Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3350
Peer-review started: November 25, 2020
First decision: January 7, 2021
Revised: January 20, 2021
Accepted: March 3, 2021
Article in press: March 3, 2021
Published online: May 16, 2021
Processing time: 154 Days and 21 Hours
MET fusion is a key driver mutation, but it is rare in gastric cancer (GC). Several MET (hepatocyte growth factor receptor) inhibitors have been approved for the treatment of MET-positive patients, but the tumor response is heterogeneous. With the development of next-generation sequencing, diverse MET fusion partner genes have been identified. We herein report a fusion variant involving KIF5B-MET in GC.
After thoracoscopic inferior lobectomy plus lymph node dissection under general anesthesia, a “tumor within a tumor” was found in the lung tumor tissue of a 64-year-old non-smoking male patient. Combining the medical history and the results of enzyme labeling, the focal area was considered to be GC. To seek potential therapeutic regimens, an intergenic region between KIF5B and MET fusion was identified. This fusion contains a MET kinase domain and coil-coiled domains encoded by KIF5B exons 1-25, which might drive the oncogenesis.
Our finding could extend the spectrum and genomic landscape of MET fusions in GC and favor the development of personalized therapy.
Core Tip: In this case report, we report the discovery of a "tumor within a tumor " in the lung tumor tissue of a 64-year-old non-smoking male patient. Combined with the medical history and the results of enzyme labeling, the focal area of the postoperative pathology was considered to be gastric cancer. To seek potential therapeutic regimens, an intergenic region between KIF5B and MET fusion was identified, which contains a MET kinase domain and a coil curl domain encoded by KIF5B exons 1-25, which may drive the tumorigenesis.
- Citation: Wu ZW, Sha Y, Chen Q, Hou J, Sun Y, Lu WK, Chen J, Yu LJ. Novel intergenic KIF5B-MET fusion variant in a patient with gastric cancer: A case report. World J Clin Cases 2021; 9(14): 3350-3355
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3350.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3350
Gastric cancer (GC) is one of the most common malignancies[1]. The incidence and mortality of GC vary by region, but more than 50% of cases occur in East Asia[2]. GC is responsible for 10.6% of new cases and 13.6% of cancer-related deaths in China[3]. Targeted therapies designed to disrupt the activity of specific oncogenic signaling pathways have recently emerged as a promising therapeutic strategy in GC, such as the ToGA trial. Trastuzumab and ramucirumab improved the overall survival of patients by targeting the HER-2 and VEGFR genes[4,5].
MET proto-oncogene receptor tyrosine kinase is a high-affinity proto-oncogene receptor tyrosine kinase that, upon activation, drives oncogenic pathways involved in cell proliferation, survival, and metastasis. As one of the major MET variants, MET fusion is a key driver mutation. The common fusion proteins are derived from chromosomal translocations, which are usually composed of N-terminal dimerization domains provided by the kinase domains to which fusion partner proteins fuse to tyrosine kinases. These fusion mutations often lead to the activation of the kinase domain and provide ideal targets for the development of anticancer therapies[6]. With the development of next-generation sequencing (NGS), diverse MET fusion partner genes, such as ETV6-MET[7], PTPRZ1-MET[8], and CD74-MET, have been identi
A 64-year-old male patient was admitted to Jingjiang Peoples’ hospital due to nodules in his right lower lung.
The patient underwent chest computed tomography (CT) 2 years ago, which revealed nodules in the right lower lung.
The patient underwent subtotal gastrectomy for GC 15 years ago, and was diagnosed with gastric adenocarcinoma by postoperative pathology. Adjuvant chemotherapy was given after operation (details are not available). During this period, the condition has been stable.
None special.
There was no abnormality in cardiopulmonary and abdominal examinations.
Blood analysis did not reveal raised levels of tumor markers.
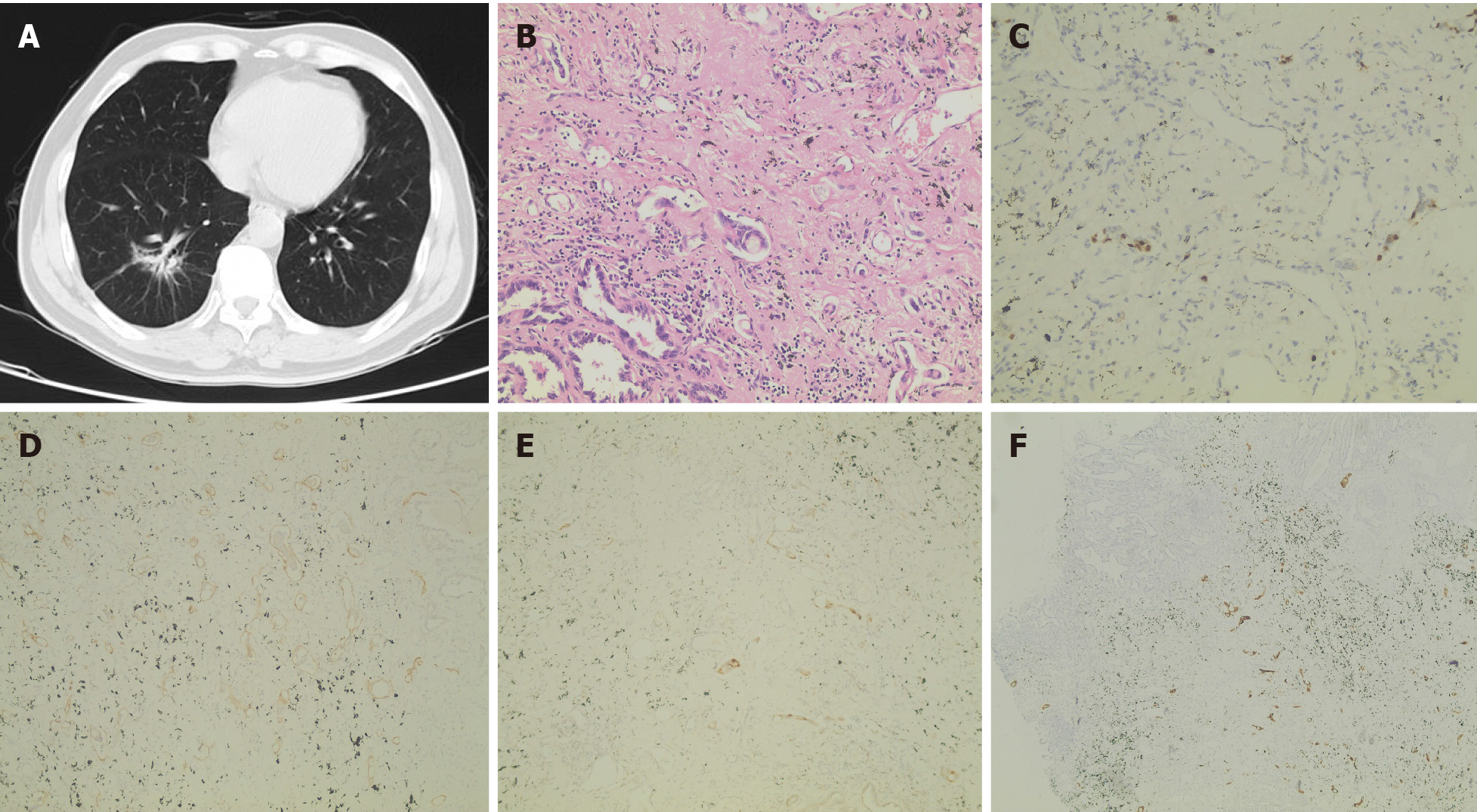
After admission, chest CT (April 18, 2019) showed irregular patchy shadows in the lower lobe of the right lung, local thickening of the bronchial wall, and narrowing of the lumen, which was slightly denser than that 2 years ago (Figure 1A).
The patient underwent thoracoscopic inferior lobectomy plus lymph node dissection under general anesthesia on April 26, 2019. Postoperative pathology revealed that the heterotype glands in the focal area were significantly different from the main tumor body, the cytoplasm was rich in mucus, and most of the tumor cells were located in lymphatics (Figure 1B). Combining the medical history and the results of immuno
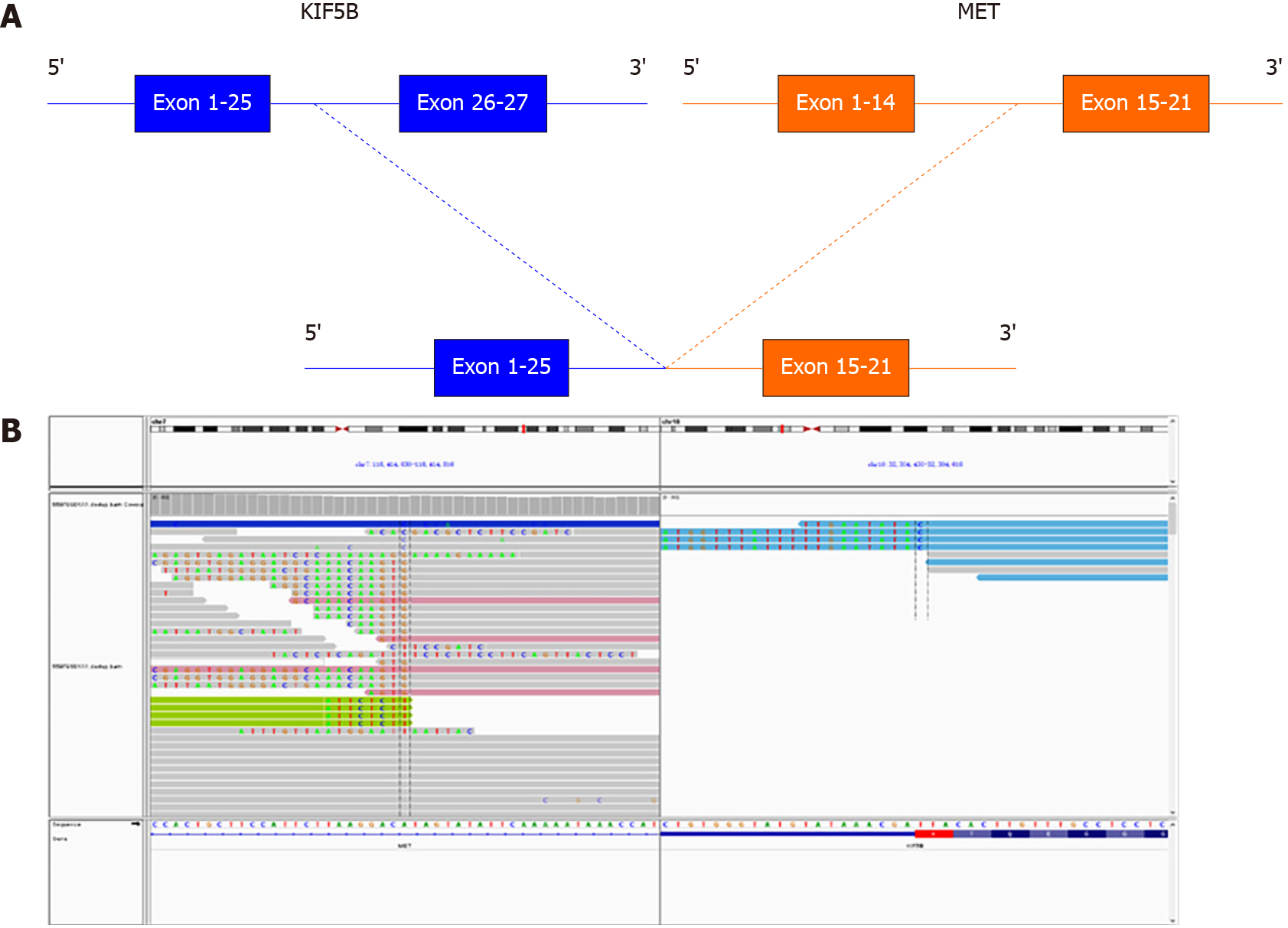
To probe the genomic profile of the tumor for targeted therapy, the tissue specimens were subjected to NGS analysis, and an intergenic region between KIF5B and MET fusion was identified (Figure 2A). The fusion of KIF5B-MET included exons 1-25 of KIF5B and exons 15-21 of MET, and the complete kinase structure of the MET (hepatocyte growth factor receptor) protein was retained (Figure 2B). The mutant allele frequency was 15.24%. No other driver gene variants were found.
Pathology of the Cancer Hospital of Fudan University: (right lower lung) adeno
Lung metastasis after resection of GC and right lung lower lobe adenocarcinoma (pT1N0M0 IA stage).
The patient took three courses of tegafur monotherapy on July 2, July 23, and August 13, 2019, with grade II gastrointestinal reaction. Then he developed right pleural effusion and were treated by thoracic catheter drainage on November 4, 2019. The carcinoembryonic antigen (CEA) level of pleural effusion was 423.4 ng/mL, which was significantly increased, suggesting it was tumor related, but no cancer cells were found in the pleural effusion exfoliative cell examination. In January 2020, the patient developed cough, expectoration, and shortness of breath. He went to Shanghai for positron emission tomography/CT examination, which revealed that there was dense shadow in right lower hilar with fluorodeoxyglucose (FDG) metabolism increased; the right pleural and peritoneal thickening with FDG metabolism increased, metastasis possible; the left lower abdominal wall metastasis possible; pleural, abdominal and pelvic effusion on both sides. The epithelioid cells in pleural effusion had a larger nucleocytoplasmic ratio and obvious nucleoli. The cells in paraffin section were arranged in an adenoid pattern and considered as adenocarcinoma according to the hematoxylin-eosin staining.
Unfortunately, the patient's cough and shortness of breath gradually worsened and he died of respiratory failure in February 2020.
MET fusions are rare in GC, and this is the first documented case of a patient with an MET fusion-positive tumor. We identified a fusion variant involving KIF5B-MET, and the variant was the same as the most recently reported KIF5B-MET fusion variant in non-small cell lung cancer[10]. This fusion gene was initially reported in one of 513 lung adenocarcinoma samples[11]. Although several MET inhibitors have been approved for the treatment of MET-positive patients, the tumor response is heterogeneous, and the overall survival ranges from 1 mo to 36 mo[10,12,13]. One explanation is that the response of different MET fusion types to MET inhibitors differs. Other possible reasons include the existence of other driver mutations or primary resistance mutations in the kinase structure of the MET protein. Therefore, it seems necessary to comprehensively understand MET fusion information, and NGS could serve as a supplementary approach for MET status detection because of its high-throughput molecular analysis that can detect gene copy number alterations, deletions, insertions, and fusions simultaneously.
To the best of our knowledge, this is the first report of breakpoints coexisting simultaneously in the intergenic region between the KIF5B gene and the MET gene, as well as a novel MET rearrangement in GC. This fusion contains a MET kinase domain and coil-coiled domains encoded by KIF5B exons 1-25, which might drive the oncogenesis. Given the recent development of several MET inhibitors and their potential therapeutic efficacy for tumors expressing the KIF5B-MET fusion protein, we expect that further investigation of KIF5B-MET fusions will enable us to identify GC patients suitable for this targeted therapy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aykan NF S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21364] [Article Influence: 2136.4] [Reference Citation Analysis (3)] |
| 2. | Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 3. | Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1122] [Article Influence: 187.0] [Reference Citation Analysis (1)] |
| 4. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy vs chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5312] [Article Influence: 354.1] [Reference Citation Analysis (3)] |
| 5. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1572] [Article Influence: 142.9] [Reference Citation Analysis (0)] |
| 6. | International Cancer Genome Consortium PedBrain Tumor Project. Recurrent MET fusion genes represent a drug target in pediatric glioblastoma. Nat Med. 2016;22:1314-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 7. | Rooper LM, Karantanos T, Ning Y, Bishop JA, Gordon SW, Kang H. Salivary Secretory Carcinoma With a Novel ETV6-MET Fusion: Expanding the Molecular Spectrum of a Recently Described Entity. Am J Surg Pathol. 2018;42:1121-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Zeng AL, Yan W, Liu YW, Wang Z, Hu Q, Nie E, Zhou X, Li R, Wang XF, Jiang T, You YP. Tumour exosomes from cells harbouring PTPRZ1-MET fusion contribute to a malignant phenotype and temozolomide chemoresistance in glioblastoma. Oncogene. 2017;36:5369-5381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Pan Y, Zhang Y, Ye T, Zhao Y, Gao Z, Yuan H, Zheng D, Zheng S, Li H, Li Y, Jin Y, Sun Y, Chen H. Detection of Novel NRG1, EGFR, and MET Fusions in Lung Adenocarcinomas in the Chinese Population. J Thorac Oncol. 2019;14:2003-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Gow CH, Liu YN, Li HY, Hsieh MS, Chang SH, Luo SC, Tsai TH, Chen PL, Tsai MF, Shih JY. Oncogenic Function of a KIF5B-MET Fusion Variant in Non-Small Cell Lung Cancer. Neoplasia. 2018;20:838-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 747] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 12. | Cho JH, Ku BM, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. KIF5B-MET Gene Rearrangement with Robust Antitumor Activity in Response to Crizotinib in Lung Adenocarcinoma. J Thorac Oncol. 2018;13:e29-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Plenker D, Bertrand M, de Langen AJ, Riedel R, Lorenz C, Scheel AH, Müller J, Brägelmann J, Daßler-Plenker J, Kobe C, Persigehl T, Kluge A, Wurdinger T, Schellen P, Hartmann G, Zacherle T, Menon R, Thunnissen E, Büttner R, Griesinger F, Wolf J, Heukamp L, Sos ML, Heuckmann JM. Structural Alterations of MET Trigger Response to MET Kinase Inhibition in Lung Adenocarcinoma Patients. Clin Cancer Res. 2018;24:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |