 9 | Dysphagia | Squamous cell carcinoma of esophagus (Poorly differentiated) | Gamma knife radiotherapy | 5 mo after MPC, died of tumor progression and multiple organ failure |
| June, 2007 | 9 | NA | Adenocarcinoma of rectum | NA | |||||||
| Peng et al[3] | 2015 | Female/77 | February, 2007 | Liver cirrhosis related to HBV, HCC | Tac + MMF + Pred, Tac monotherapy 3 mons after LT | March,2011 | 49 | RBC (+) in urine samples | Left renal clear cell carcinoma | Laparoscopic partial nephrectomy | Graft function is stable, and no tumor recurrence and metastasis |
| July, 2013 | 77 | Ground glass nodule in left upper lobe of lung | Adenocarcinoma of the upper lobe of the right lung (Well differentiated) | Thoracoscopic right upper lobectomy | |||||||
| Wang et al[4] | 2017 | Female/62 | February, 2010 | Liver cirrhosis related to HBV | NA | April, 2013 | 38 | NA | Renal clear cell carcinoma | NA | NA |
| June, 2015 | 59 | NA | Adenocarcinoma of lung | NA | |||||||
| Present case | 2021 | Male/47 | September, 2015 | Liver cirrhosis related to HBV | Tac + MMF + Pred, Tac monotherapy 3 mons after LT | January, 2019 | 39 | Stomach distress | Signet ring cell carcinoma of gastric body (Poorly differentiated), pT1aN0M0 | Radical distal gastrectomy | Graft function is stable, and no tumor recurrence and metastasis |
| February, 2019 | 40 | NA | Adenocarcinoma in situ in right lower lobe of lung | Limited thoracoscopic lymphadenectomy and right thoracoscopic lobectomy | |||||||
No obvious complaints.
A 47-year-old man underwent orthotopic LT due to hepatitis B liver cirrhosis with portal hypertension on September 19, 2015.
The patient had a history of hepatitis B virus (HBV) infection for more than 30 years.
The patient had a history of smoking for nearly 20 years and drinking for nearly 15 years, but had no family history of gastrointestinal tumors before LT.
Physical examination showed no positive signs.
The blood concentration of FK506 was 3.3 ng/mL, and the tumor markers and graft function showed no obvious abnormalities, as the de novo MPCs were diagnosed.
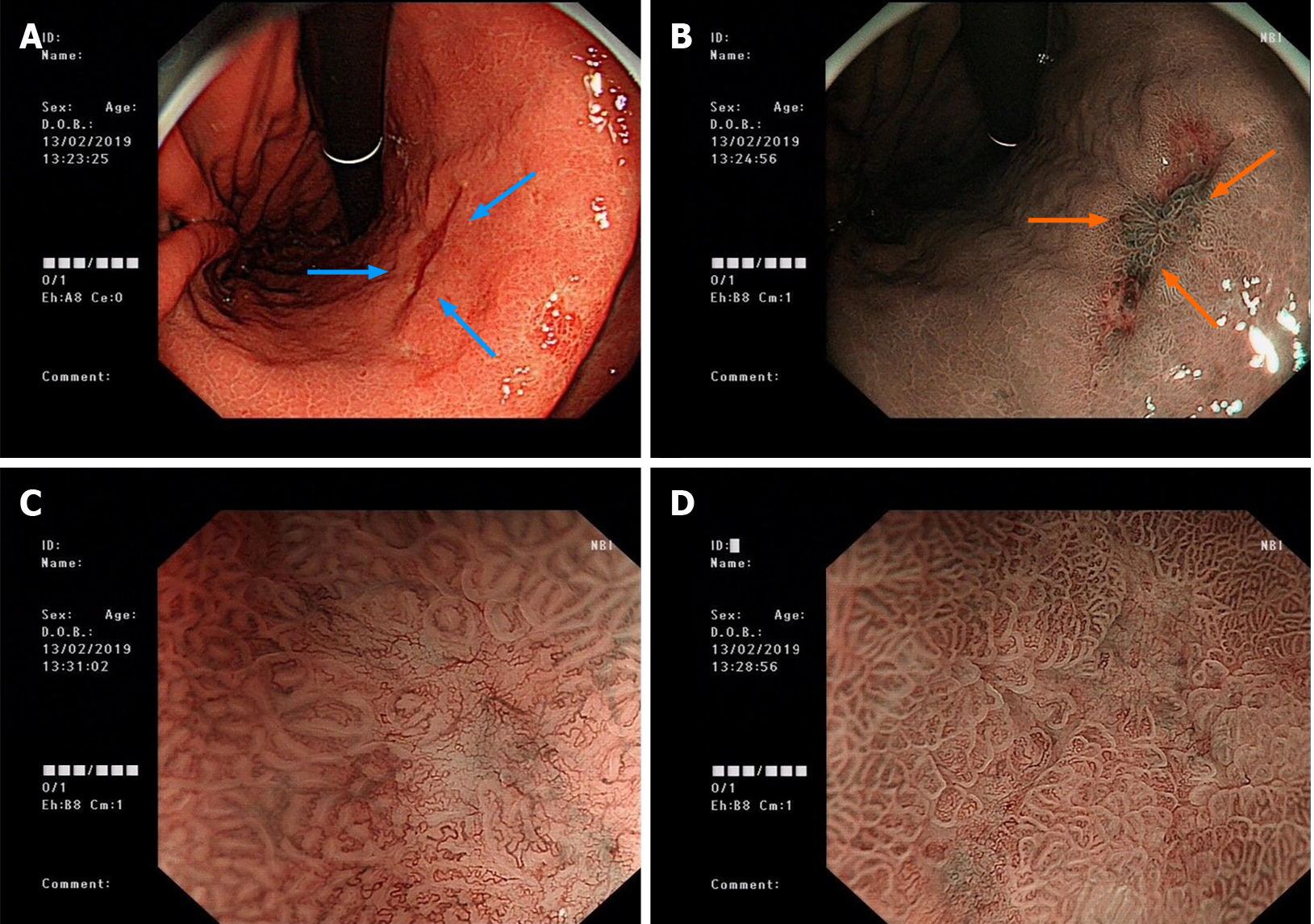
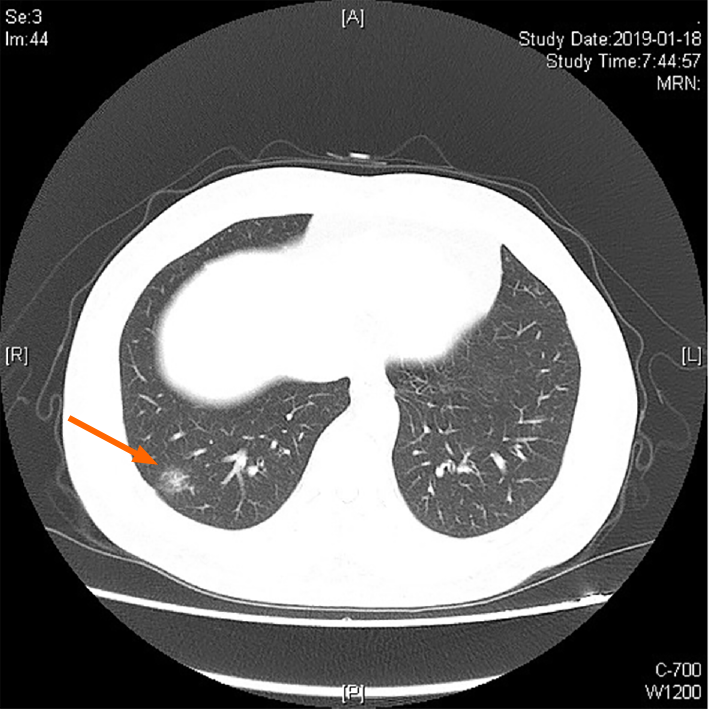
In January 2019, the patient underwent electronic gastroscopy due to stomach illness (Figure 1). The results showed a 2.0 cm × 1.0 cm flat concave mucosa on the lesser curvature of the lower stomach, a rough mucosa of the central gastric angle, and a 0.5 cm × 0.6 cm flat concave mucosa on the anterior wall of the gastric antrum. Histopathological examination showed poorly differentiated carcinoma (signet ring cell carcinoma) of the gastric antrum, gastric horn, and gastric body with HP (+), and immunohistochemistry (IHC) showed CK (+) and CD56 (-). Given all these results, combined with the results of narrow band imaging and magnifying gastroscopy, the patient was diagnosed with early gastric cancer (IIb + IIc) in February 2019. So the patient underwent radical distal gastrectomy (Billroth II) in February 2019 (41 mo after LT). Postoperative histopathological examination revealed intramucosal signet ring cell carcinoma (1.5 cm × 1.3 cm; Lauren type: diffuse), without invading the muscularis mucosa and metastasis. IHC showed CK (+), Her-2 (+), TOPO2 (level I), CD31 detecting for intravascular tumor thrombus (D2-40, -), S-100 detecting for nerve invasion (-), Ki-67 (20%), PD-1 (interstitial lymphocytes +, < 1%), and thick-walled vascular invasion (-). TNM stage was determined as pT1aN0M0. Two months later, the patient underwent high-resolution computed tomography (CT) of the chest. The result showed a nodule in the lower lobe of the right lung, which might be lung cancer (Figure 2). Further examination by positron emission tomography (PET)-CT showed the ground glass and solid mixed density nodules in the posterior basal segment of the right lower lobe of the lung, around 15 mm in length, with slightly higher metabolism than the background of the lung and an SUVmax value of about 1.3, suggesting to be lung tumors, and no tumor metastasis was found in other sites. Therefore, the patient underwent thoracoscopic wedge resection of the right lower lobe of the right lung and localized lymph node dissection in April 2019 (43 mo after LT). Histopathological examination revealed adenocarcinoma in situ, without lymph node or distant metastasis. IHC results showed CK (+) and elastic fiber staining detecting for visceral pleura invasion (-).
De novo intramucosal signet ring cell carcinoma of the gastric body and de novo adenocarcinoma in the right lower lobe of the right lung post LT.
Radical distal gastrectomy (Billroth II) for the intramucosal signet ring cell carcinoma of the gastric body, and thoracoscopic wedge resection and localized lymph node dissection for the de novo adenocarcinoma in the right lower lobe of the right lung.
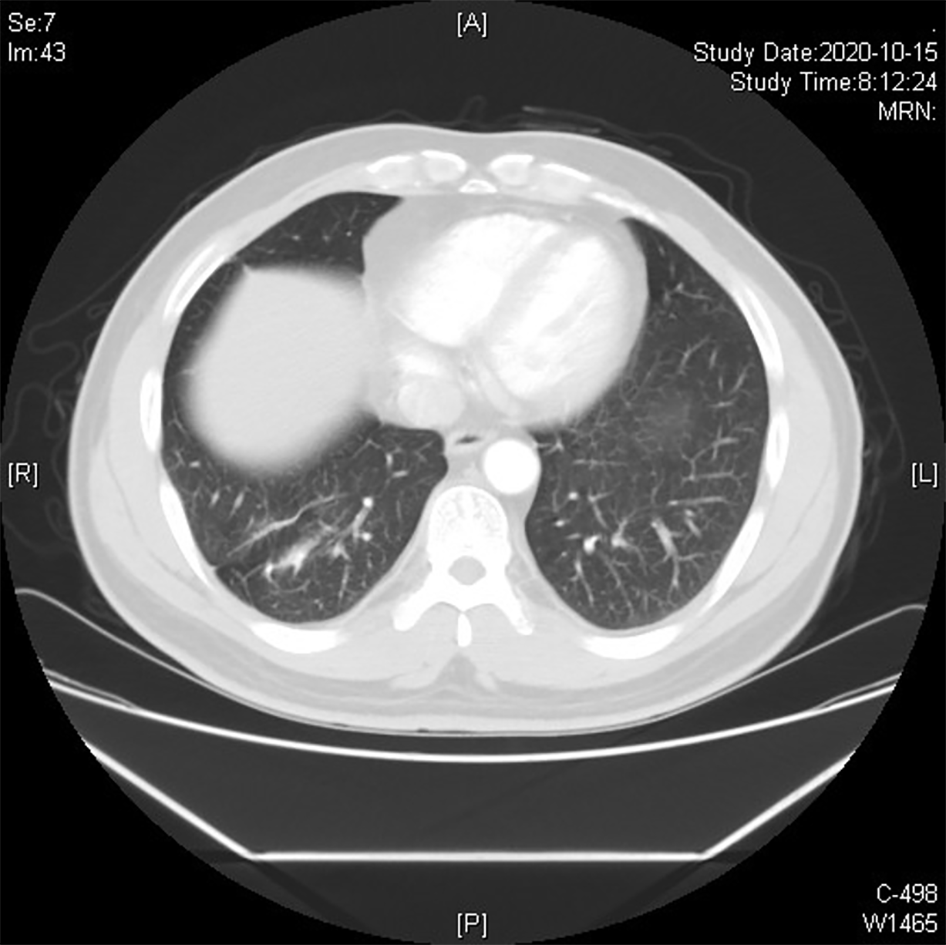
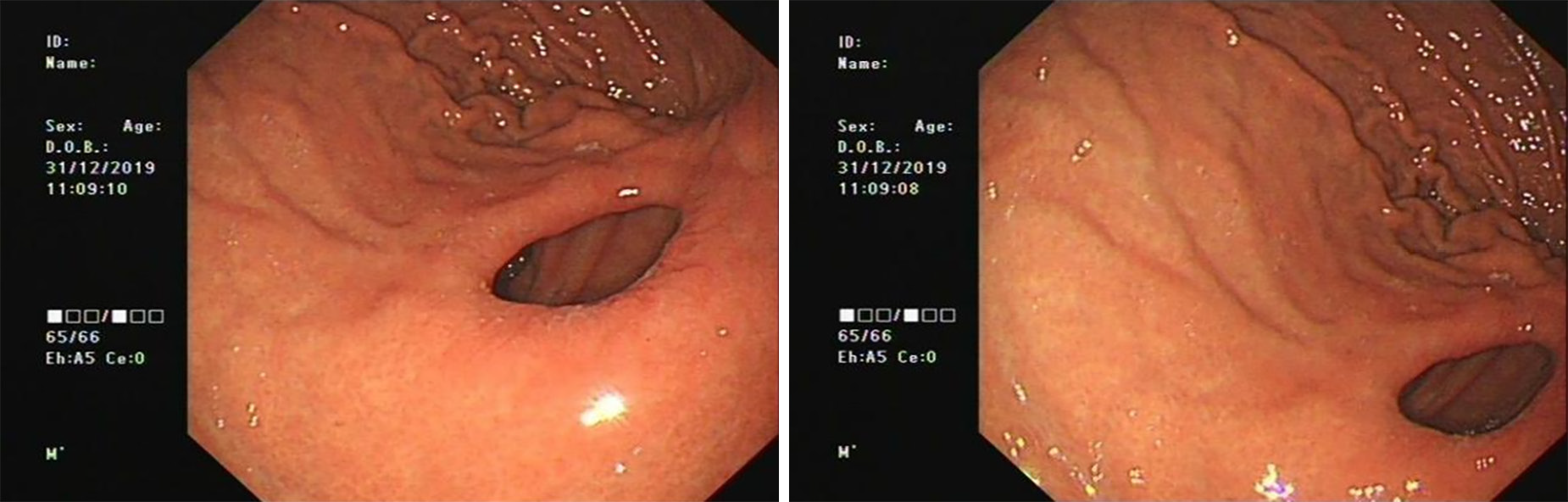
The patient has survived till now, and the graft has been in good condition (Figures 3 and 4).
In Europe and the United States, skin cancer and lymphoproliferative diseases are the most common primary malignant tumors after LT[1]. Whereas in Asia including China, gastrointestinal cancers are of the highest incidence[5-9], which may indicate the different epidemiological characteristics of tumors between Western and Asian countries. According to the 2019 statistics of the National Cancer Registry in China, the incidence of malignant tumors was 285.83/100000 in 2015. Among them, lung cancer and gastric cancer ranked the top two malignant tumors, accounting for 30.29% of common tumors[10]. And the 5-year survival rates were 19.7% and 35.7%, respectively[11]. The incidence of primary cancers in LT recipients has increased significantly, ranging from 0.76% to 16% depending on the follow-up time, age, and immunosuppressant regimens[5-9]. Only a few papers reported MPC after LT[2-4]. The definition of MPC is two or more primary malignant tumors that occur in single or multiple tissues simultaneously or sequentially in the same individual[12]. When the interval between the occurrence of two tumors is within 6 mo, they are considered as synchronous MPC (SC), and when the interval is less than 6 mo, they are considered as metachronous MPC (MC). Here we report a rare MPC case with newly developed gastric cancer and lung cancer after LT. The interval between gastric cancer and lung cancer was only 1 mo, and it therefore can be diagnosed as SC after LT. Genetically, the two primary tumors in this case exhibited totally different somatic mutation features, which further confirmed that the two tumors derived independently. Generally, the prognosis of MPC is poor, and the prognosis of SC after LT is worse[11,12]. However, this case has survived till now owing to early diagnosis and early intervention, and the graft has been in good condition.
The patient in this study was a man with a history of smoking and drinking. The primary disease was hepatitis B liver cirrhosis in decompensated period, and LT was performed at the age of 47 years old. The newly developed MPC after LT was diagnosed at the age of 51 years old. The interval between LT and diagnosis of MPC was 44 mo. No inherent germline mutation involved in tumorigenesis was detected in this patient. It has been reported that new malignant tumors after LT are associated with age[6], male gender[13], immunosuppressive regimens[14], time after LT[8,15], smoking history[15,16], drinking history[6], etc. Among them, long-term usage of immunosuppressive agents of liver transplant recipients keeps the immune system in a suppressed state, which helps tumor cells evade immune surveillance and then proliferate[14]. Meanwhile, postoperative immunosuppression will increase the incidence of infection, trigger insulin resistance, and help tumor cells to proliferate. Moreover, immunosuppressive agents such as calcineurin inhibitor (CNI) and mycophenolic acid are inherently carcinogenic. All the factors mentioned above may lead to the occurrence of MPC after LT.
A retrospective study of second primary cancer in HCC carried out in Western countries reported that 7.3% of patients with HCC developed extrahepatic primary malignancy[17], while liver transplant recipients are at high risk of new malignant tumors. Although it is crucial to screen for new malignant tumors during postoperative follow-up, due to the higher operational risk and poorer compliance of the patients, it is very rare to perform electronic gastro-duodenoscopy screening before LT in most centers of China. Our case reports gastric cancer and lung cancer after LT, which are the top two common malignant tumors with high mortality in China. Owing to early detection and effective treatment, the patient achieved a good prognosis. At present, there are no consensus recommendations regarding the screening of newly developed gastric cancer after LT in China, however, liver transplant recipients are an immunosuppressed population at high risk of gastric cancer[18]. It has been suggested that for patients after LT[7], those who are < 40 years old need to undergo gastroscopy once every 2 to 3 years, and those who are ≥ 40 years old need to undergo gastroscopy once every 2 years. Patients with gastric mucosal atrophy with metaplasia are recommended to perform gastroscopy once a year, patients with low-grade intraepithelial neoplasia are recommended to be followed every 6 mo, and patients with high-grade intraepithelial neoplasia are recommended to undergo endoscopic or surgical treatment depending on the disease stage and the patient's physical condition[8,18,19]. Lung cancer is the most common new solid malignant tumors after LT, and the lung is the second most metastatic organ of hepatocellular carcinoma. Therefore, it is necessary to screen the recipients after LT by chest CT. For liver transplant recipients > 40 years old, especially smokers, low-dose spiral CT should be routinely performed for lung cancer screening[20]. If the pulmonary nodule is > 8 mm in diameter, low-dose CT follow-up, PET-CT, and non-surgical biopsy and/or surgical resection are required depending on how malignant the tumor is. If the pulmonary nodule is 4-6 mm in diameter, follow-up should be conducted between 6-12 mo, then conducted again between 18-24 mo if the nodule does not enlarge, thereafter the routine annual inspection should be performed[20].
Early intervention with drugs and/or surgical resection is essential for new malignant tumors after LT to improve the survival rate and quality of life. A number of studies have confirmed that CNI can stimulate tumor growth and increase the incidence of tumors after transplantation, while mammalian target of rapamycin (mTOR) inhibitors may have advantages in preventing and treating gastrointestinal cancer[14,21]. Thimonier et al[21] reported that LT recipients developed new malignant tumors postoperatively, and converting immunosuppressive agents into mTOR inhibitors can effectively improve the prognosis. Therefore, early screening of new malignant tumors after LT and adjusting the immunosuppressive regimen into mTOR inhibitors are effective strategies to delay the progression of disease. The patient was diagnosed with MPC 4 years ago, therefore, immunosuppressive agent was given at a low dose and the blood concentration of immunosuppressant was maintained at a low level. No adjustment was made here for personal reason. For the treatment of MPC after LT, minimally invasive or surgical resection should be considered. Korean scholars reported that early endoscopic treatment of gastric cancer after LT can reduce the occurrence of perioperative complications[22]. Mangus et al[15] reported that it is safe to perform lobectomy in patients with lung cancer after LT when receiving immunosuppressive therapy. In this report, the patient with MPC after LT was successfully treated by partial gastrectomy and partial lung resection, and achieved a good prognosis. The results confirmed the effectiveness of surgical resection for MPC after LT.
Besides, depending on the relatively benign results of DNA targeted sequencing for resected gastric and lung tissues with matching blood, which showed lower TMB and MSS with no germline mutation in this patient, no further anti-tumor therapy was applied and no carcinoma recurrence was found in the follow-up period for 2 years.
De novo MPC after LT is rare, and the prognosis is poor. However, early detection and related intervention can significantly improve the prognosis of patients. Therefore, we recommend that liver transplant recipients be followed and screened for newly developed malignant tumors to improve the survival rate and quality of life.
We thank all the staff working in the Liver Disease Center, Department of Organ Transplantation, The Affiliated Hospital of Qingdao University.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rompianesi G S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Li X
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 713] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 2. | Wang X, Chen H, Wang LT, Zhang Q, Tian Y, Zhao FL. De novo malignant tumor after orthotopic liver transplantation: a report of three cases and literature review. Zhongguo Jijiu Fusu Yu Zaihai Yixue Zazhi. 2009;7:484-486. [DOI] [Full Text] |
| 3. | Peng SS, Chen H, Li J, Zhang Q, Fan TY, Wang X. A case of new renal cell carcinoma and lung cancer after liver transplantation. Zhongguo Qiguan Yizhi Zazhi. 2015;36:296-297. [DOI] [Full Text] |
| 4. | Wang X, Chen H, Fan TY, Zhao C, Li J. Clinical analysis of new malignant tumor after liver transplantation. Zhongguo Zhongliu. 2017;26:410-414. [DOI] [Full Text] |
| 5. | Zhang ZJ, Zhong HG, Zhang W, Zhang ML, Wang WL, Zheng SS. Diagnosis and treatment of new malignant tumors of digestive system after liver transplantation: a report of 10 cases. Zhongguo Qiguan Yizhi Zazhi. 2016;37:513-517. [DOI] [Full Text] |
| 6. | Gao PJ, Gao J, Li Z, Hu ZP, Zhu JY. De novo malignancy after liver transplantation: a single-center experience of 14 cases. Ann Surg Treat Res. 2015;88:222-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Jung DH, Hwang S, Song GW, Ahn CS, Moon DB, Ha TY, Kim KH, Park GC, Kim BS, Park IJ, Lim SB, Kim JC, Yoo MW, Byeon JS, Jung HY, Lee GH, Myung SJ, Choe J, Choi JY, Park HW, Lee SG. Survival Benefit of Early Cancer Detection Through Regular Endoscopic Screening for De Novo Gastric and Colorectal Cancers in Korean Liver Transplant Recipients. Transplant Proc. 2016;48:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Rao W, Xie M, Liu DY, Gong YQ, Fu XY, Zhu XD, Guo Y, Li ZQ, Zang YJ. Literature analysis of new malignancies after liver transplantation in China. Zhonghua Yizhi Zazhi. 2018;12:165-169. [DOI] [Full Text] |
| 9. | Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J. [Report of cancer epidemiology in China, 2015]. Zhonghua Zhong Liu Za Zhi. 2019;41:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 277] [Reference Citation Analysis (0)] |
| 10. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 977] [Article Influence: 139.6] [Reference Citation Analysis (2)] |
| 11. | Ibrahim ME, Saleh M, Ali A, Alavi N. Multiple Primary Malignant Neoplasms: An Unusual Case of Metachronous Breast Ductal and Squamous Cell Carcinomas. Cureus. 2020;12:e6954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Gong CS, Yoo MW, Kim BS, Hwang S, Kim KH, Yook JH, Lee SG. De Novo Gastric Cancer After Liver Transplantation. Ann Transplant. 2016;21:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Ji L, Zhao G. [Occurrence, diagnosis and treatment of de novo gastrointestinal malignancies after organ transplantation]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Shoji F, Toyokawa G, Harada N, Itoh S, Harimoto N, Ikegami T, Okamoto T, Soejima Y, Yoshizumi T, Maehara Y. Surgical Treatment and Outcome of Patients with De Novo Lung Cancer After Liver Transplantation. Anticancer Res. 2017;37:2619-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Mangus RS, Fridell JA, Kubal CA, Loeffler AL, Krause AA, Bell JA, Tiwari S, Tector J. Worse Long-term Patient Survival and Higher Cancer Rates in Liver Transplant Recipients With a History of Smoking. Transplantation. 2015;99:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | National Center for Clinical Medicine of Digestive Diseases; Digestive endoscopy branch of Chinese Medical Association; Health Management Branch of Chinese Medical Association; China Medical Association Endoscopic Physicians Committee; Committee of Health Management and Physical Examination of Endoscopic Digestive Endoscopy; Endoscopic Physicians Branch; Chinese Medical Association; National Quality Control Centre for Digestive Endoscopy; China Anticancer Association Cancer Endoscopy Professional Committee. China experts consensus on the protocol of early gastric cancer screening (2017, Shanghai). Zhonghua Xiaohua Zazhi. 2018;38:87-92. [DOI] [Full Text] |
| 17. | Fernández-Ruiz M, Guerra-Vales JM, Castelbón-Fernández FJ, Llenas-García J, Caurcel-Díaz L, Colina-Ruizdelgado F. Multiple primary malignancies in Spanish patients with hepatocellular carcinoma: analysis of a hospital-based tumor registry. J Gastroenterol Hepatol. 2009;24:1424-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Chinese Society of Gastroenterology. Consensus on chronic gastritis in China (Shanghai, 2017). Zhonghua Xiaohua Zazhi. 37:712-738. [DOI] [Full Text] |
| 19. | Chinese Alliance Against Lung Cancer; Chinese Medical Association of Respiratory Disease Branch Lung Cancer Study Group; Lung Cancer Working Committee of respiratory physicians branch of Chinese Medical Doctor Association. Expert consensus on lung cancer screening and management in China. Guoji Huxi Zazhi. 2019;39:1604-1615. [DOI] [Full Text] |
| 20. | Lung cancer group, society of respiratory diseases; Chinese Medical Association; Expert group of China lung cancer prevention and treatment alliance. Consensus of Chinese experts on diagnosis and treatment of pulmonary nodules (2018). Zhonghua Jiehe He Huxi Zazhi. 2018;41: 763-771. [DOI] [Full Text] |
| 21. | Thimonier E, Guillaud O, Walter T, Decullier E, Vallin M, Boillot O, Dumortier J. Conversion to everolimus dramatically improves the prognosis of de novo malignancies after liver transplantation for alcoholic liver disease. Clin Transplant. 2014;28:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Na S, Lee GH, Song JH, Ahn JY, Kim SO, Park SJ, Park SE, Kim MY, Lee J, Choi KS, Kim DH, Song HJ, Choi KD, Jung HY, Kim JH. Endoscopic resection of gastric neoplasm in solid-organ transplant recipients. Transplantation. 2014;97:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |