Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3458
Peer-review started: December 22, 2020
First decision: January 7, 2021
Revised: January 15, 2021
Accepted: February 26, 2021
Article in press: February 26, 2021
Published online: May 16, 2021
Processing time: 127 Days and 17.5 Hours
Diabetic mastopathy is a rare benign disease in clinical practice that mainly occurs in young and middle-aged women with type 1 diabetes. It has also been reported that this disease can be found in patients with type 2 diabetes and other autoimmune diseases, such as Hashimoto's thyroiditis, as well as in men. The pathogenesis of diabetic mastopathy is not yet clear, and it is easily confused with breast cancer due to their similar clinical manifestations and imaging features.
A 69-year-old female patient was admitted because of painless breast masses, with a history of type 2 diabetes. The imaging and physical examination suggested a high risk of breast cancer. Further histopathological analysis showed dense lymphocytes infiltrating around the lobules of the breast, and extensive fibrosis of the surrounding stroma. Finally, diabetic mastopathy was diagnosed.
The diagnosis of diabetic mastopathy in elderly patients with painless breast masses is difficult to distinguish from breast cancer, and its imaging manifest
Core Tip: Diabetic mastopathy is a rare benign disease in the clinic, and its pathogenesis is not yet clear. We report a case of diabetic mastopathy in a female patient. Combined with clinical and pathological findings, we analyzed its imaging findings to better understand diabetic mastopathy and to provide a reference for the clinical diagnosis of this disease in elderly individuals.
- Citation: Chen XX, Shao SJ, Wan H. Diabetic mastopathy in an elderly woman misdiagnosed as breast cancer: A case report and review of the literature. World J Clin Cases 2021; 9(14): 3458-3465
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3458.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3458
Diabetic mastopathy, also known as lymphocytic mammary disease, was firstly reported by Sloer and Khardori in 1984[1]. It was believed that breast masses may be another manifestation of connective tissue abnormalities and related to immunological thyroid disease. Premenopausal women with diabetic complications, such as hand joint disease, are more likely to develop this disease. The clinical manifestations of diabetic mastopathy are painless masses with unclear boundaries and irregular shapes, and its imaging manifestations are also easily confused with those of breast cancer. Therefore, an accurate diagnosis is hard to made, unless a puncture to obtain pathology was performed.
In 1989, diabetic mastopathy was officially named by Logan and Hoffman[2], and three conditions for the diagnosis of this disease were proposed[2]. The pathogenesis of this disease is not clear, and there is no effective diagnosis and treatment at present. In addition, some studies have pointed out a high recurrence rate after surgery[3]. In recent years, cases of diabetic mastopathy have rarely been reported. The present case is an elderly female patient who was misdiagnosed with breast cancer before surgery, providing a reference for the clinical diagnosis of the disease.
This study was combined with a literature review for a better understanding of diabetic mastopathy and to provide a reference for the clinical diagnosis of diabetic mastopathy in elderly individuals. Key words diabetic mastopathy and lymphocytic mastopathy were used for a search in PubMed.
A 69-year-old unemployed woman from Asia developed symptoms of painless bilateral breast masses. Given these persistent symptoms, the patient opted for a further treatment at our hospital.
The patient first found bilateral breast masses without pain in June 2020. Mammog
The patient had a history of type 2 diabetes for 20 years. She was given long-term treatment with 22 U Humalog 25R in the morning and 20 U Humalog 25R in the evening via subcutaneous injection as well as voglibose tablets (0.2 mg) three times a day. With a poor glucose control, no diabetic complications were found till now.
The patient had a free personal and family history.
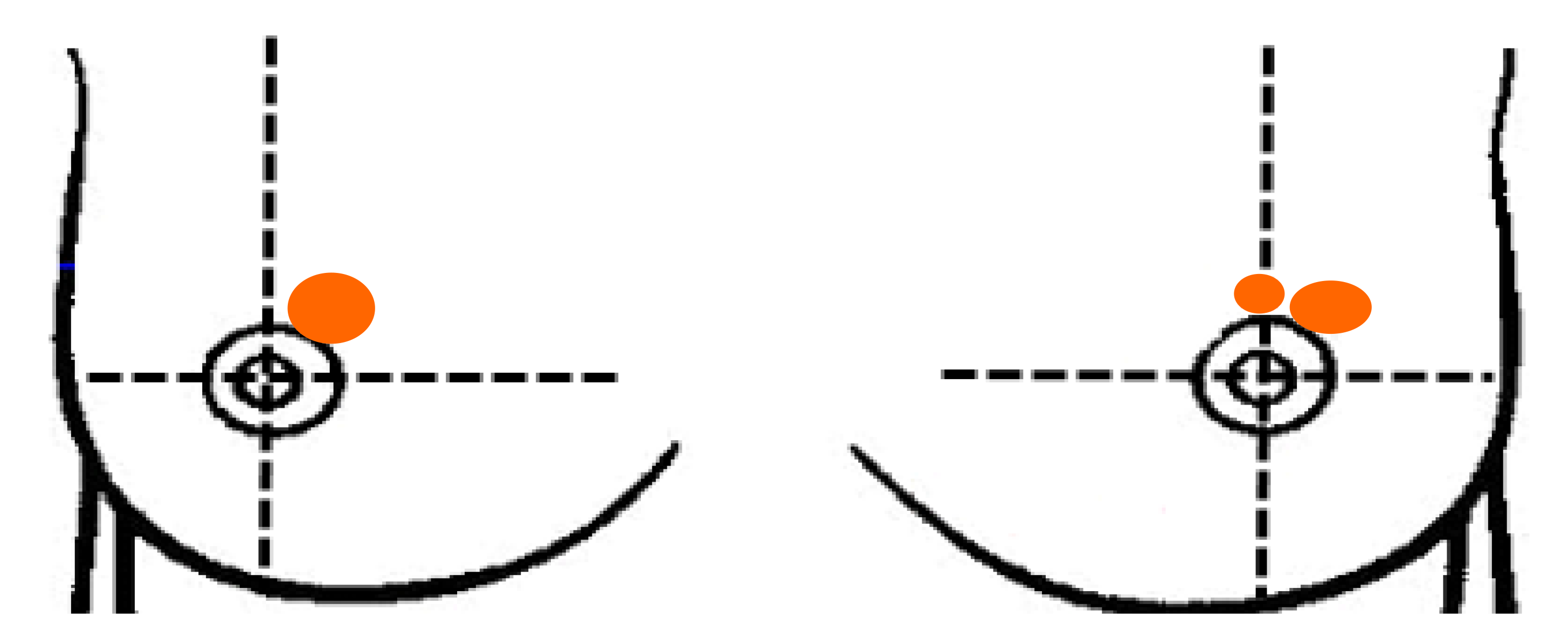
The physical examination revealed symmetrical breasts, no orange peel appearance change, no dimpling, and double nipples without retraction or discharge. At the 1 o'clock position on the right breast, a mass (size 2 cm × 2 cm) was present with no tenderness but an unclear boundary, irregular shape, poor mobility, and skin adhesion (-). At the 12 o'clock and 1 o'clock positions on the left breast, masses (approximate sizes of 0.5 cm × 0.5 cm and 1.5 cm × 2 cm, respectively) were present with no tenderness but an unclear boundary, irregular shape, poor mobility, and skin adhesion (-). Enlarged lymph nodes were not found in the supraclavicular and subclavian regions and bilateral axilla (Figure 1).
Glycosylated hemoglobin in serum was 8.3%, which exceeded the reference range of 3.6%-6.0%. Cholesterol in serum was 8.53 mmol/L, surpassing the upper limit reference 5.18 mmol/L. Triglyceride in serum was 2.89 mmol/L, also higher than the upper limit reference of 1.70 mmol/L. Other serum test results including routine blood tests, coagulation function tests, liver function tests, and tumor markers were normal.
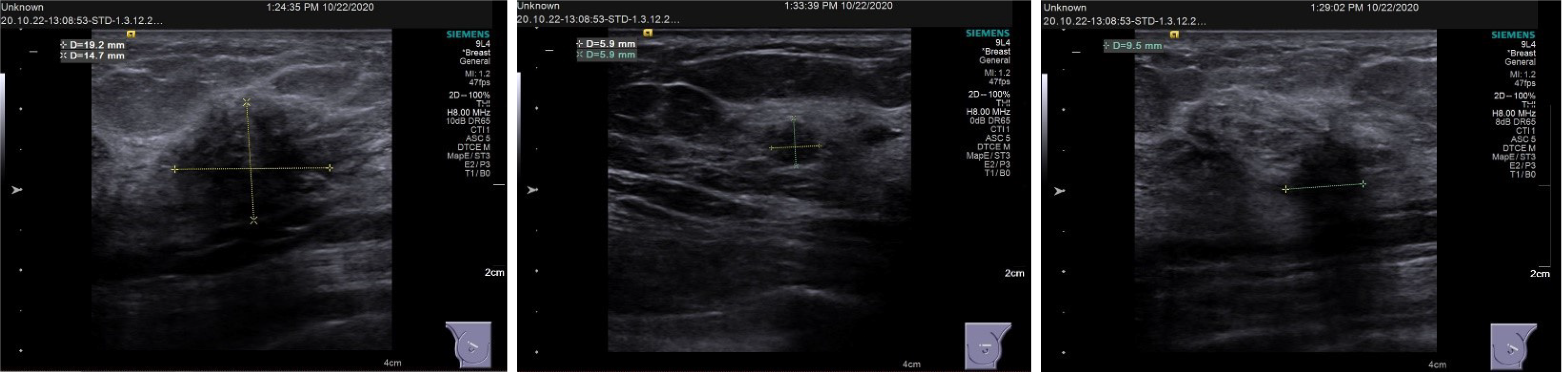
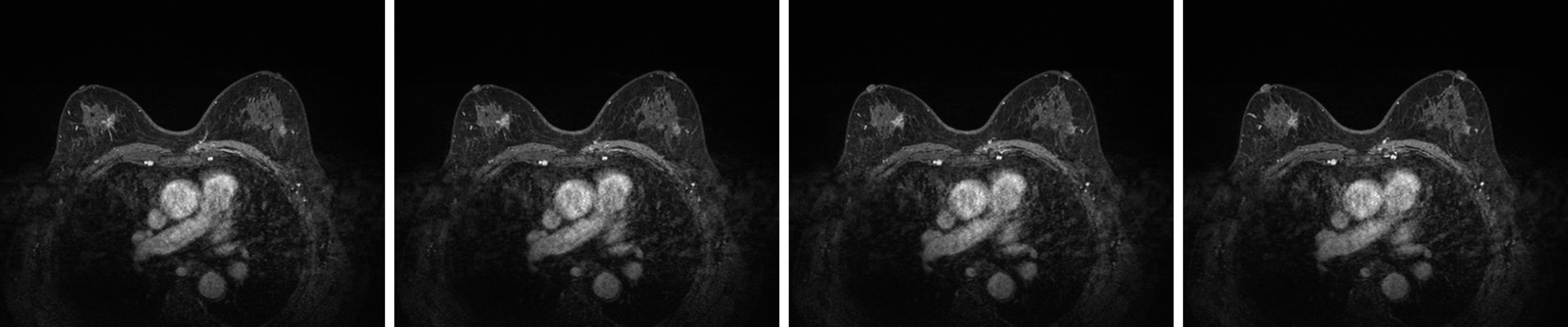
Breast ultrasound showed the following features: (1) Solid occupation of the upper outer quadrant of both breasts (BI-RADS 4C); and (2) A solid mass at the 12 o'clock position in the left breast (BI-RADS 4B). Lymph nodes were found in the bilateral axilla and left supraclavicular region. No enlarged lymph nodes were observed in the right clavicle (Figure 2). Contrast-enhanced magnetic resonance imaging (MRI) of the breast showed the following features: Irregularly enhanced images in the upper inner quadrant of the right breast (BI-RADS 4C); patchy abnormal signals in the upper outer quadrants of the left breast (BI-RADS 4B); and fibrocystic changes in both breasts (BI-RADS 2) (Figure 3).
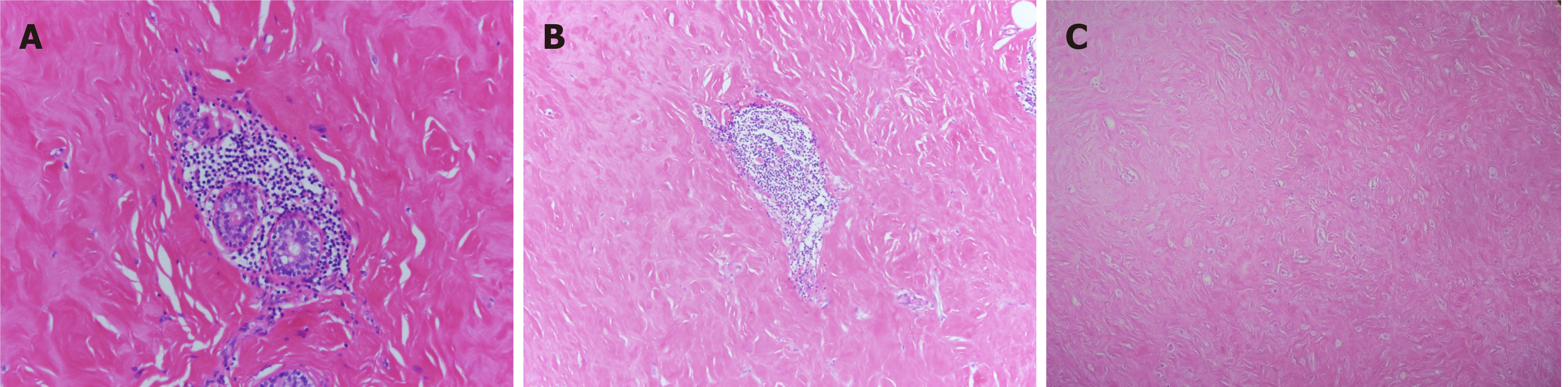
Macroanatomy indicated that the lesion of the right breast was 9 cm × 8 cm × 3 cm in size with a grayish-red medium area with a size of 1 cm × 0.5 cm × 0.5 cm. The remaining breast tissue was grayish white and soft. The left breast lesion was 4 cm × 2.5 cm × 2.2 cm in size with a grayish-white area that was soft in texture. Histopathological result showed dense lymphocyte infiltration around the lobules, extensive fibrosis of the surrounding stroma, and epithelioid myofibroblasts (Figure 4). A diagnosis of diabetic mastopathy was made.
During the perioperative period, the hypoglycemic plan was adjusted to the following treatment: 22 U Humalog 25R in the morning and 20 U Humalog 25R in the evening via hypodermic injection as well as metformin sustained release tablets (0.5 g) in the morning and evening and Empagliflozin tablets (10 mg) in the morning. Blood glucose was closely monitored and blood glucose control was good. Combined with the patient’s imaging examination and age, the breast masses were considered to be malignant. During the operation, masses and the surrounding part of the gland tissue were completely removed. The sizes of the masses ranged from approximately 3 cm × 3 cm and 2 cm × 2 cm, respectively, and the glands of bilateral breasts were stiff and degenerative. The border was not clear without obvious capsule, the color was gray and white, and the texture was hard. Intraoperative frozen pathology suggested sclerosing gland disease with small focal epithelial hyperplasia in the right breast, and adenopathy with collagen fiber hyperplasia in the left breast. A gland flap around the residual cavity of the right breast was used to reshape the breast.
The patient recovered well after the operation and had no recurrence during 2 mo of follow-up.
The prevalence of diabetic breast disease is not known. Sloer and Khardori reported in 1984 that 13% of 88 women with type 1 diabetes had the disease[1]. Logan and Hoffman[2] estimated that there was 1 case of diabetic mastopathy per 1700 persons in their clinic[2]. Byrd et al[4] found 8 cases in thousands of puncture specimens[4]. In 2015, Moschetta et al[5] reported that the incidence of the disease was 7% (9 out of 120 diabetic patients). A previous study[1] indicated that the onset age of diabetic breast disease is in the range of 20-40; however, exceptions can occur such as a case report of an 80-year-old female patient[6].
Diabetic breast disease is often accompanied by immunological thyroid disease when it is first reported; however, its pathogenesis remains unclear and is generally thought to be related to autoimmunity. In previous studies, some autoimmune disease related class II human leukocyte antigens were expressed, such as human leukocyte antigen (HLA)-DR3, HLA-DR4, and HLA-DR5. In addition, a previous study[7] found that 3 out of 8 cases had follicular centers with B cell proliferation, and surface immunoglobulin analysis showed that B cells were polyclonal. In addition, lobular cell HLA-DR expression was detected in two cases, which was also observed in parotid lymphoepithelial lesions and Hashimoto's thyroiditis. In a study in 1991[8], 13 patients with lesions characterized by lobular fibrosis and abnormal lymphocyte infiltration were included. Consistent with previous study, immunophenotypic analysis of breast lymphocyte infiltration showed that the majority of infiltrating lymphocytes were B cells. Several subsequent research reports agreed with previous conclusions[9]. Tomaszewski et al[10] reported that the application of exogenous insulin is a common single clinical factor in diabetic mastopathy and proposed the following hypothesis for the pathogenesis of diabetic mastopathy: Fibroinflammatory lesions can be attributed to the expansion of the extracellular matrix followed by increased collagen production and reduced degradation, which are partly related to hyperglycemia. According to their model, the lesions form advanced glycosylated end products that act as antigens, triggering autoimmune B cell proliferation and autoantibody production. The release of cytokines causes the matrix to swell. In addition, Seidma et al[11] also found that diabetic mastopathy[11] is relatively specific to patients with insulin-dependent diabetes. Therefore, it is believed that the disease may be related to the application of insulin and may be caused by inflammation because of insulin. A study compared the prevalence of type 1 diabetes mellitus with insulin therapy to type 2 diabetes mellitus to assess whether insulin therapy is associated with sclerosing lymphocytic lobulitis, and the results showed no significant difference. In addition, it is also believed that diabetic mastopathy, such as diabetic nephropathy and retinopathy, should be regarded as a chronic complication of diabetes[12]. Miura et al[13] reported an elderly female patient whose autoantibodies in serum reacted positively against her ductal epithelium as well as other diabetic and nondiabetic breast ductal cells. The results of the insulin antigen absorption test showed a decreasing insulin concentration with the increasing antigen absorption intensity. Therefore, it is suggested that these insulin antibodies produced in diabetic patients may cause ductitis via antigen cross reaction.
Logan and Hoffman[2] proposed for the first time that the diagnosis of diabetic mastopathy should meet the following three conditions: (1) A long history of insulin-dependent diabetes mellitus; (2) Painless, hard, irregular, poorly demarcated, and mobile breast masses that are often bilateral or unilateral; and (3) Fine needle aspiration indicating benign lesions. Rollins[14] suggested that fine needle aspiration is a good tool for diagnosis and lesions should be evaluated by fine needle aspiration in patients with a prior diagnosis of diabetic breast lesions.
If cytology and clinical presentation are consistent with diabetic breast lesions, conservative clinical treatment may be considered. Subsequent studies[15] have shown that fine needle aspiration is usually nondiagnostic and that core needle biopsy is necessary for a clear diagnosis. This known advantage should be conducive in selecting core needle biopsy rather than fine needle aspiration in ultrasound-guided biopsy. In their study, 64% of core-needle samples showed lymphocytic lobulitis, 73% showed lymphocytic ductitis, 100% showed dense keloid fibrosis, and 91% showed epithelioid fibroblasts. In 2012, a clinicopathological correlation analysis of 34 patients with diabetic breast disease showed that 85.3% of patients with diabetes clinically showed palpable breast masses[16]. Ultrasound often shows an irregular hypoechoic mass (44.4%), and mammography often shows a negative or nonspecific density (67.6%). Histologically, we found that most cases of diabetes mastopathy occurred in the upper lateral/central part of the breast (76%) with sizes ranging from 0.5 to 3.7 cm. All patients presented with lymphocytic lobulitis, vasculitis, scar fibrosis, lobular atrophy, and varying degrees of epithelioid fibroblasts. Examination of the normal tissue around the resected specimen revealed that the margins of the diseased tissue in diabetic mastopathy were often ill-defined and irregularly separated from the normal mammary tissue. Vascular calcification was found in 10 of the 24 resected specimens. A follow-up study was also consistent with the ultrasound manifestation mentioned in this report[5,17]. With updated examination techniques, MRI plays an important role in the diagnosis of diabetic mastopathy. An increasing number of studies have reported the value of MRI in the diagnosis of diabetic mastopathy. It was reported for the first time in 2002[18] that the MRI characteristic of diabetic breast disease was uneven dot enhancement but that it could not be completely differentiated from breast cancer in terms of imaging manifestations, indicating the inevitable role of puncture and surgery.
In addition, studies have reported[19] the application of diffusion weighted MRI in the differentiation of this disease from malignant tumors. MRI showed uneven segmental enhancement, similar to malignant lesions, but no abnormalities were found in DWI diffusion weighted imaging. In addition, the value of apparent dispersion coefficient did not decrease. In addition, studies[20] have also reported the application of dynamic contrast-enhanced MRI and magnetic resonance spectroscopy in diabetic breast disease. Post-MRI diabetic mastopathy showed pitted contrast uptake, while breast cancer showed strong contrast enhancement. In the magnetic resonance spectrum, the choline peak was absent in the type 1 imaging uptake curve and the proton spectrum. Despite the improvement of imaging techniques, there are still great challenges in the diagnosis of diabetic breast disease[9,21-23]. An increasing number of studies have reported cases of diabetic breast disease complicated with malignant tumors[24,25], and some studies[26,27] have also shown that neither clinical examination nor imaging studies can clearly distinguish diabetic breast disease from breast cancer. Therefore, the diagnosis of this disease needs improvement and requires further research.
In terms of treatment and prognosis, studies[28] have shown that diabetic breast lesions are prone to single or multiple recurrences in the same or opposite breast after surgical resection, and one patient was found to have spontaneous regression during the 5-year follow-up period. In addition, Camuto et al[29] reported that 60% of diabetic breast patients relapsed after surgical resection and the masses tended to be observed in the same location, even involving more breast tissue than the previous lesion. Therefore, surgical biopsy should not be considered. Ely et al[3] showed that 6 of 19 patients with diabetic breast disease experienced recurrence, either unilateral, contralateral, or bilateral. Previous studies[30,31] have suggested that conservative treatment should be provided after the exclusion of malignant lesions and that surgical biopsy or resection is not recommended.
No relevant studies have reported that diabetic breast disease can develop into malignant lesions, such as breast cancer.
Diabetic mastopathy is a rare benign disease in the clinic, and its pathogenesis is not yet clear. The clinical manifestations are painless, irregular, unilateral or bilateral breast masses. The diagnosis of diabetic mastopathy needs to be distinguished from breast cancer, mucosa-related lymphoma, and invasive lobular carcinoma. Sometimes surgery and biopsy cannot be avoided, causing psychological burden to patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fakhr I, Zeng YQ S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Soler NG, Khardori R. Fibrous disease of the breast, thyroiditis, and cheiroarthropathy in type I diabetes mellitus. Lancet. 1984;1:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Logan WW, Hoffman NY. Diabetic fibrous breast disease. Radiology. 1989;172:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 86] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Ely KA, Tse G, Simpson JF, Clarfeld R, Page DL. Diabetic mastopathy. A clinicopathologic review. Am J Clin Pathol. 2000;113:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Byrd BF Jr, Hartmann WH, Graham LS, Hogle HH. Mastopathy in insulin-dependent diabetics. Ann Surg. 1987;205:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 60] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Moschetta M, Telegrafo M, Triggiani V, Rella L, Cornacchia I, Serio G, Ianora AA, Angelelli G. Diabetic mastopathy: a diagnostic challenge in breast sonography. J Clin Ultrasound. 2015;43:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Kojima T, Kammori M, Hashimoto M, Ogawa T, Yasuda H, Takazawa Y, Takubo K, Kaminishi M. Diabetic mastopathy in an advanced elderly woman with insulin-dependent type 2 diabetes mellitus. Breast Cancer. 2003;10:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Schwartz IS, Strauchen JA. Lymphocytic mastopathy. An autoimmune disease of the breast? Am J Clin Pathol. 1990;93:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Lammie GA, Bobrow LG, Staunton MD, Levison DA, Page G, Millis RR. Sclerosing lymphocytic lobulitis of the breast--evidence for an autoimmune pathogenesis. Histopathology. 1991;19:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Ng WK, Chan SK, Kwok KM, Fung PY. Diabetic mastopathy: a breast carcinoma mimic. Hong Kong Med J 2019; 25: 251.e1-251. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Tomaszewski JE, Brooks JS, Hicks D, Livolsi VA. Diabetic mastopathy: a distinctive clinicopathologic entity. Hum Pathol. 1992;23:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 121] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Seidman JD, Schnaper LA, Phillips LE. Mastopathy in insulin-requiring diabetes mellitus. Hum Pathol. 1994;25:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 12. | Ricart Selma V, Camps Herrero J, Martínez Rubio C, Cano Muñoz R, González Noguera PJ, Forment Navarro M, Cano Gimeno J. [Diabetic mastopathy: clinical presentation, imaging and histologic findings, and treatment]. Radiologia. 2011;53:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Miura K, Teruya C, Hatsuko N, Ogura H. Autoantibody with cross-reactivity between insulin and ductal cells may cause diabetic mastopathy: A case study. Case Rep Med. 2012;2012:569040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Rollins SD. Fine-needle aspiration cytology of diabetic fibrous mastopathy. Diagn Cytopathol. 1993;9:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Andrews-Tang D, Diamond AB, Rogers L, Butler D. Diabetic mastopathy: adjunctive use of ultrasound and utility of core biopsy in diagnosis. Breast J. 2000;6:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Dorokhova O, Fineberg S, Koenigsberg T, Wang Y. Diabetic mastopathy, a clinicopathological correlation of 34 cases. Pathol Int. 2012;62:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Suvannarerg V, Claimon T, Sitthinamsuwan P, Thiravit S, Muangsomboon K, Korpraphong P. Clinical, mammographic, and ultrasonographic characteristics of diabetic mastopathy: A case series. Clin Imaging. 2019;53:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Sakuhara Y, Shinozaki T, Hozumi Y, Ogura S, Omoto K, Furuse M. MR imaging of diabetic mastopathy. AJR Am J Roentgenol. 2002;179:1201-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Isomoto I, Wada T, Abe K, Uetani M. Diagnostic utility of diffusion-weighted magnetic resonance imaging in diabetic mastopathy. Clin Imaging. 2009;33:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Kim J, Kim EK, Kim MJ, Moon HJ, Yoon JH. Diabetic mastopathy: imaging features and the role of image-guided biopsy in its diagnosis. Ultrasonography. 2016;35:140-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Nasu H, Ikeda A, Ogura H, Teruya C, Koizumi K, Kinoshita M, Tsuchida T, Baba S, Miura K, Takehara Y, Sakahara H. Two cases of diabetic mastopathy: MR imaging and pathological correlation. Breast Cancer. 2015;22:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Alkhudairi SS, Abdullah MM, Alselais AG. Diabetic mastopathy in a patient with high risk of breast carcinoma: A management dilemma. Cureus. 2020;12:e7003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Allué M, Arribas MD, Guemes A. Diabetic mastopathy: Differential diagnosis of breast carcinoma. Breast J. 2020;26:1416-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Yamashita M, Ogawa T, Hanamura N, Kashikura Y, Mitsui T, Zhang X, Fujii K, Shiraishi T. An uncommon case of T1b breast cancer with diabetic mastopathy in type II diabetes mellitus. Breast Cancer. 2013;20:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Mackey SP, Sinha S, Pusey J, Chia Y, McPherson GA. Breast carcinoma in diabetic mastopathy. Breast. 2005;14:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Gold HT, Shao H, Oratz R, Yu O, Hammer M, Richardson S, Boudreau D. Association of diabetes and other clinical and sociodemographic factors with Guideline-concordant breast cancer treatment for breast cancer. Am J Clin Oncol. 2020;43:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Schairer C, Gadalla SM, Pfeiffer RM, Moore SC, Engels EA. Diabetes, Abnormal Glucose, Dyslipidemia, Hypertension, and risk of inflammatory and other breast cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Bayer U, Horn LC, Schulz HG. Bilateral, tumorlike diabetic mastopathy-progression and regression of the disease during 5-year follow up. Eur J Radiol. 1998;26:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Camuto PM, Zetrenne E, Ponn T. Diabetic mastopathy: a report of 5 cases and a review of the literature. Arch Surg. 2000;135:1190-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Croce S, Chaney G, Bretz-Grenier MF, Wittersheim A, Casnedi S, Mathelin C. [Diabetic mastopathy: a recurrent benign breast disease]. Gynecol Obstet Fertil. 2010;38:686-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Accurso A, Della Corte GA, Rocco N, Varone V, Buonaiuto R, Compagna R, Tari DU, Amato B, Riccardi A. Unusual breast lesion mimicking cancer: diabetic mastopathy. Int J Surg. 2014;12 Suppl 1:S79-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |