Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3506
Peer-review started: January 19, 2021
First decision: February 9, 2021
Revised: February 21, 2021
Accepted: March 17, 2021
Article in press: March 17, 2021
Published online: May 26, 2021
Processing time: 111 Days and 20.2 Hours
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disorder characterized by recurrent nodules, abscesses, and sinus tracts. Crohn’s disease (CD) is characterized by inflammation of the entire digestive tract and belongs to the group of inflammatory bowel diseases, and there are many extraintestinal manifestations, among which hidradenitis suppurativa is one of the rare extraintestinal manifestations. There appears to be a strong association between CD and HS based on clinical and histological similarities (sinus tract development, granulomatous inflammation, and scarring), intersections in pathogenesis (genetic loci, immune dysregulation mechanisms, and microbiome changes), and commonality in treatment. In this review, we summarize recent studies on the association between HS and CD.
Core Tip: In this review, we summarize the recent studies on the association between hidradenitis suppurativa and Crohn's disease, based on clinical and histological similarities (sinus tract development, granulomatous inflammation, and scarring), intersections in pathogenesis (genetic loci, immune dysregulation mechanisms, and microbiome changes), and commonality in treatment.
- Citation: Zhang M, Chen QD, Xu HX, Xu YM, Chen HJ, Yang BL. Association of hidradenitis suppurativa with Crohn’s disease. World J Clin Cases 2021; 9(15): 3506-3516
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3506.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3506
Hidradenitis suppurativa (HS), known as acne inversa, is a chronic inflammatory skin disorder characterized by recurrent nodules, abscesses, and sinus tracts. HS most commonly affects the axillary, inguinal, and anogenital regions. The course of the disease can persist for decades.
The chronic and recurrent inflammation of HS, which is similar to that in Crohn’s disease (CD), finally leads to fistula and sinus tracts. Ostlere et al[1] first noticed a high risk of HS in patients with CD in the 1990s. Subsequently, several reported cases indicated similar pathologic features[2,3] and genetic susceptibility[4] between HS and CD. Later, patients with HS with simultaneous CD symptoms were significantly relieved by treatment with anti-tumor necrosis factor-alpha inhibitors (anti-TNF-α). Anti-TNF-α therapy not only promoted great progress in the treatment of HS but also manifested the possible common pathogenesis of HS and CD[5]. In addition, some studies found that both HS and CD shared the same immune dysregulation mechanism, such as significantly increased interleukin-1 (IL-1), IL-6, IL-17, IL-23, and TNF[6,7]. According to similar clinical and histological characteristics, as well as the intersections of pathogenesis and treatment, it is widely accepted that there may be a strong association between CD and HS. In this review, we summarize the recent studies focused on the association between HS and CD.
The incidence of HS ranges from 0.03% to 4%[8-11]. However, due to the sparse clinical epidemiological data and misdiagnosis of HS, the actual incidence may be higher. With the improvement in diagnosis, recent research data have shown that the incidence of HS has been increasing to 10 per 100000[12].
Numerous studies have indicated that patients with CD have a significantly higher risk of HS than ordinary people. In a population-based cohort study from Minnesota, the incidence of HS among 679 patients with inflammatory bowel disease (IBD) was 1.2%. The relative risk of HS in patients with IBD is 9 times that of the general population, while the risk of HS in patients with CD is higher than that of ulcerative colitis (UC) patients[13]. Three cohort studies from the Netherlands revealed that the incidence of HS in patients with CD was 17% (17/102), 15% (96/634), and 26% (181/688)[14-16]. Interestingly, the literature results showed that the incidence of HS in patients with CD was higher than that of CD in patients with HS. According to Garg et al[17], the incidence of CD in patients with HS was 2.0% (1025/51340).
In addition, HS with CD is more common in women and blacks. A large cohort study indicated that female sex was the most independent risk parameter for HS formation in IBD (odds ratio [OR] = 3.494, P < 0.001)[16]. Kamal et al[18] retrospectively analyzed 18 patients with HS combined with IBD (CD vs UC = 15: 3). Among the 15 patients with CD, 11 (73%) were female and 9 (60%) were black. However, related studies remain inadequate. Other future studies are needed to further explore the risk parameters.
At present, the pathogenesis of HS and CD remains unclear. It is believed that HS is a multifactor-related systemic inflammatory disease that may be caused by autoimmune inflammation[19]. Studies have shown that HS and CD carry similar risk factors, immune dysregulation mechanisms, genetic loci, and microbiome changes.
It is accepted that smoking increases the risk of CD and reduces treatment effects. Recent studies have also found a strong association between smoking and HS. Garg et al[20] retrospectively measured the prevalence of newly diagnosed HS in nearly 4 million smokers over three years. Among tobacco smokers, the overall odds of new HS diagnosis was increased by 91% (OR = 1.91, 95%CI: 1.85–1.97) compared with nonsmokers (P < 0.001). The potential pathogenic effects of smoking in HS include epidermal hyperplasia and follicular plugging[21]; neutrophil chemotaxis[22]; TNF-α secretion[23]; and induction of Th17 cell differentiation in keratinocytes[24]. Prospective cohort studies have shown that smoking cessation is the safest and least expensive option for HS. However, it is difficult to change a smoking habit according to clinical results.
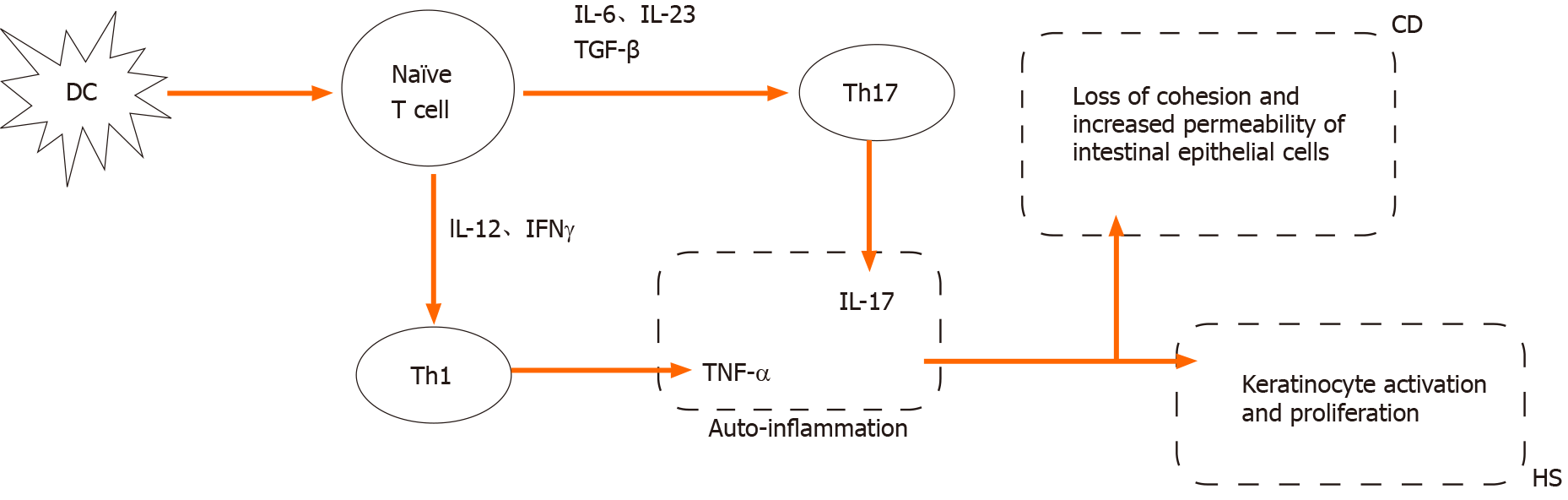
The TNF-α and IL-23/Th17 signaling pathways were associated with HS and CD, suggesting the same immune-mediated origins (Figure 1). TNF-α, associated with many other inflammatory diseases such as psoriasis and IBD, is secreted by innate and adaptive immune cells. Several studies have shown that TNF-α increases in the serum and lesion tissues of patients with HS. As an effective therapeutic target, adalimumab was the first approved anti-TNF-α agent for the treatment of HS. Similarly, increased proinflammatory IL-23/Th17 cells are associated with many chronic inflammatory diseases. IL-12 and IL-23 were largely expressed by macrophages in the lesion skin of HS, accompanied by Th17 and CD4+ T cell infiltration. As a cell activator and inflammatory regulator of keratinization, IL-17 cells were produced in the lesions and surrounding skin of HS. It has been clinically confirmed that the IL-12 and IL-17 inhibitor ustekinumab has certain efficacy in the treatment of HS[25].
Few studies have focused on the correlation between HS and CD at the microbiota and gene levels. The microbiota influences the immunologic and physiologic homeostasis of the skin epithelia and gut mucosa by activating Toll-like receptors to recognize pathogens and repair damage. However, a variety of environmental factors can alter microbial balance, leading to a decrease in microbial diversity. Such alterations in the microbiota may cause immune dysregulation and susceptibility to HS and IBD[26]. Gower-Rousseau et al[4] reported three cases of HS occurring in two first-degree relatives of patients with CD, which might suggest common genetic susceptibility for the two diseases. Janse et al[16] identified the protective gene ELOVL7 and the risk-related genes SULT1B1 and SULT1E1 in HS combined with IBD. However, these genetic associations need further exploration.
HS, with the characteristics of chronicity and recurrency, usually involves the axilla, inguina, perianal area, perineum, buttocks, and the area under the breast fold with typical skin lesions, including deep painful nodules, abscesses, sinuses, and bridge scars. Extensive sinus tracts and scars are the result of continuous disease progression and recurrent attacks. Usually, the Hurley stage is used to assess the severity of HS and guide treatment during clinical practice (Table 1)[27,28]: Stage I (mild)-abscess (single or multiple) without sinus tracts and cicatrization/scarring; stage II (moderate)-recurrent abscesses with tract formation and cicatrization, single or multiple, and widely separated lesions; stage III (severe)-diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses across the entire area
| Clinical manifestations | |
| Grade I | Abscess (single or multiple) without sinus tracts and cicatrization/scarring |
| Grade II | Recurrent abscess with sinus tracts and scarring, single or multiple widely separated lesions |
| Grade Ш | Diffuse or almost diffuse involvement, or multiple interconnected sinus tracts and abscesses across the entire area |
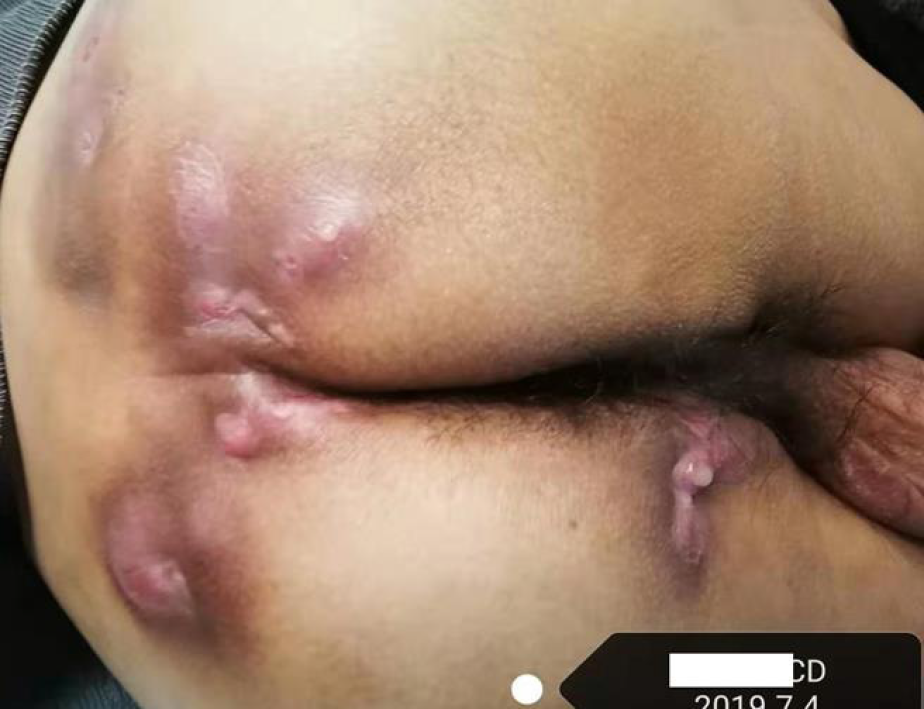
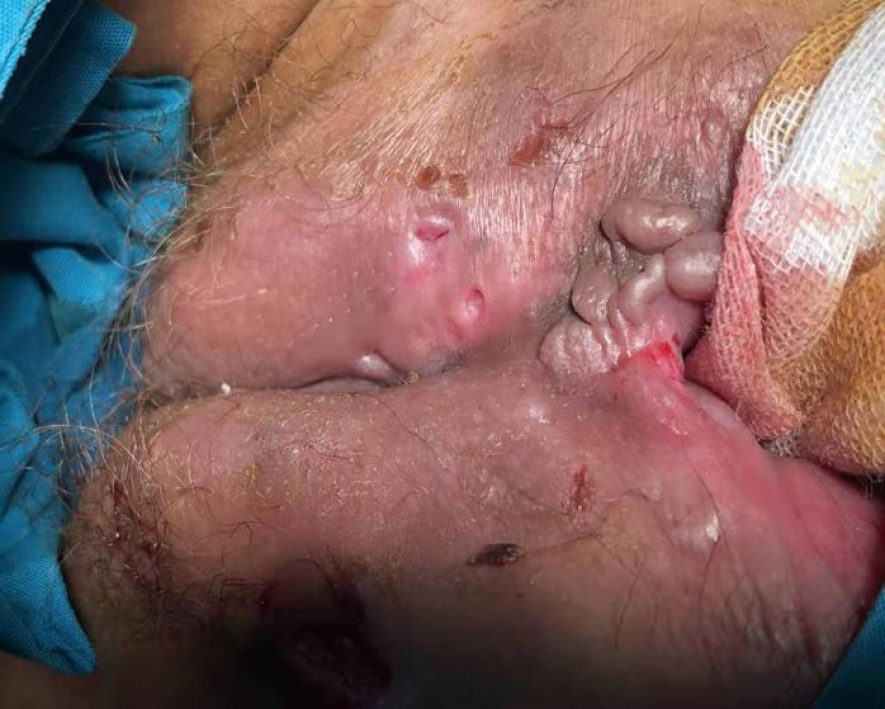
Sometimes, it is difficult to differentiate perianal fistulizing CD (PFCD) from HS only by clinical features and pathological manifestations. Both HS and PFCD have abscesses or sinus tracts with perianal pain, redness, itching, bleeding, and increased purulent secretions. HS lesions are usually far from the anal canal and rectum. Most fistula lesions do not extend to the dentate line of the anal canal (no apocrine glands at or above the dental line). These may be helpful for identification of HS. On the other hand, patients with PFCD may have gastrointestinal symptoms or unexplained biological abnormalities such as abdominal pain, diarrhea, anemia, hypoferritinemia, and elevated C-reactive protein (CRP). This may put doctors on the alert and distinguish them from HS. However, some patients may have fistulas connecting to the anal canal due to the apocrine glands at the distal end of the anal canal. Approximately 17%-40% of patients with CD have perianal HS, where symptoms often overlap with PFCD (Figures 2 and 3). In addition, the histological features of CD and HS may all be granulomas and lymphatic follicles. Hence, imaging assessment, especially magnetic resonance imaging (MRI), is extremely important. Monnier et al[29] compared pelvic MRI characteristics of 23 patients with HS and 46 patients with CD with anoperineal disease. The results showed that the specific characteristics of HS mainly included a predominance of the ‘absence of features’ in the perianal area, the absence of rectal wall thickening, and bilaterality of features. Other features included subcutaneous edema, less involvement of the anal sphincter, and sinus passages without connection to the anorectal canal.
Patients with simultaneous CD and HS usually present with severe pathogenetic conditions and increased colonic involvement of CD. Kamal et al retrospectively analyzed 15 patients with CD with HS[18]: 47% of patients presented with colonic CD, 53% with ileocolonic CD, there were no cases of isolated ileal disease or upper gastrointestinal CD, and 67% with perianal lesions. The average age at HS diagnosis was 34 years old. The lesion sites of HS were axillary (53%), inguinal (47%), and perianal/perineal (73%). Thirteen (93%) patients had Hurley II or III disease. One patient developed squamous cell carcinoma.
Like CD, HS is incurable and few treatments are available to maintain long-term remission, which imposes a huge medical and social burden. Treatment is even more difficult when patients also have CD. Traditional HS therapies include antibiotics, hormonal therapy, and especially surgical therapy. However, the surgical wounds of patients with CD combined with HS are usually difficult to heal. Fortunately, a variety of drugs and surgical methods have been shown to alleviate the disease and prevent new lesions with the rapid clinical application of biological agents. Thus, systemic drug therapy should be used first. Steroids are not recommended in the acute phase of CD-related anal fistula and HS because of limited efficacy and increased risk of infection. Once local sepsis is effectively controlled, the application of immuno-suppressive agents and biological agents needs to be evaluated. On the basis of effective control of active intestinal lesions, surgical treatment can be considered.
Smoking and obesity are strongly correlated with the severity of HS. Smoking is a risk factor for CD onset and recurrence. It is strongly recommended that patients with HS quit smoking. Loose clothing is preferred to avoid mechanical stress and friction[30,31].
Antibiotics: Antimycobacterial therapy has shown no effects on induction and maintenance of remission or mucosal healing in patients with CD and should not be used as primary therapy[32]. There are currently no high-quality data on the treatment of HS with antibiotics, especially patients with CD and HS. Antibiotics are routinely used for the treatment of infection. The combination of clindamycin (300 mg, 2/d) and rifampicin (300 mg, 2/d) is currently used by clinicians. In a prospective study, clindamycin and rifampicin were combined for 12 wk and the patients were followed for 1 year. Clinical response was observed in 73% (19/26) of patients at the outset but decreased to 41% (7/17) after the 1-year follow-up. The mean recurrence time was 4.2 mo. The most common side effects were diarrhea and nausea[33]. Although many other antibiotic application studies have been carried out clinically, antibiotics are generally not a practical solution to HS.
Biological agents: The application of biological agents has provided new therapeutic approaches and significantly reduced the incidence[34]. TNF-α, as the initiating factor of inflammatory progression, is mainly concentrated in the serum and skin lesions of patients with HS and is significantly higher than that of normal people. Kelly et al[35] confirmed the increased levels of TNF-α, IL-17, and IL-1 in HS lesions. Despite the wide variety of inflammatory cytokines associated with the pathogenesis of HS, anti-TNF-α therapy can effectively control most proinflammatory cytokines.
Adalimumab: As an effective therapeutic target, adalimumab is the first and only anti-TNF-α agent approved by the Food and Drug Administration for the treatment of moderate to severe HS[36,37]. The standard regimen was 160 mg at week 0, 80 mg at week 2, and then 40 mg weekly by subcutaneous injection. The efficacy of adalimumab in the treatment of HS reported in the literature varies greatly. In an open clinical trial, ten patients with HS received adalimumab treatment at week 0 at a dose of 160 mg, 80 mg at week 1, and then 40 mg every 2 wk (every other week, EOW) for 12 wk. The primary endpoint was HiSCR-50 (the counts of abscesses and inflammatory nodules were reduced by 50% compared with baseline). At the end of the study, no patients were considered to be responsive to the treatment, and four participants quitted due to lack of efficacy[38]. However, a double-blind, placebo-controlled randomized clinical trial (RCT) showed that 21 patients receiving adalimumab [80 mg at baseline and 40 mg every other week (EOW) for 12 wk] had better efficacy than the placebo group. The efficacy endpoint was a change in Sartorius score and Hurley stage at 12 wk. The most significant reduction in the Sartorius score was observed at the 12th week (P = 0.07)[39]. In general, adalimumab is effective for the treatment of HS and has a sustained clinical response. Weekly administration is better than EOW administration[38-43].
Infliximab (IFX): The efficacy for HS was first observed in patients with CD treated with IFX[5]. An RCT involving 38 patients determined the efficacy of IFX in the treatment of HS, with standard treatment regimens of 0, 2, and 6 wk of 5 mg/kg followed by maintenance therapy every 8 wk for 22 wk. The primary endpoint was that the Hidradenitis Suppurativa Severity Index (HSSI) score decreased by 50% from baseline after treatment. The results showed that the HSSI, erythrocyte sedimentation rate, CRP, and pain were significantly relieved. Among 15 patients receiving IFX treatment, the HSSI decreased by more than 50% in 26.7% of patients, 60% of patients had a 25%-50% decrease, and 13.3% of patients had a less than 25% decrease. The HSSI improvement was less than 25% in most patients who received placebo (88.9%)[44]. A comparative study of IFX and adalimumab showed that IFX had better assessment indicators, including the Dermatology Life Quality Index, clinical evaluation, and efficacy duration. This study indicated that IFX might be more suitable for the treatment of severe HS than adalimumab[45].
Ustekinumab: Studies have shown that specific genetic variants of IL-12 and IL-23 receptor subunits are associated with severe HS. In a prospective study, 17 patients with HS received ustekinumab (≤ 100 kg: 45 mg; > 100 kg: 90 mg) and were followed for 40 wk. Twelve (70%) patients completed the treatment. At the 40th week, 8 (47%) patients obtained a clinical response (total abscesses and inflammatory nodule counts were reduced by 50%; abscesses or drainage fistula counts did not increase); 14 (82%) had a modified sartorius score that was moderately improved[25]. In 2019, Cline et al[46] reported that a 39-year-old woman with CD was unable to effectively control HS with IFX, adalimumab, and azathioprine treatment. After a combination of adalimumab (80 mg loading dose followed by 40 mg/wk maintenance) and ustekinumab (90 mg/8 wk), HS was effectively controlled for the first time at the follow-up 1 mo later.
Chronic sinuses and scars formed by HS usually require surgical intervention. A systematic review[47] showed the recurrence rate following various surgical procedures for HS: The total recurrence rate of extensive resection was 13%, direct suture was 15%, transfer flap was 8%, and skin graft was 6%. The local resection recurrence rate was 22%, and the window drainage recurrence rate was 27%. Although surgery has removed a large amount of tissue, it cannot prevent recurrence.
Incision and drainage: Incision and drainage are not effective in treating HS. When fluctuating abscesses occur locally in patients with HS, incision and drainage can quickly relieve pain. Some patients may be cured, but the recurrence rate is quite high. Incision and drainage are usually ineffective for inflammatory nodules and sinus tracts. For patients with active CD with HS, simple incision and drainage is a relatively rational treatment that can control local inflammation, avoid sepsis, and provide opportunities for the application of steroids, immunological agents, or biological agents.
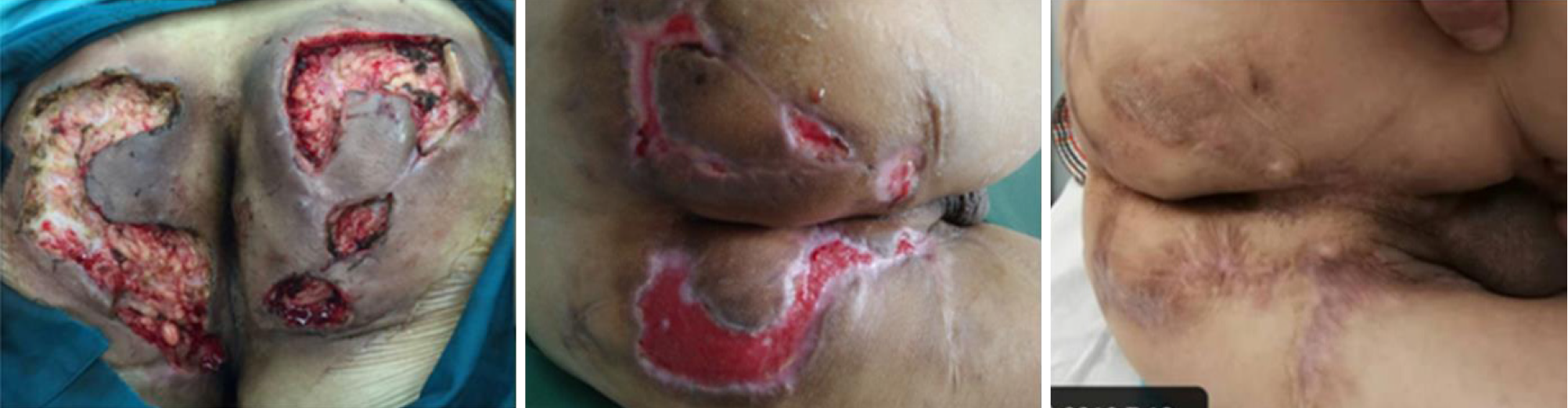
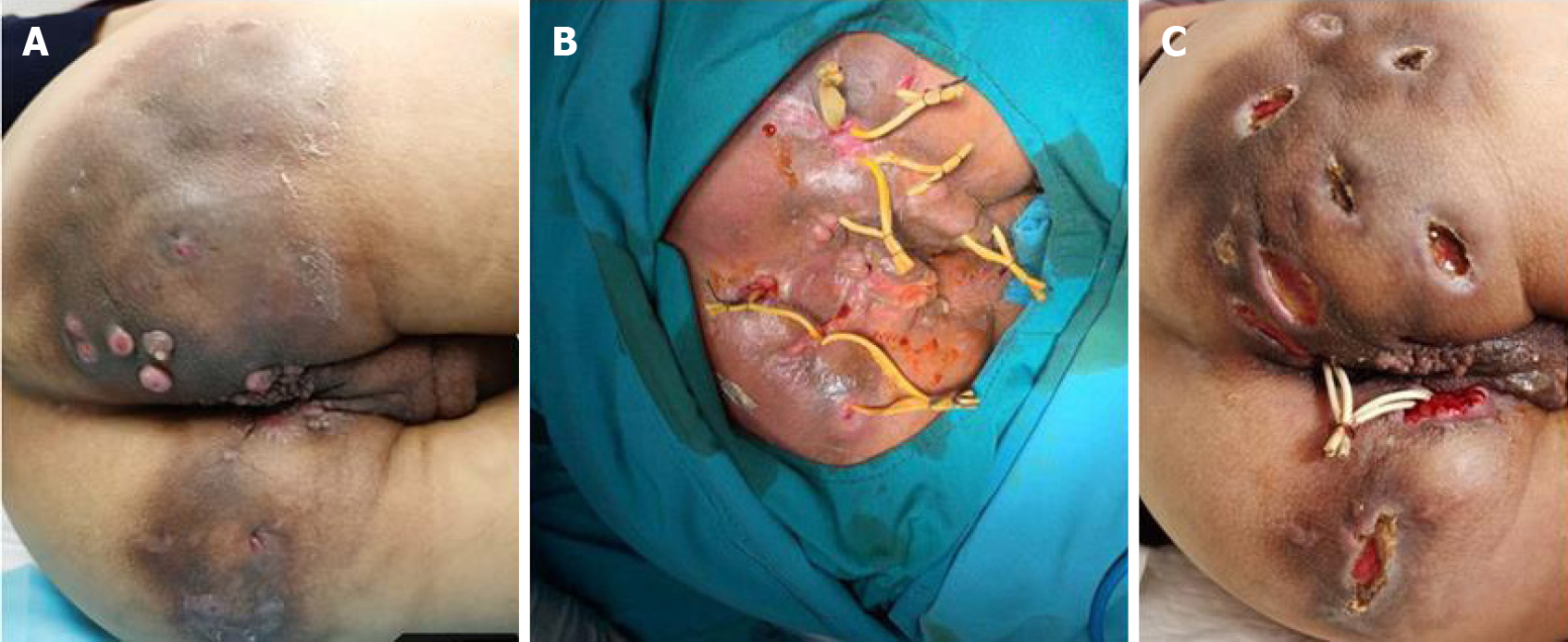
Deroofing (local or extensive incision): Deroofing (local or extensive incision) can be used in the treatment of moderately severe HS (Hurley stage II or III). Under local or regional anesthesia, inflammatory nodules or sinus passages are fully incised. The skin covering the surface of each sinus tract should be completely open to explore additional sinus openings at its base and edges. The granulation and necrotic tissue in the open inflammatory nodules and sinus passages are cleared with coarse gauze or a curette. The wound should be completely open or make a marsupialization for secondary healing (Figure 4).
Extensive resection + reconstruction: After the failure of drug treatment or con-servative surgical treatment, serious HS (Hurley III) may require extensive resection of lesions. Extensive excision generally removes hair follicles and sebaceous gland tissues while preserving normal subcutaneous fat. When deeper sinus passages are encountered, more skin with accessory organs, sinuses, and scar tissue is resected simultaneously. Wide excision with skin closure is controversial and depends mainly on the extent of excision of the lesion. According to the resected wound surface, the skin can be closed by free and direct flap sutures (usually with extension-reduction sutures), local flap transfer, and skin grafts. Although HS is not technically curable, most patients can control their clinical symptoms for a long time after extensive resection.
HS usually requires multimodal therapy-a combination of surgery and drugs. Biologics could potentially augment the surgical intervention results. A retrospective study showed that a combination of surgery with biologics was significantly better than single surgical treatment for recalcitrant HS. The recurrence rates (19% vs 38.5%; P < 0.01) were significantly lower. Disease progression (18% vs 50%; P < 0.001) among patients with adjuvant biologic therapy was continued for at least 6 mo[48]. There is no current literature regarding adverse events integrating biologic therapy and surgery in patients with HS.
Kamal et al[18]analyzed the treatment of 15 patients with CD with perianal HS at Icahn Medical College from 2003 to 2013. The results were as follows: 14 patients (93%) received medication for CD, including antibiotics (100%), steroids (64%), mesalamine (71%), azathioprine/6-mercaptopurine (78%), and anti-TNFs (80%). Among the 12 patients treated with anti-TNFs [IFX (n = 11) and adalimumab (n = 4)], 75% required an increased dose (based on weight gain or shorter intervals) and 11 (92%) exhibited a response to anti-TNF treatment. In addition, 67% of patients required surgery during treatment. These results suggest that compared with patients with simple HS, CD-associated HS has a poor response to anti-TNF treatment. A combination of surgery and medication may be necessary.
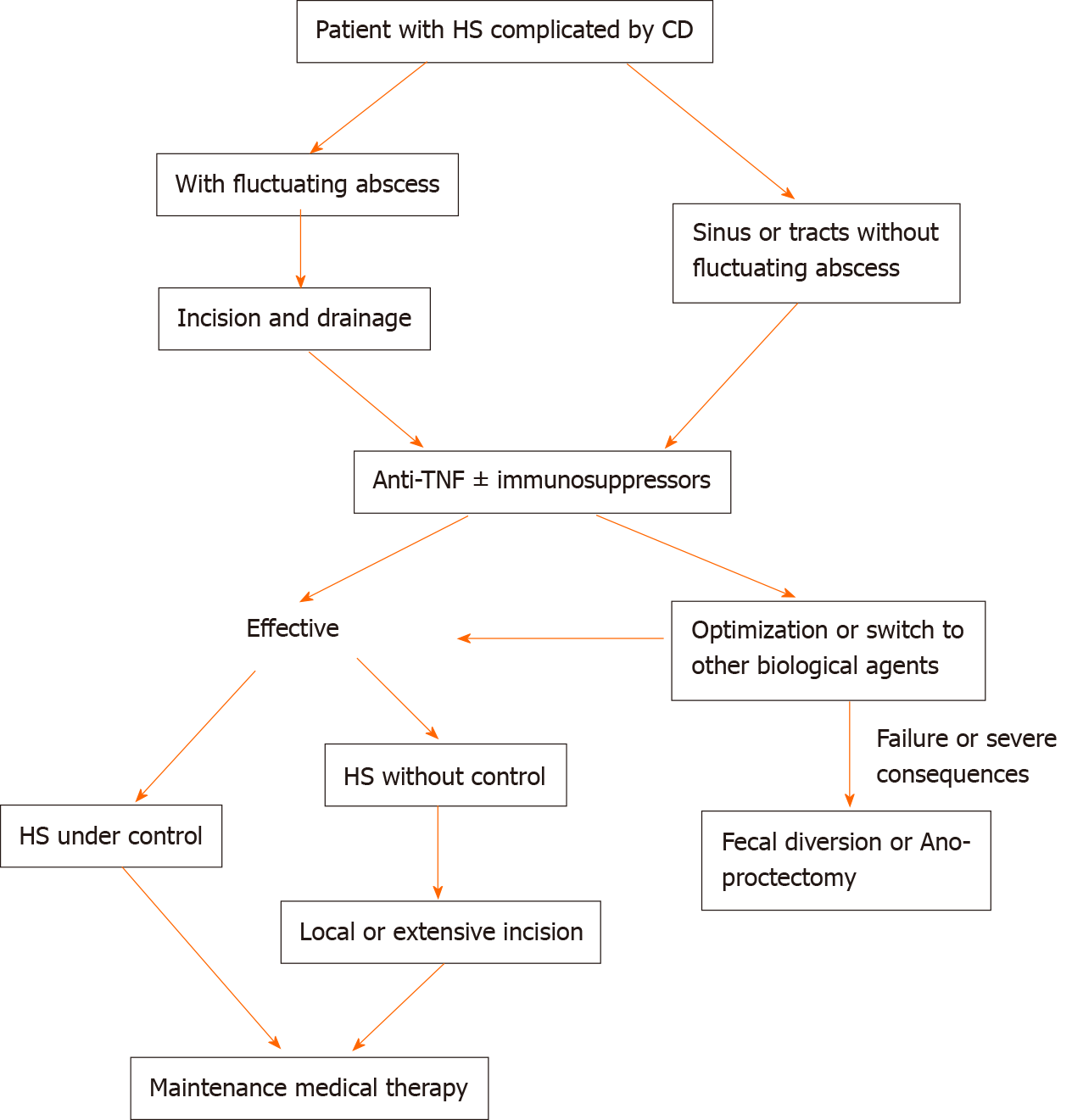
Based on the existing clinical evidence, we propose a clinical strategy for HS combined with CD (Figure 5). Meanwhile, we present a case of multimodal treatment of HS combined with CD: A 37-year-old man with CD, Hurley stage III. Due to recurrent perianal abscesses and fistulas, the patient received drainage and seton of the perianal lesion and IFX therapy after surgery. At the 1-mo follow-up, the wound healed well, and the local pain drainage, CRP, and white blood cell count were significantly reduced. Abdominal symptoms are almost relieved (Figure 6).
Both HS and CD can influence the quality of life and daily activities of patients. According to the consistency in pathogenesis, clinical manifestations, and treatment between HS and CD, HS may be another extraintestinal manifestation of CD.
When physicians encounter patients with HS accompanied by digestive symptoms or unexplained biological abnormalities, such as anemia, hypoferritinemia, and elevated CRP, gastrointestinal examination should be performed to determine the intestinal lesions. Similarly, in patients with CD with accompanying HS-like skin lesions, especially in the perianal and perineum areas, further attention is required to differentiate HS from anal fistula.
Although there is a large amount of literature on the treatment of the two diseases, there is still no unified conclusion on the treatment of patients with HS combined with CD. Antibiotics have limited therapeutic effects on CD and HS unless the bacterial culture of the lesions is positive or some immunomodulatory antibiotics (such as tetracycline and ciprofloxacin) are applied. The application of biological agents in the treatment of HS combined with CD has opened up new treatment methods and greatly relieved symptoms. As wounds are difficult to heal, surgical treatment is usually used as an adjunct to medication in patients with HS and CD. Multimodal treatment, surgical approach, and medical treatment can help postoperative healing. This may be the main direction for the future treatment of comorbidities.
In summary, early diagnosis and timely comprehensive treatment are important for patients with CD-related HS to improve quality of life. Clinicians should be aware of this link to avoid delayed diagnosis and ensure adequate management and follow-up.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheifetz AS S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Ostlere LS, Langtry JA, Mortimer PS, Staughton RC. Hidradenitis suppurativa in Crohn's disease. Br J Dermatol. 1991;125:384-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Attanoos RL, Appleton MA, Hughes LE, Ansell ID, Douglas-Jones AG, Williams GT. Granulomatous hidradenitis suppurativa and cutaneous Crohn's disease. Histopathology. 1993;23:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Roy MK, Appleton MA, Delicata RJ, Sharma AK, Williams GT, Carey PD. Probable association between hidradenitis suppurativa and Crohn's disease: significance of epithelioid granuloma. Br J Surg. 1997;84:375-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Gower-Rousseau C, Maunoury V, Colombel JF, Coulom P, Piette F, Cortot A, Paris JC. Hidradenitis suppurativa and Crohn's disease in two families: a significant association? Am J Gastroenterol. 1992;87:928. [PubMed] |
| 5. | Martínez F, Nos P, Benlloch S, Ponce J. Hidradenitis suppurativa and Crohn's disease: response to treatment with infliximab. Inflamm Bowel Dis. 2001;7:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2428] [Cited by in RCA: 2310] [Article Influence: 121.6] [Reference Citation Analysis (1)] |
| 7. | Schlapbach C, Hänni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | Jemec GB, Kimball AB. Hidradenitis suppurativa: Epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73:S4-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 9. | Saunte DML, Jemec GBE. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA. 2017;318:2019-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 317] [Article Influence: 39.6] [Reference Citation Analysis (1)] |
| 10. | Delany E, Gormley G, Hughes R, McCarthy S, Kirthi S, Markham T, Tobin AM, Murphy M, Kirby B. A cross-sectional epidemiological study of hidradenitis suppurativa in an Irish population (SHIP). J Eur Acad Dermatol Venereol. 2018;32:467-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Ingram JR, Jenkins-Jones S, Knipe DW, Morgan CLI, Cannings-John R, Piguet V. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 12. | Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 13. | Yadav S, Singh S, Edakkanambeth Varayil J, Harmsen WS, Zinsmeister AR, Tremaine WJ, Davis MD, Wetter DA, Colombel JF, Loftus EV Jr. Hidradenitis Suppurativa in Patients With Inflammatory Bowel Disease: A Population-Based Cohort Study in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2016;14:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | van der Zee HH, van der Woude CJ, Florencia EF, Prens EP. Hidradenitis suppurativa and inflammatory bowel disease: are they associated? Br J Dermatol. 2010;162:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | van der Zee HH, de Winter K, van der Woude CJ, Prens EP. The prevalence of hidradenitis suppurativa in 1093 patients with inflammatory bowel disease. Br J Dermatol. 2014;171:673-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 16. | Janse IC, Koldijk MJ, Spekhorst LM, Vila AV, Weersma RK, Dijkstra G, Horváth B. Identification of Clinical and Genetic Parameters Associated with Hidradenitis Suppurativa in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 17. | Garg A, Hundal J, Strunk A. Overall and Subgroup Prevalence of Crohn Disease Among Patients With Hidradenitis Suppurativa: A Population-Based Analysis in the United States. JAMA Dermatol. 2018;154:814-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Kamal N, Cohen BL, Buche S, Delaporte E, Colombel JF. Features of Patients With Crohn's Disease and Hidradenitis Suppurativa. Clin Gastroenterol Hepatol. 2016;14:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Smith MK, Nicholson CL, Parks-Miller A, Hamzavi IH. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Garg A, Papagermanos V, Midura M, Strunk A. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 21. | Hana A, Booken D, Henrich C, Gratchev A, Maas-Szabowski N, Goerdt S, Kurzen H. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 2007;80:2214-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Kurzen H, Kurokawa I, Jemec GB, Emtestam L, Sellheyer K, Giamarellos-Bourboulis EJ, Nagy I, Bechara FG, Sartorius K, Lapins J, Krahl D, Altmeyer P, Revuz J, Zouboulis CC. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-6; discussion 457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Jeong SH, Park JH, Kim JN, Park YH, Shin SY, Lee YH, Kye YC, Son SW. Up-regulation of TNF-alpha secretion by cigarette smoke is mediated by Egr-1 in HaCaT human keratinocytes. Exp Dermatol. 2010;19:e206-e212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Torii K, Saito C, Furuhashi T, Nishioka A, Shintani Y, Kawashima K, Kato H, Morita A. Tobacco smoke is related to Th17 generation with clinical implications for psoriasis patients. Exp Dermatol. 2011;20:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Blok JL, Li K, Brodmerkel C, Horvátovich P, Jonkman MF, Horváth B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 26. | Chen WT, Chi CC. Association of Hidradenitis Suppurativa With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA Dermatol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 27. | van der Zee HH, Jemec GB. New insights into the diagnosis of hidradenitis suppurativa: Clinical presentations and phenotypes. J Am Acad Dermatol. 2015;73:S23-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus. In: Roenigk RH, Roenigk HH Jr, eds. Dermatologic surgery: principles and practice. New York, NY: Marcel Dekker; 1989: 729-739. |
| 29. | Monnier L, Dohan A, Amara N, Zagdanski AM, Drame M, Soyer P, Hoeffel C. Anoperineal disease in Hidradenitis Suppurativa : MR imaging distinction from perianal Crohn's disease. Eur Radiol. 2017;27:4100-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Vekic DA, Cains GD. Hidradenitis suppurativa, a review of pathogenesis, associations and management. Part 2. Australas J Dermatol. 2018;59:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Hunger RE, Laffitte E, Läuchli S, Mainetti C, Mühlstädt M, Schiller P, Lapointe AK, Meschberger P, Navarini AA. Swiss Practice Recommendations for the Management of Hidradenitis Suppurativa/Acne Inversa. Dermatology. 2017;233:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 932] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 33. | Dessinioti C, Zisimou C, Tzanetakou V, Stratigos A, Antoniou C. Oral clindamycin and rifampicin combination therapy for hidradenitis suppurativa: a prospective study and 1-year follow-up. Clin Exp Dermatol. 2016;41:852-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Savage KT, Flood KS, Porter ML, Kimball AB. TNF-α inhibitors in the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2019;10:2040622319851640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Kelly G, Hughes R, McGarry T, van den Born M, Adamzik K, Fitzgerald R, Lawlor C, Tobin AM, Sweeney CM, Kirby B. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 36. | van der Zee HH, Laman JD, de Ruiter L, Dik WA, Prens EP. Adalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo study. Br J Dermatol. 2012;166:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Kimball AB, Okun MM, Williams DA, Gottlieb AB, Papp KA, Zouboulis CC, Armstrong AW, Kerdel F, Gold MH, Forman SB, Korman NJ, Giamarellos-Bourboulis EJ, Crowley JJ, Lynde C, Reguiai Z, Prens EP, Alwawi E, Mostafa NM, Pinsky B, Sundaram M, Gu Y, Carlson DM, Jemec GB. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med. 2016;375:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 38. | Amano M, Grant A, Kerdel FA. A prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativa. Int J Dermatol. 2010;49:950-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Miller I, Lynggaard CD, Lophaven S, Zachariae C, Dufour DN, Jemec GB. A double-blind placebo-controlled randomized trial of adalimumab in the treatment of hidradenitis suppurativa. Br J Dermatol. 2011;165:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 40. | Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Kimball AB, Kerdel F, Adams D, Mrowietz U, Gelfand JM, Gniadecki R, Prens EP, Schlessinger J, Zouboulis CC, van der Zee HH, Rosenfeld M, Mulani P, Gu Y, Paulson S, Okun M, Jemec GB. Adalimumab for the treatment of moderate to severe Hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 300] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 42. | Kimball AB, Sundaram M, Shields AL, Hudgens S, Okun M, Foley C, Ganguli A. Adalimumab alleviates skin pain in patients with moderate-to-severe hidradenitis suppurativa: Secondary efficacy results from the PIONEER I and PIONEER II randomized controlled trials. J Am Acad Dermatol. 2018;79:1141-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Zouboulis CC, Okun MM, Prens EP, Gniadecki R, Foley PA, Lynde C, Weisman J, Gu Y, Williams DA, Jemec GBE. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol 2019; 80: 60-69. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 44. | Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 295] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 45. | van Rappard DC, Leenarts MF, Meijerink-van 't Oost L, Mekkes JR. Comparing treatment outcome of infliximab and adalimumab in patients with severe hidradenitis suppurativa. J Dermatolog Treat. 2012;23:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Cline A, Pichardo RO. Successful treatment of hidradenitis suppurativa in the setting of Crohn disease with combination adalimumab and ustekinumab. Dermatol Online J. 2019;25. [PubMed] |
| 47. | Mehdizadeh A, Hazen PG, Bechara FG, Zwingerman N, Moazenzadeh M, Bashash M, Sibbald RG, Alavi A. Recurrence of hidradenitis suppurativa after surgical management: A systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:S70-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 48. | DeFazio MV, Economides JM, King KS, Han KD, Shanmugam VK, Attinger CE, Evans KK. Outcomes After Combined Radical Resection and Targeted Biologic Therapy for the Management of Recalcitrant Hidradenitis Suppurativa. Ann Plast Surg. 2016;77:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |