Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3308
Peer-review started: November 12, 2020
First decision: January 23, 2021
Revised: January 30, 2021
Accepted: March 10, 2021
Article in press: March 10, 2021
Published online: May 16, 2021
Processing time: 167 Days and 18.1 Hours
Endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) is a safe and accurate technique to confirm the diagnosis of pancreatic cancers. Recently, numerous studies comparing the diagnostic efficacy of smear cytology (SC) and liquid-based cytology (LBC) for pancreatic lesions yielded mixed results.
To compare and identify the better cytology method for EUS-FNA in pancreatic lesions.
A comprehensive search of PubMed, Embase, and Cochrane was undertaken through July 18, 2020. The primary endpoint was diagnostic accuracy (sensitivity and specificity). Secondary outcomes included sample adequacy and post procedure complications. In addition, factors affecting diagnostic efficacy were discussed.
Data on a total of 1121 comparisons from 10 studies met the inclusion criteria. Pooled rates of sensitivity for SC and LBC were 78% (67%-87%) vs 75% (67%-81%), respectively. In any case, both SC and LBC exhibited a high specificity close to 100%. Inadequate samples more often appeared in LBC compared with SC. However, the LBC samples exhibited a better visual field than SC. Very few post procedure complications were observed.
Our data suggested that for EUS-FNA in pancreatic lesions (particularly solid lesions), SC with Rapid On-Site Evaluation represents a superior diagnostic technique. If Rapid On-Site Evaluation is unavailable, LBC may replace smears. The diagnostic accuracy of LBC depends on different LBC techniques.
Core Tip: Numerous studies comparing the diagnostic efficacy of smear cytology and liquid-based cytology (LBC) for endoscopic ultrasonography-guided fine-needle aspiration in pancreatic lesions yielded mixed results. Therefore, we conducted this systematic review and meta-analysis, finding that for endoscopic ultrasonography-guided fine-needle aspiration in pancreatic lesions (particularly solid lesions), smear cytology with Rapid On-Site Evaluation represents a superior diagnostic technique. If Rapid On-Site Evaluation is unavailable, LBC may replace smears. The diagnostic accuracy of LBC depends on different LBC techniques.
- Citation: Zhang XH, Ma SY, Liu N, Wei ZC, Gao X, Hao YJ, Liu YX, Cai YQ, Wang JH. Comparison of smear cytology with liquid-based cytology in pancreatic lesions: A systematic review and meta-analysis. World J Clin Cases 2021; 9(14): 3308-3319
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3308.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3308
Pancreatic cancer is a very lethal disease, and the 5-year relative survival rate for all stages combined is one of the lowest at only 9%[1]. Most patients are inoperable at diagnosis. After careful pretherapeutic evaluation, only 15%-20% of patients are eligible for upfront radical surgery[2]. Therefore, early diagnosis is important to improve the survival rate. Endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) is a safe and accurate technique to confirm the diagnosis of pancreatic cancer[3,4], which is important for the initiation of appropriate treatment. However, the diagnosis of lesion specimens obtained by EUS-FNA is influenced by many factors, including the experience of the endoscopic operator, the nature of the lesion, the needle type, the tissue processing procedure, the quality of the sample, and the experience of the pathologist[5]. Our study mainly compares the differences between two different cytological methods.
Traditionally, the cytological samples collected are smeared onto glass slides for so-called smear cytology (SC). This technique is cheap, easy to use, and available to the majority of EUS centers[6]. However, it is very sensitive to insufficient cells, uneven smears, and smears filled with inflammatory and blood cells, dry artifacts, crushing artifacts, or thick tissue fragments, which can obscure the cytological features and result in a suboptimal diagnosis[7,8]. To overcome the abovementioned problems, liquid-based cytology (LBC) was developed. LBC was originally applied in the field of gynecology and was gradually applied in non-gynecology fields in many countries[9]. In the diagnosis of gynecological diseases, LBC is considered superior to SC in many aspects. A similar conclusion has been drawn for FNA samples obtained from thyroid[10], parathyroid[11], breast, and other organs[12]. The process of LBC is time-saving and easier than smears and has the advantage of overcoming cell congestion and blood contamination[12]. The process also allows pathologists to perform ancillary tissue tests that could previously only be performed on the histological sample[13]. However, LBC is not common in pancreatic specimens obtained by EUS-FNA, and the diagnostic efficacy is unknown[8]. On the other hand, inadequate samples for EUS-FNA obtained from pancreatic lesions are a common problem in LBC. A sample processed with LBC may lose some background information that is useful for the correct diagnosis, such as mucus of intraductal papillary mucinous neoplasms or mucinous cyst-adenoma and the bloody background of a solid pseudopapillary tumor[12].
Recently, numerous studies comparing the diagnostic efficacy of SC and LBC for EUS-FNA in pancreatic lesions reported mixed results. Therefore, we performed this systematic and meta-analysis to compare and identify the better method.
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement[14] (Supplementary Table 1).
A systematic search of PubMed, Embase, and Cochrane was performed for all published studies that compared the diagnostic accuracy of SC with LBC for EUS-FNA in pancreatic lesions. The following search strategy was used: ["Pancreas" (Mesh)] OR [pancreatic (Title/Abstract)] OR {[Pancreatic biliary (Title/Abstract)] and [(liquid-based cytology) OR Thin Prep] OR (Cell Prep Plus) OR Sure Path} and {["Cytological Techniques"(Mesh)] OR [smear cytology (Title/Abstract)] OR [conventional smear Cytology (Title/Abstract)] OR [smears (Title/Abstract)] OR [Technique, Cytological (Title/Abstract)] OR [Cytologic Technics (Title/Abstract)] OR [Cytologic Method (Title/Abstract)]}. The latest search was performed on July 18, 2020. Two authors independently examined the title and abstract of citations. Records of potentially eligible studies were obtained, and disagreements regarding inclusion in the review were resolved by discussion and consensus. The reference lists of retrieved papers were further screened for additional eligible publications. Only English records were included.
Only studies meeting the following criteria were included: Studies directly comparing the samples of SC with LBC and those with complete raw data on diagnostic accuracy (or data useful for its calculation). The final diagnosis was defined as the diagnosis obtained from the integration of all of the results of SC, LBC, biopsy, or surgical pathology, if available, or clinical follow-up. Studies with the following features were excluded: Abstracts or published studies with incomplete or repeated data, conference articles, reviews, and meta-analyses; and description of only one index test in a patient cohort (SC or LBC).
A validated quality assessment tool for diagnostic accuracy studies was performed to assess the methodological quality of included studies[15]. The tool contains 14 items used to appraise the risk of bias and the applicability of results from included studies to the research question.
The primary outcome was diagnostic accuracy (sensitivity and specificity). Secondary outcomes were sample adequacy (defined as the ability to procure cytological samples adequate for interpretation) and post procedure complications.
Two authors (Zhang XH and Wei ZC) used a structured data collection sheet to extract data from studies independently. Quantitative variables extracted for the index test (SC and LBC) included the following: Number of true-positive, false-positive, true-negative, and false-negative results (Supplementary Table 2); the number of patients; and the number of punctures. Other quality-related variables extracted included the following: Type of study, with or without Rapid On-Site Evaluation (ROSE), whether the two tests were performed on different patients or in the same patient consecutively, whether inadequate samples were excluded in the final analysis, sample adequacy, and condition of the follow-up. All reported complications after the procedure were also extracted from included studies.
Statistical analysis was performed according to Cochrane guidelines[16]. Study-specific estimates of pooled sensitivity and specificity with 95% confidence intervals were calculated using a Mantel–Haenszel random-effects model and the bivariate mixed-effects regression model.
Summary receiver operating characteristic curves (SROC) were used to illustrate the relationship between sensitivity and specificity and to convey the diagnostic test performance of SC and LBC. Trapezoidal integration was used in SROC curves to calculate the pooled area under the curve (AUC); an AUC score of 1.0 indicates a perfect diagnostic test.
The I² statistic was used to assess the proportion of total variation in reported outcomes due to intrastudy heterogeneity. An I² score of 25%-49% indicates low heterogeneity, 50%-74% moderate, and greater than 75% a high degree of heterogeneity[17]. If heterogeneity among studies was recorded, the potential source of heterogeneity was investigated by meta-regression.
We assessed the possibility of publication bias by constructing a Deek’s funnel plot of each trial’s effect size against the standard error (Supplementary Figure 1) and defined significant publication bias as a P < 0.1. Publication bias was not present in LBC groups but present in SC groups. This difference could be due to great difference in the operational process in included studies. We could not exclude a publication bias in our meta-analysis. Meta-regression (subgroup analysis) was conducted according to study design (prospective and retrospective), matching methods, ROSE availability, LBC techniques, and cytological analysis classification methods.
All statistical analyses were conducted using the MIDAS module of STATA Stata/MP version 16.0 from http://www.stata.com (College Station, TX, United States). For all calculations, a 2-tailed P < 0.05 was considered statistically significant. Quality assessment tool for diagnostic accuracy study was performed using Review Manager (Rev Man) [Computer program] version 5.4 (The Cochrane Collaboration, 2020).
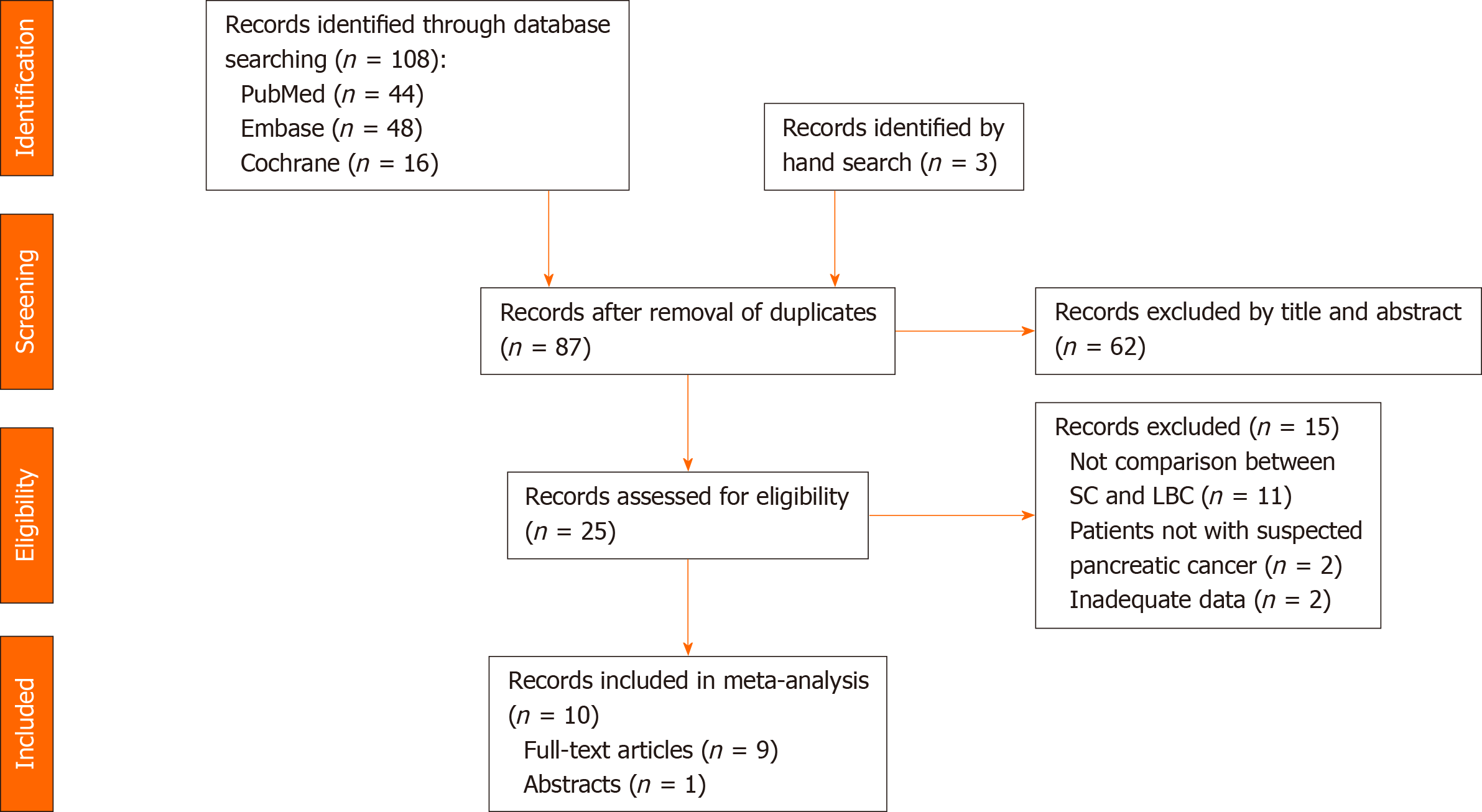
The electronic search of the PubMed, Embase, and Cochrane databases identified a total of 108 studies, and three additional studies were identified through extended bibliographic searches. In total, 24 studies were excluded as duplicates. In addition, 62 studies did not qualify for full-text review based on title and abstract. In total, 25 studies were assessed individually by both reviewers, and 15 were excluded. Thus, 10 studies describing comparative data on preoperative SC and LBC of pancreatic lesions were finally included in the analysis[5,8,12,13,18-23] (Figure 1).
Ten studies with a total of 1184 patients were included in this review with a total of 1121 comparisons between SC and LBC[5,8,12,13,18-23]. In two of the studies, inadequate cytological samples were excluded from the final analysis[8,18]. One of the studies also included a few patients who performed cell block exclusively in the final diagnostic accuracy analysis of LBC groups, but the LBC (Thin Prep) group comprised greater than half of all subjects[13]. Of the 10 studies included, seven were prospective[5,8,13,19-21,23], and three were retrospective[12,18,22]. Publication dates ranged from 2004 to 2020. The main characteristics of the included studies are reported in Table 1. All studies were well-balanced between groups based on homology or heterology matching[22]. ROSE was available in two studies[18,19], and the needle caliper was mainly 22 gauge. The most frequently used LBC technique was Thin Prep (TP)[13,18-21], whereas newer techniques were tested in four studies. Cytological analysis is generally based on the common classification: Inadequate specimens, negative and atypical specimens were classified as benign, and suspicious and malignant specimens were classified as malignant. Specimens classified as other, including Bethesda classification, which was scored as nondiagnostic, benign, atypical, or malignant, and nondiagnostic were excluded in the final analysis, and only malignant specimens were classified as "malignant"[13]. Classification based on the Papanicolaou Society of Cytopathology classification[24] yielded six categories[12].
| Ref. | Year | LBC technique | Cytological diagnosis classification | Match method | ROSE available | Research type | Outcome | Sample size1 | Number of puncture | FNA needle size, gauge |
| van Riet et al[6] | 2016 | TP and/or CB | Bethesda | Homology | No | Prospective | LBC = SC | 71 | 1 | 19/22/25 |
| Chun et al[8] | 2020 | SP | Common | Homology | NA1 | Prospective | LBC = SC | 169 (160/166) | ≥ 3 | 19/22 |
| Zhou et al[12] | 2020 | SP | PSC | Homology | No | Retrospective | SC + LBC > LBC > SC | 514 | ≥ 3 | 22/25 |
| Shih et al[23] | 2019 | NA | Common | Homology | NA | Prospective | LBC > SC | 9 | NA | NA |
| Yeon et al[5] | 2018 | CP and CB | Common | Homology | No | Prospective | LBC < SC | 48 | ≥ 3 | 22 |
| Hashimoto et al[22] | 2017 | SP and CB | Common | Heterology | No | Retrospective | LBC > SC | 63 | ≥ 3 | 19/22/25 |
| Qin et al[21] | 2014 | TP and CB | Common | Homology | No | Prospective | LBC > SC | 72 | ≥ 3 | 22 |
| Lee et al[20] | 2011 | TP | Common | Homology | No | Prospective | LBC < SC | 58 | ≥ 3 | 22/25 |
| LeBlanc et al[19] | 2010 | TP | Common | Homology | Yes | Prospective | LBC < SC | 50 | ≥ 3 | 22 |
| de Luna et al[18] | 2004 | TP | Common | Homology | Yes | Retrospective | LBC < SC | 67 (62/51) | NA | NA |
All of the studies were normal procedures of FNA, but there were some operator preference, e.g., number of punctures. All of the studies preferred ≥ 3 times if the sample in the field of vision was not sufficient. Van Riet et al[13] also performed the common practice, but samples obtained only after one single pass were included in their comparative studies[13].
The procedures were performed by several endoscopists with years of experience in three studies[5,8,13] and performed by experts or trainees in another study, depending on the difficulty of each EUS-FNA procedure[22]. The remaining studies did not report whether the procedures were performed by the same endoscopists or how experienced they were. In addition, most slides were prepared by endoscopists or nurses and transferred to pathology laboratories, where the slides were reviewed by patholo
Risk of bias and applicability concerns for all included studies are summarized in Supplementary Figure 2. Four studies had a high risk of bias for flow and timing and a high applicability of concerns for the index test[12,18,22,23]. This bias was largely because not all patients were included in the final analysis. For example, when analyzing the diagnostic accuracy, some inadequate samples may be removed. Regardless of whether SC or LBC was performed, the conduct and interpretation of the technique differ from the common practice primarily based on the aspects of cytological analysis classification methods and LBC techniques. These factors are important to consider because they affect sensitivity. However, we deliberately decided to restrict inclusion criteria to studies directly comparing samples by SB and LBC to provide more robust and homogenous outcome estimates.
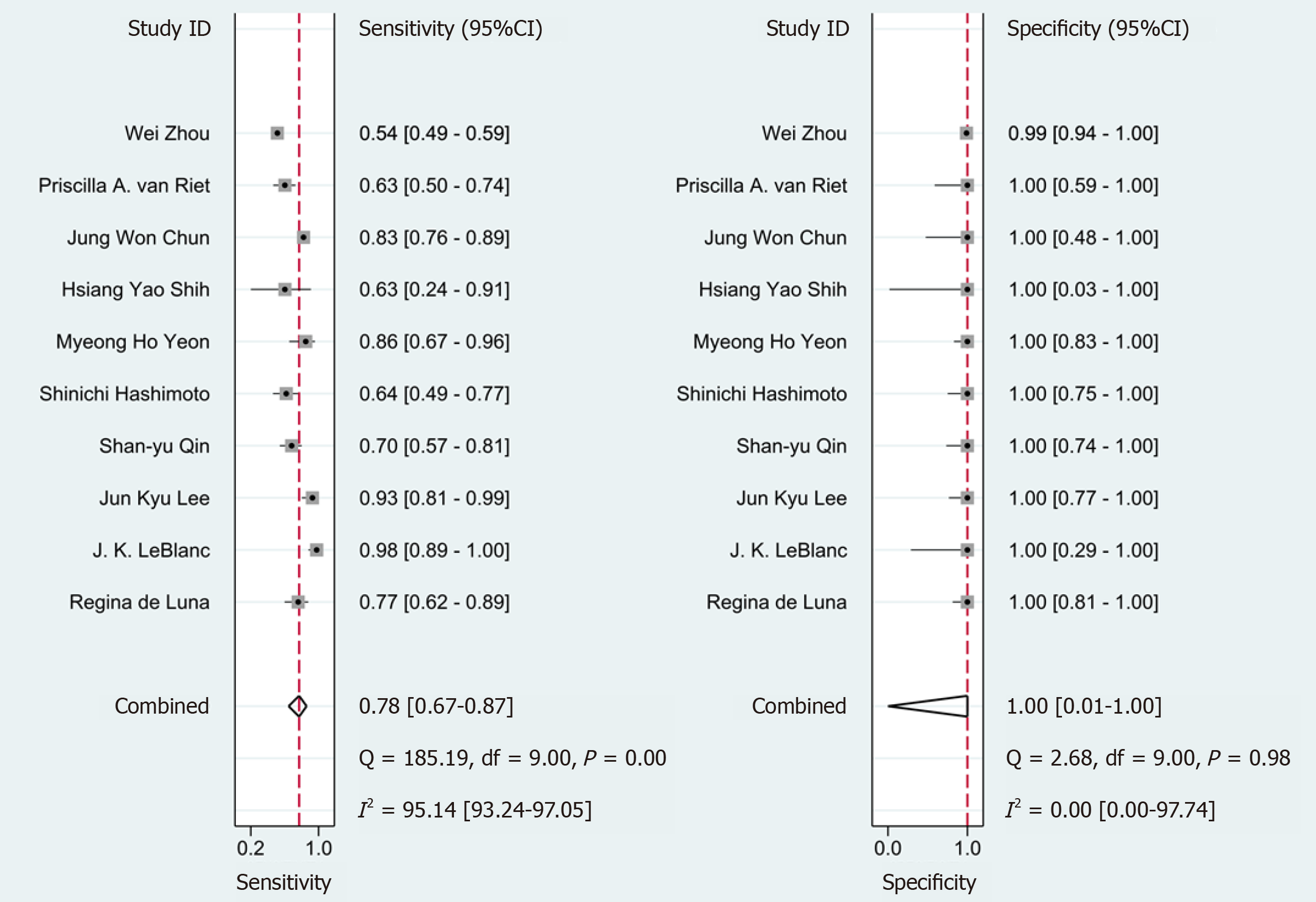
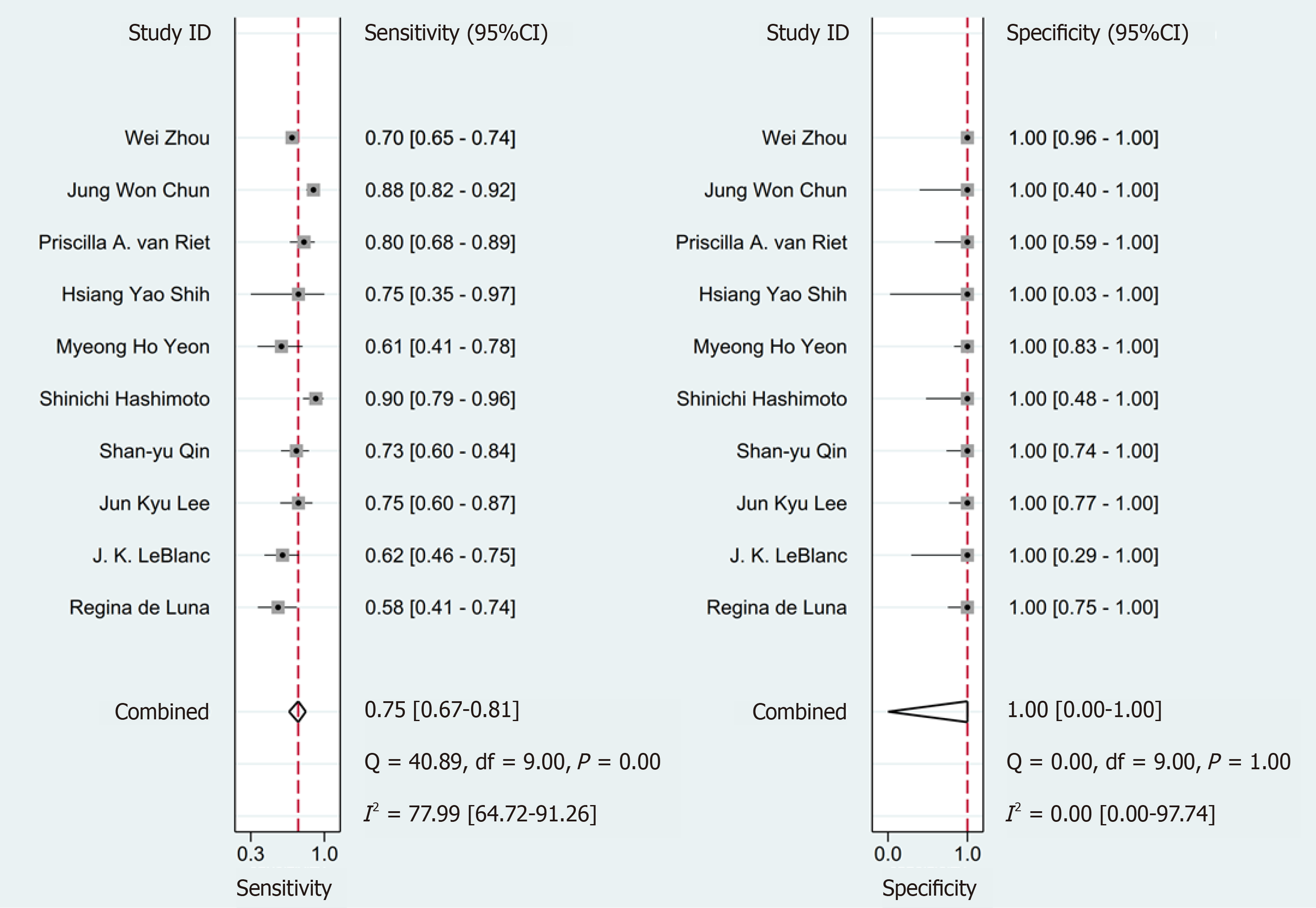
The pooled sensitivity of SC was superior compared with LBC at 78% [95% confidence interval (CI): 67%-87%] vs 75% (95%CI: 67%-81%), respectively. No difference in the pooled specificity of SC compared with LBC was noted [100% (95%CI: 1%-10%) and 100% (95%CI: 0%-100%), respectively] (Figures 2 and 3).
The SROC provides a global summary of the diagnostic accuracy of the different studies. The AUC for SC was 0.97 (95%CI: 0.95-0.98) and that for LBC was 1.00 (95%CI: 0.99-1.00). Both SC and LBC exhibited excellent test performance (Supplementary Figure 3). The accuracy of SC and LBC was similar in identifying patients with pancreatic lesions in EUS-FNA.
The pooled of diagnosis sensitivity presented substantial heterogeneity (SC: I² = 95.14 (93.24, 97.05); LBC: I² = 77.99 (64.72, 91.26)). To identify the source of heterogeneity, we performed meta-regression and subgroup analyses (Tables 2 and 3).
| Parameter | Category | Studies, n | Sensitivity | P value |
| Type | Prospective | 7 | 0.83 (0.74-0.92) | 0.71 |
| Retrospective | 3 | 0.65 (0.47-0.84) | ||
| Subject | ≥ 50 | 8 | 0.78 (0.68-0.89) | 0.67 |
| < 50 | 2 | 0.80 (0.56-1.00) | ||
| Match | Homology | 9 | 0.80 (0.70-0.89) | 0.48 |
| Heterology | 1 | 0.64 (0.27-1.00) | ||
| ROSE | Without ROSE | 8 | 0.74 (0.64-0.85) | 0.03 |
| With ROSE | 2 | 0.90 (0.80-1.00) | ||
| LBC | TP | 5 | 0.83 (0.72-0.94) | 0.87 |
| SP/CP | 5 | 0.73 (0.58-0.88) | ||
| Classification | Common | 5 | 0.86 (0.77-0.95) | 0.94 |
| Other1 | 5 | 0.71 (0.58-0.84) |
| Parameter | Category | Studies, n | Sensitivity | P value |
| Type | Prospective | 7 | 0.75 (0.67-0.84) | 0.12 |
| Retrospective | 3 | 0.75 (0.63-0.87) | ||
| Subject | ≥ 50 | 8 | 0.77 (0.70-0.83) | 0.85 |
| < 50 | 2 | 0.68 (0.46-0.89) | ||
| Match | Homology | 9 | 0.74 (0.67-0.80) | 0.01 |
| Heterology | 1 | 0.90 (0.80-1.00) | ||
| ROSE | Without ROSE | 8 | 0.79 (0.73-0.84) | 0.80 |
| With ROSE | 2 | 0.60 (0.44-0.76) | ||
| LBC | TP | 5 | 0.70 (0.61-0.80) | 0.00 |
| SP/CP | 5 | 0.80 (0.73-0.88) | ||
| Classification | Common | 5 | 0.74 (0.64-0.85) | 0.05 |
| Others1 | 5 | 0.76 (0.67-0.85) |
This review included two studies with ROSE[18,19]. Meta-regression analysis showed that the diagnostic sensitivity of the SC groups with and without ROSE was 90% (95%CI: 80%-100%) and 74% (95%CI: 64%-85%) (P = 0.03), respectively, revealing a significant difference. No significant difference was noted in the LBC groups.
In nine institutions[5,8,12,13,18-21,23], SC and LBC were concurrently compared to allow a head-to-head comparison of the two techniques. However, Hashimoto et al[22] compared two separate cohorts of patients: Those who with SC between January 2009 to May 2012 and LBC between June 2012 to August 2014. In total, 112 and 153 individuals, respectively, were included. The individuals were matched in preference, and 63 individuals were finally included in each group. The meta-regression analysis showed that the diagnostic sensitivity of the LBC groups with homology and heterology matching was 74% (95%CI: 67%-80%) and 70% (95%CI: 80%-100%) (P = 0.01), respectively, revealing a significant difference.
Among the 10 studies, five studies used TP technology, three studies used Cell Prep Plus (CP) technology, and one used Sure Path (SP) technology. The technology used in one was not mentioned. The diagnostic accuracy of LBC groups were related to LBC techniques. The accuracy of the earliest TP was 70% (95%CI: 61%-80%), and that noted with the others was 80% (95%CI: 73%-88%) (P < 0.01).
A smaller cell population appeared more often in LBC, resulting in a large number of inadequate samples. Compared with SC, the mucin protein on LBC slides was significantly reduced[8]. However, LBC samples exhibited better visibility than SC. For instance, these samples were cleaner with less blood in the background and free of air artifacts and thicknesses irregularities that were commonly found in SC sampl
Four of the 10 studies described complications after EUS-FNA[8,13,19,20]. No procedure-related complications (pancreatitis, infection, bleeding, or other) were recorded in three of the four studies[13,19,20]. A total of 13 of 170 (7.6%) patients had post procedure complications. The details of the complications were described in one[8] of the studies, including four patients with abdominal pain, five with bleeding, three with fever, and one with perforation.
The best cytological method for EUS-FNA in pancreatic lesions is controversial. Our findings clearly describe the factors that influence the diagnostic accuracy of SC or LBC and provide a reasonable and comprehensive comparison. The pooled diagnostic sensitivity for SC and LBC is 78% (95%CI: 67%-87%) and 75% (95%CI: 67%-81%), respectively.
Meta-regression analysis results indicated that the diagnostic sensitivity of the SC groups with or without ROSE is 90% (95%CI: 80%-100%) and 74% (95%CI: 64%-85%), respectively (P = 0.03). The diagnostic sensitivity of LBC groups was mainly related to LBC techniques. The earliest TP exhibits 70% accuracy (95%CI: 61%-80%). For LBC techniques with CP/SP, the value is 80% (95%CI: 73%-88%) (P < 0.01). Moreover, LBC groups were categorized based on matching methods. Homology matching yields 74% (95%CI: 67%-80%) accuracy, whereas heterology matching yields 90% (95%CI: 80%-100%) (P = 0.01).
ROSE refers to a clinical practice method that aims to improve the efficiency of biopsy diagnosis through real-time cell morphological analysis of specimens during FNA operations, which is mainly done by cytopathologists. It is concluded that the existence of ROSE is of great significance to improve the diagnosis accuracy of SC, which was also confirmed in two meta-analyses[25,26]. This finding is associated with a significant reduction in the number of inadequate samples and fewer needle passes[20,27]. However, the need for more staff and material resources has limited the use of this technique in some institutions[6].
LBC is largely divided into filtration methods (Thin Prep, Cell Prep Plus, E-Prep) and precipitation methods (Sure Path, Liquid-PREP)[5]. LBC methods have a great influence on the diagnostic accuracy of LBC. TP is the earliest and most common technology. CP is an automated LBC method developed in Korea that utilizes a vacuum filtration system for cell filtration. Its usefulness has been reported particularly for obtaining exfoliative cells during cervicovaginal cytology, and this method has been applied to various cytological specimens, including body fluid, urine, and sputum, yielding favorable outcomes[5]. In addition, unsatisfactory rates have been reported to be reduced by the SP method compared with the TP method for cervicovaginal cytology specimens[28]; however, direct comparisons have not been performed for pancreatic specimens obtained by EUS-FNA[28-31]. Therefore, we believe that it makes more sense to evaluate the diagnostic performance of different LBC methods for pancreatic lesions.
It is possible that some advanced hospitals can combine SC with LBC to achieve complementary advantages to improve further the diagnostic accuracy. A recent retrospective study found that LBC combined with SC was superior to SC alone based on sensitivity, accuracy, and nucleopolyhedrovirus[22]; the combined sensitivity and accuracy of SC and LBC were greater than that of LBC alone in another two studies[12,32]. Yeon et al[5] reported that the biggest problem with LBC is the insufficient number of cells and inadequate samples. In addition, if the blood is heavily contaminated, the LBC may be a good complement to the stain.
Recently, Pan et al[33] published a similar study[33] that included eight studies and expounded that LBC may be more sensitive than SC in the cytological diagnosis of pancreatic lesions. However, the authors further emphasized the superior diagnostic performance of SC combined with LBC. On one hand, the study ignored the influence of ROSE in some studies. On the other hand, although they noted that the LBC methods were different, which may lead to bias, the meta-regression and subgroup analysis performed in our study revealed that the sensitivity of LBC could be improved by improving the LBC method. It may not be necessary to combine SC with LBC, which may increase the workload.
There are several potential limitations to this study. First, this finding should be interpreted with caution given the high heterogeneity observed. We used a random-effects model (regardless of the level of heterogeneity) as recommended by Cochrane guidelines. Meta-regression and subgroup analyses were performed. Moreover, sufficient interpretation of heterogeneity sources was explored, and this aspect represents a nearly unique analysis in this field. Second, one of the reasons for the difference in the accuracy of literature reports is that EUS-FNA operations are not completely unified. Thus, direct comparisons are limited. In addition to different LBC techniques and the lack of a unified cytological analysis classification method, wide variation in needle sizes was noted across different studies. However, we deliberately decided to restrict inclusion criteria to studies directly comparing samples by SB and LBC to provide more robust and homogenous outcome estimates. Third, the nature of the lesions was not involved given the lack of relevant data. A few studies included some cystic or cystic-solid lesions but most were solid lesions. The study of Hashimoto et al[22] reported that for pancreatic solid lesions, LBC is helpful in the diagnosis of pancreatic ductal adenocarcinoma and larger malignancies. The diagnostic sensitivity may be influenced by the type and size of pancreatic lesions, which still lacks relevant studies[22]. Therefore, our findings were specific for whole pancreatic lesions (particularly solid lesions). Fourth, in the subset analysis, only two studies included ROSE, which may result in a bias that SC with ROSE is better as confirmed in two other meta-analyses mentioned above.
Our study suggested that for EUS-FNA in pancreatic lesions (particularly solid lesions), SC with ROSE potentially represents a superior diagnostic technique. If ROSE is unavailable, LBC may replace smears, and the diagnostic accuracy of LBC depends on the different LBC techniques used.
Endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) is a safe and accurate technique to confirm the diagnosis of pancreatic cancers. The best cytological method for EUS-FNA in pancreatic lesions is controversial.
Recently, numerous studies comparing the diagnostic efficacy of smear cytology (SC) and liquid-based cytology (LBC) for pancreatic lesions yielded mixed results.
To compare and identify the better cytology method for EUS-FNA in pancreatic lesions.
A comprehensive search of PubMed, Embase, and Cochrane was undertaken through July 18, 2020.
Data on a total of 1121 comparisons from 10 studies met the inclusion criteria. Pooled rates of sensitivity for SC and LBC were 78% (67%-87%) vs 75% (67%-81%), respectively. In any case, both SC and LBC exhibited a high specificity close to 100%. Inadequate samples more often appeared in LBC compared with SC. However, the LBC samples exhibited a better visual field than SC. Very few post procedure complications were observed.
For EUS-FNA in pancreatic lesions (particularly solid lesions), SC with Rapid On-Site Evaluation (ROSE) represents a superior diagnostic technique. If ROSE is unavailable, LBC may replace smears. The diagnostic accuracy of LBC depends on different LBC techniques.
The need for more staff and material resources has limited the use of ROSE in some institutions. It makes sense to evaluate the diagnostic performance of different LBC methods for EUS-FNA in pancreatic lesions.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kai K S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15468] [Article Influence: 2578.0] [Reference Citation Analysis (2)] |
| 2. | Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Maréchal R, Van Laethem JL, Ducreux M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer. 2016;57:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Hasan MK, Hawes RH. EUS-guided FNA of solid pancreas tumors. Gastrointest Endosc Clin N Am. 2012;22:155-167, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Yeon MH, Jeong HS, Lee HS, Jang JS, Lee S, Yoon SM, Chae HB, Park SM, Youn SJ, Han JH, Han HS, Lee HC. Comparison of liquid-based cytology (CellPrepPlus) and conventional smears in pancreaticobiliary disease. Korean J Intern Med. 2018;33:883-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | van Riet PA, Cahen DL, Poley JW, Bruno MJ. Mapping international practice patterns in EUS-guided tissue sampling: outcome of a global survey. Endosc Int Open. 2016;4:E360-E370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Yamabe A, Irisawa A, Bhutani MS, Shibukawa G, Fujisawa M, Sato A, Yoshida Y, Arakawa N, Ikeda T, Igarashi R, Maki T, Yamamoto S. Efforts to improve the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic tumors. Endosc Ultrasound. 2016;5:225-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Chun JW, Lee K, Lee SH, Kim H, You MS, Hwang YJ, Paik WH, Ryu JK, Kim YT. Comparison of liquid-based cytology with conventional smear cytology for EUS-guided FNA of solid pancreatic masses: a prospective randomized noninferiority study. Gastrointest Endosc 2020; 91: 837-846. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Zeppa P. Liquid-based cytology: a 25-year bridge between the pap smear and molecular cytopathology. Acta Cytol. 2014;58:519-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Nagarajan N, Schneider EB, Ali SZ, Zeiger MA, Olson MT. How do liquid-based preparations of thyroid fine-needle aspiration compare with conventional smears? Thyroid. 2015;25:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Park GS, Lee SH, Jung SL, Jung CK. Liquid-based cytology in the fine needle aspiration of parathyroid lesions: a comparison study with the conventional smear, ThinPrep, and SurePath. Int J Clin Exp Pathol. 2015;8:12160-12168. [PubMed] |
| 12. | Zhou W, Gao L, Wang SM, Li F, Li J, Li SY, Wang P, Jia FZ, Xu JJ, Zhou CH, Zou DW, Jin ZD, Wang KX. Comparison of smear cytology and liquid-based cytology in EUS-guided FNA of pancreatic lesions: experience from a large tertiary center. Gastrointest Endosc. 2020;91:932-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | van Riet PA, Quispel R, Cahen DL, Snijders-Kruisbergen MC, van Loenen P, Erler NS, Poley JW, van Driel LMJW, Mulder SA, Veldt BJ, Leeuwenburgh I, Anten MGF, Honkoop P, Thijssen AY, Hol L, Hadithi M, Fitzpatrick CE, Schot I, Bergmann JF, Bhalla A, Bruno MJ, Biermann K. Diagnostic yield and agreement on fine-needle specimens from solid pancreatic lesions : comparing the smear technique to liquid-based cytology. Endosc Int Open. 2020;8:E155-E162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17508] [Article Influence: 1094.3] [Reference Citation Analysis (1)] |
| 15. | Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.0.0. The Cochrane Collaboration, 2008. [cited 15 January 2021]. Available from: www.cochrane-handbook.org. |
| 16. | Deeks JJ, Davenport C. Chapter 4: Guide to the contents of a Cochrane Diagnostic Test Accuracy Protocol. In: Deeks JJ, Bossuyt PM, Gatsonis C. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration; 2013. |
| 17. | Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 1994] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 18. | de Luna R, Eloubeidi MA, Sheffield MV, Eltoum I, Jhala N, Jhala D, Chen VK, Chhieng DC. Comparison of ThinPrep and conventional preparations in pancreatic fine-needle aspiration biopsy. Diagn Cytopathol. 2004;30:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | LeBlanc JK, Emerson RE, Dewitt J, Symms M, Cramer HM, McHenry L, Wade CL, Wang X, Musto P, Eichelberger L, Al-Haddad M, Johnson C, Sherman S. A prospective study comparing rapid assessment of smears and ThinPrep for endoscopic ultrasound-guided fine-needle aspirates. Endoscopy. 2010;42:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Lee JK, Choi ER, Jang TH, Chung YH, Jang KT, Park SM, Lee JK, Lee KT, Lee KH. A prospective comparison of liquid-based cytology and traditional smear cytology in pancreatic endoscopic ultrasound-guided fine needle aspiration. Acta Cytol. 2011;55:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Qin SY, Zhou Y, Li P, Jiang HX. Diagnostic efficacy of cell block immunohistochemistry, smear cytology, and liquid-based cytology in endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: a single-institution experience. PLoS One. 2014;9:e108762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Hashimoto S, Taguchi H, Higashi M, Hatanaka K, Fujita T, Iwaya H, Nakazawa J, Arima S, Iwashita Y, Sasaki F, Nasu Y, Kanmura S, Ido A. Diagnostic efficacy of liquid-based cytology for solid pancreatic lesion samples obtained with endoscopic ultrasound-guided fine-needle aspiration: Propensity score-matched analysis. Dig Endosc. 2017;29:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Shih HY, Wu CC. Liquid-based cytology superior to smear cytology of endoscopic ultrasonography-fine needle aspiration (EUS-FNA) for solid pancreatic and biliary tumor. Gastrointest Endosc. 2019;89:AB303. |
| 24. | Pitman MB, Centeno BA, Ali SZ, Genevay M, Stelow E, Mino-Kenudson M, Fernandez-del Castillo C, Max Schmidt C, Brugge W, Layfield L; Papanicolaou Society of Cytopathology. Standardized terminology and nomenclature for pancreatobiliary cytology: the Papanicolaou Society of Cytopathology guidelines. Diagn Cytopathol. 2014;42:338-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Chen J, Yang R, Lu Y, Xia Y, Zhou H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: a systematic review. J Cancer Res Clin Oncol. 2012;138:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, Eltoum IA. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 27. | Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, Forteza-Vila J. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | Fontaine D, Narine N, Naugler C. Unsatisfactory rates vary between cervical cytology samples prepared using ThinPrep and SurePath platforms: a review and meta-analysis. BMJ Open. 2012;2:e000847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Bigras G, Rieder MA, Lambercy JM, Kunz B, Chatelain JP, Reymond O, Cornaz D. Keeping collecting device in liquid medium is mandatory to ensure optimized liquid-based cervical cytologic sampling. J Low Genit Tract Dis. 2003;7:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Rozemeijer K, Penning C, Siebers AG, Naber SK, Matthijsse SM, van Ballegooijen M, van Kemenade FJ, de Kok IM. Comparing SurePath, ThinPrep, and conventional cytology as primary test method: SurePath is associated with increased CIN II+ detection rates. Cancer Causes Control. 2016;27:15-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Naeem RC, Goldstein DY, Einstein MH, Ramos Rivera G, Schlesinger K, Khader SN, Suhrland M, Fox AS. SurePath Specimens Versus ThinPrep Specimen Types on the COBAS 4800 Platform: High-Risk HPV Status and Cytology Correlation in an Ethnically Diverse Bronx Population. Lab Med. 2017;48:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Itonaga M, Murata SI, Hatamaru K, Tamura T, Nuta J, Kawaji Y, Maekita T, Iguchi M, Kato J, Kojima F, Yamaue H, Kawai M, Okada KI, Hirono S, Shimokawa T, Tanioka K, Kitano M. Diagnostic efficacy of smear plus liquid-based cytology for EUS-FNA of solid pancreatic lesions: A propensity-matched study. Medicine (Baltimore). 2019;98:e15575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Pan HH, Zhou XX, Zhao F, Chen HY, Zhang Y. Diagnostic value of liquid-based cytology and smear cytology in pancreatic endoscopic ultrasound-guided fine needle aspiration: A meta-analysis. World J Clin Cases. 2020;8:3006-3020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |