Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2890
Peer-review started: December 5, 2020
First decision: January 7, 2021
Revised: January 18, 2021
Accepted: March 3, 2021
Article in press: March 3, 2021
Published online: April 26, 2021
Processing time: 130 Days and 12.3 Hours
Convalescent plasma therapy is used for the treatment of critically ill patients for newly discovered infectious diseases, such as coronavirus disease 2019 (COVID-19) pneumonia, under the premise of lacking specific treatment drugs and corresponding vaccines. But the best timing application of plasma therapy and whether it is effective by antiviral and antibiotic treatment remain unclear.
We describe a patient with COVID-19, a 100-year-old, high-risk, elderly male who had multiple underlying diseases such as stage 2 hypertension (very high-risk group) and infectious pneumonia accompanied by chronic obstructive pulmonary disease and emphysema. We mainly describe the diagnosis, clinical process, and treatment of the patient, including the processes of two plasma transfusion treatments.
This provides a reference for choosing the best timing of convalescent plasma treatment and highlights the effectiveness of the clinical strategy of plasma treatment in the recovery period of patients with COVID-19 pneumonia.
Core Tip: A 100-year-old coronavirus disease 2019 (COVID-19) patient with several underlying diseases including hypertension (stage 2 hypertension) accompanied by chronic obstructive pulmonary disease and emphysema was treated using convalescent plasma. Besides the effective maintenance of supportive treatment and no antiviral and antibiotic treatment, the convalescent plasma was infused into the patient in the early stage of the infection. This choice of time points (days 7 and 11 of hospitalization) provides a reference for choosing the best timing of convalescent plasma treatment and highlights the effectiveness of the clinical strategy of plasma treatment in the recovery period of patients with COVID-19 pneumonia.
- Citation: Liu B, Ren KK, Wang N, Xu XP, Wu J. Timing of convalescent plasma therapy-tips from curing a 100-year-old COVID-19 patient using convalescent plasma treatment: A case report . World J Clin Cases 2021; 9(12): 2890-2898
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2890.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2890
The outbreak of coronavirus disease 2019 (COVID-19) pneumonia in Wuhan in 2019 has garnered intense attention. Most patients with this type of pneumonia have a good prognosis. A small percentage of patients fall critically ill, and critical illness and death occur mostly in the elderly. Therefore, elderly people are the high-risk group of COVID-19 pneumonia patients. Understanding the medical treatment of elderly patients with severe COVID-19 pneumonia can greatly reduce the mortality from novel coronavirus infection. According to a press release issued by the National Health Commission of the People's Republic of China on February 4, 2020, an analysis of current death cases found that most deaths (two-thirds) are in men and in senior citizens (> 80% are over 60 years old). Over 75% of patients who die from it have more than one underlying disease, mainly including cardiovascular and cerebrovascular diseases, diabetes, and cancer. Once infected with pneumonia, elderly people with underlying diseases are considered high-risk in clinical practice, and their mortality rate is very high[1]. Although numerous trials have reported the timing of convalescent plasma therapy[2,3], to date, no trials have been conducted on extremely old persons or newly infected persons with multiple underlying diseases.
Here, we present a case of a 100-year-old (super elderly) patient with multiple underlying diseases (i.e. high-risk patient) who was cured of a novel coronavirus infection. We mainly describe the diagnosis, clinical process, and treatment of this case, including a description of two plasma transfusion treatments. The cure of this case provides a reference for formulating the treatment plans and the best timing of convalescent plasma treatment for elderly patients with various underlying diseases. This report was approved by Ethics Committee of Guanggu Branch of Hubei Maternal and Child Health Hospital.
The patient had a history of exposure and had repeated coughing and shortness of breath for 2 mo. He tested positive for the novel coronavirus using a nucleic acid test in Wuhan Community Health Center on February 22, 2020.
The patient had repeated coughing and shortness of breath for 2 mo.
The patient had a history of underlying diseases including hypertension for approximately 30 years, dementia, abdominal aortic aneurysm, cerebral infarction, and prostate hyperplasia.
The patient had lived in Wuhan, China for a long time, and had contact with COVID-19-confirmed patients (not confirmed at that time).
Physical examination of the patient upon admission showed stable vital signs, with a body temperature of 36.6 °C, a pulse of 87 beats/min (regular), a respiratory rate of 18 breaths/min (regular), and blood pressure of 125/63 mmHg.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test results in patient at different time points (on hospitalization days 5, 9, 10, 12 and 13) are shown in Table 1. The results of blood biochemistry (hepatic function, renal function, electrolytes, lipid, and glucose), blood cell analysis, and coagulation function are shown in Table 1.
| Hospital day | Reference range | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 |
| Feb 25, 2020 | Feb 26, 2020 | Feb 27, 2020 | Feb 28, 2020 | Feb 29, 2020 | Mar 1, 2020 | Mar 2, 2020 | Mar 3, 2020 | Mar 4, 2020 | Mar 5, 2020 | Mar 6, 2020 | Mar 7, 2020 | ||
| Nucleic acid testing | P | P | P | N | N | ||||||||
| Antibody | IgG+ | IgG+ | Detection IgM+ | - | IgM+ | ||||||||
| IL6 in pg/mL | 0-10 | - | 24.63↑ | 66.21↑ | 21.47↑ | - | |||||||
| BNP in pg/mL | 0-100 | 78.8 | 135.4↑ | 242.1↑ | |||||||||
| Albumin in g/L | 35-52 | 29.4 | 29.1 | 33.4 | 35 | 31.6 | 34.7 | 35.7 | |||||
| Blood clean | 1-2.4 | 0.95↓ | 0.8↓ | 0.95 | 0.91 | 0.93 | 1.05 | 1.08 | |||||
| Globulin, A/GGlutamic pyruvic | 0-55 | 49.3 | 34.2 | 17.4 | 20.7 | 16.4 | 13.7 | 17.3 | |||||
| Transaminase, ALT in U/L, GGT in U/L | 12-64 | 96↑ | 75↑ | 57 | 52 | 42 | 35 | 34 | |||||
| TBA in μmol/L | 0-9.67 | 4 | 2.5 | 19.3 | 14.5 | 29.7 | 42.8 | 11.2 | |||||
| CRE in μmol/L | 64-104 | 73.1 | 62↓ | 50.8↓ | 55.1↓ | 53.2↓ | 42.6↓ | 48.4 | |||||
| UA in μmol/L | 210-420 | 293 | 346 | 246 | 256 | 241 | 118↓ | 116 | |||||
| hs-cTn in pg/mL | 0-34.2 | 19 | |||||||||||
| MYO in ng/mL | 0-106 | 498.7↑ | |||||||||||
| CK-MB in ng/mL | 0-3.1 | 11.1 ↑ | |||||||||||
| D-Dimer in mg/L | 0-0.55 | 3.17 | 4.38 | ||||||||||
| LYM, % | 20-50 | 12.1↓ | 14.9↓ | 12.8↓ | 14.4 | ||||||||
| LYM, × 109/L | 1.1-3.2 | 0.63↓ | 0.69↓ | 0.84↓ | 1.08 | ||||||||
| RBC, × 1012/L | 4.3-5.8 | 3.29↓ | 3.35↓ | 3.27↓ | 3.22 | ||||||||
| HGB in g/L | 130-175 | 100↓ | 100↓ | 97↓ | 95 | ||||||||
| Red blood cell specific volume, HCT, % | 40-50 | 29.3↓ | 29.7↓ | 29.3↓ | 28.7 | ||||||||
| hs-CRP in mg/L | 0-10 | 108.43↑ | 49.18↑ | 27.03 | 24.97 | ||||||||
| tHb in g/L | 117-174 | 70↓ | 94↓ | 115 | |||||||||
| Carbon monoxide | 0.5-1.5 | 2.2↑ | 1.3 | 1.5 | |||||||||
| COHb, % un-ionized HHb, % | 0--5 | -0.4 | 0.6 | 0.2 | |||||||||
| Glucose in mmol/L | 3.6-5.2 | 10.9↑ | 6.2↑ | 8.1 | |||||||||
| Base excess of blood in mmol/L | -2-3 | -5.1↓ | 4.6↑ | 4.1 | |||||||||
| Beecf in mmol/L | -2-3 | -5.6↓ | 5↑ | 4.7 |
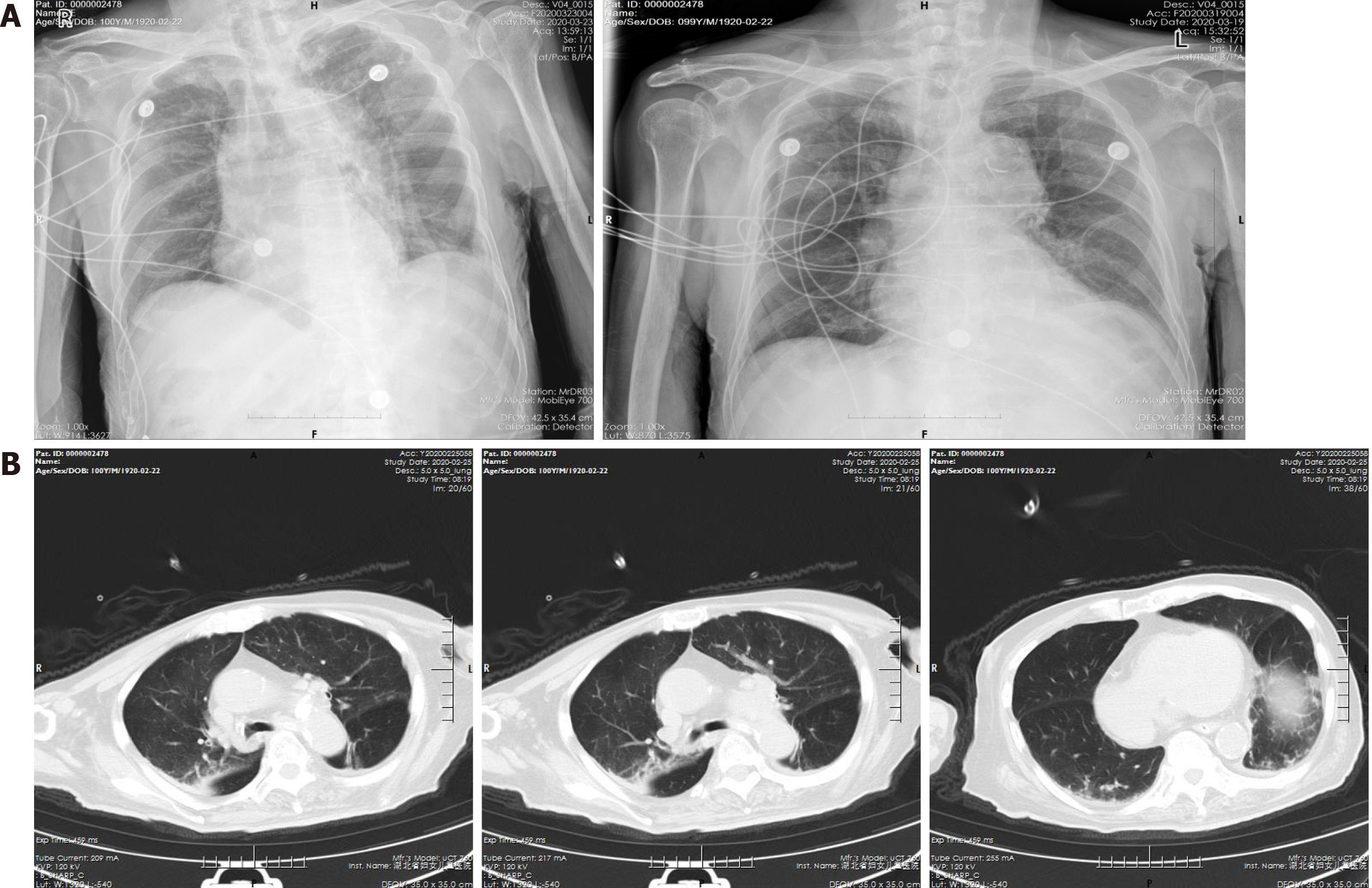
On the second day after admission (February 25, 2020), a chest computed tomography (CT) scan revealed small amounts of abnormally dense shadows in both lungs, suggesting infectious pneumonia accompanied by chronic obstructive pulmonary disease and emphysema (Figure 1).
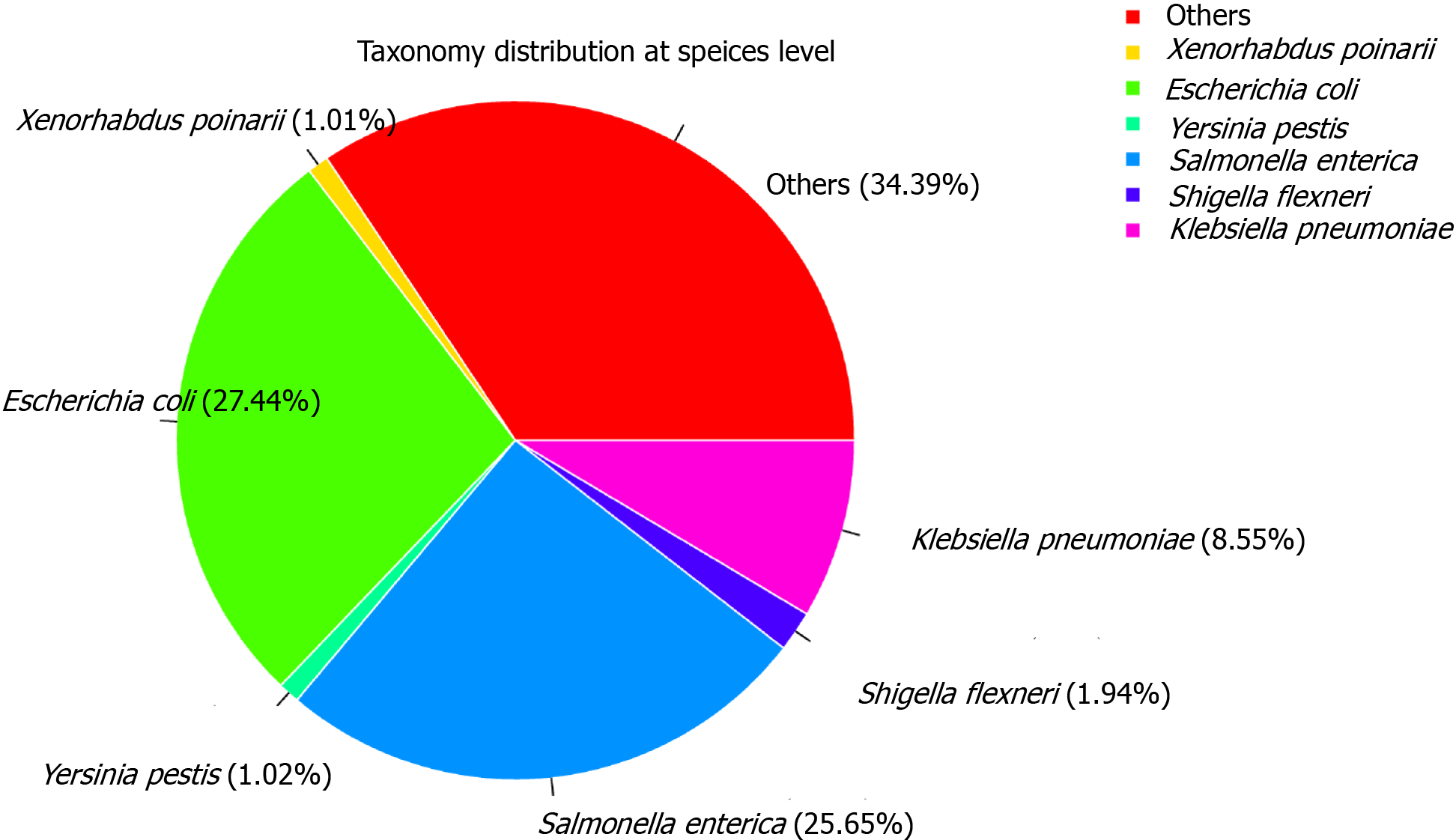
We sequenced the metatranscriptome of the anal swab from the patient on the Illumina NextSeq 500 PE150 platform, producing over 18 Million Pair-End reads (Supplementary Table 1). The sample contained approximately 3.21% human reads. For taxonomic classification, filtered reads were searched against the NCBI Ref-Seq database. This classified 99.24% of the filtered reads to the bacteria domain and 0.05% to the viral domain (Supplementary Table 2 and Figure2). No SARS-CoV-2 reads were detected. This result was consistent with the clinical outcome that the patient had recovered completely from SARS-CoV-2 infection. In addition, the anal swab sample exhibited a high abundance of reads of the putative bacterial pathogens, 27.44% classified to Escherichia coli, 25.65% Salmonella enterica, and 8.55% Klebsiella pneumoniae (Figure 2, Supplementary Figure 1 and Supplementary Table 3).
The patient was confirmed with COVID-19 by positive SARS-CoV-2 oropharyngeal swab test and suggested infectious pneumonia accompanied by chronic obstructive pulmonary disease and emphysema, and a history of underlying diseases.
The treatment plan adopted was supportive treatment with oxygen inhalation and frequent, low-volume nasal-feeding nutritional support with a daily maintenance of 1000 mL of Fresubin Diabetes (Enteral Nutritional Emulsion [Tpf-D]). Because lung changes due to COVID-19 pneumonia were mild and the patient was very old, no antiviral treatment was given at first. Intravenous supplementation with hypertonic sodium chloride was given to correct his hyponatremia, and intravenous infusion of human albumin was given to correct the hypoproteinemia. The antihypertensive drugs that the patient routinely took (telmisartan tablets and nifedipine sustained release tablets) kept stabilizing blood pressure.
On day 5 of hospitalization, the patient tested positive for SARS-CoV-2 nucleic acid. The patient showed repeated coughing and shortness of breath. An urgent blood test showed hemoglobin at 100 g/L and absolute lymphocyte value at 0.63 × 109/L, suggesting that the patient had anemia and low immunity. CT showed infectious pneumonia with chronic obstructive pulmonary disease and emphysema. The above clinical indications together with a confirmed diagnosis of COVID-19 pneumonia and the patient’s age qualified the patient for infusion of convalescent plasma for COVID-19 patients. On day 7 of hospitalization (March 1, 2020), plasma transfusion therapy using convalescent plasma of an infected patient was started. Intravenous infusion started at 21:45, and 200 mL plasma was infused at 01:30. Throughout the infusion process, the patient had no special discomfort and no adverse reactions to blood transfusion such as fever, chills, rash, or allergies. The patient’s vital signs were stable, and the blood oxygen saturation was stabilized at above 95% under low flow oxygen inhalation. The patient continued to receive nutritional support treatment.
On day 11 of hospitalization (March 5, 2020), we conducted the second round of convalescent plasma treatment for SARS-CoV-2 infection using 200 mL plasma, which was infused first slowly and then quickly and ended at 20:40. There was no transfusion reaction during plasma infusion. The day after the blood transfusion, the blood routine test was conducted to evaluate the effects of plasma therapy. The laboratory test results showed that the red blood cell count and hemoglobin had improved and remained stable. This confirmed that the blood transfusion was effective. At the same time, the patient's B-type natriuretic peptide was higher than before, so hydrochlorothiazide tablet was added for diuretic supportive treatment.
On day 11 (March 5, 2020) after admission, a throat swab was taken again, and the novel coronavirus nucleic acid test was negative. The SARS-CoV-2 antibody test showed positive for the novel coronavirus immunoglobulin (Ig) G and IgM. On day 12 after admission, the results of physical examination, blood routine, and biochemical tests indicated that the vital signs were stable, the novel coronavirus nucleic acid test was again negative, and the patient's condition had improved. On day 13 after admission, a nasopharyngeal swab sample was collected, and the patient was subjected to metagenomic sequencing using next-generation sequencing. The SARS-CoV-2 reads were not detected in the sequencing results, which was consistent with the nucleic acid test results.
Elderly people with underlying diseases, when they are infected with COVID-19 pneumonia, belong to the high-risk group in clinic practice, and their mortality rate is very high, especially patients over 60 years of age with hypertension, heart disease, diabetes, or cancer. In addition, the probability of becoming severely ill or critically ill is higher[4-7]. The treatment of severely ill and critically ill patients has put much pressure on Chinese medical teams at this time. When China’s epidemic situation was at its peak, there were nearly 10000 severely ill and critically ill patients in Wuhan with critically ill patients accounting for approximately 5%. The material and manpower requirements were huge. Faced with this situation, we had to treat the patients scientifically and effectively, reduce the number of patients becoming critically ill, and ultimately reduce the mortality.
For the treatment of COVID-19 pneumonia, there are no particularly effective anti-disease drugs. In this case, treatment mainly includes strengthening nutritional therapy, applying oxygen therapy, such as oxygen masks, establishing early warnings for the functions of important organs, and administering intravenous immunoglobulin infusion or convalescent plasma infusion at early stages according to the disease condition. Convalescent plasma is a plasma product collected from patients who have been infected with the virus but have recovered after treatment, and its active ingredient is immunoglobulin. Convalescent plasma usually contains high-potency pathogen-specific antibodies. The collected plasma undergoes virus inactivation and is prepared after neutralizing antibody test and multiple pathogenic microorganism detection. It is used for the treatment of critically ill patients with infectious diseases. Compared with intravenous injection of human immunoglobulin, convalescent plasma contains more specific immunoglobulins for pathogenic microorganisms. Convalescent plasma from recovered patients contains more specific immunoglobulin than the ordinary human immunoglobulin for intravenous injection. For newly discovered infectious diseases, such as COVID-19 pneumonia, under the premise of lacking specific treatment drugs and corresponding vaccines, infusion of special plasma products collected from patients who have recovered from COVID-19 pneumonia can provide instant, massive, and passive immune support and eliminate pathogens. This greatly reduces the mortality of critically ill patients[8]. Convalescent plasma therapy has also been used in the past for the treatment of SARS, 2009 influenza A, avian influenza A, Ebola hemorrhagic fever, and other viral infections[9-11]. Therefore, convalescent plasma therapy has practical significance for the treatment of COVID-19 pneumonia in the absence of specific drugs and vaccines.
Recently, researchers at the Third People’s Hospital of Shenzhen, China, treated five critically ill patients with convalescent plasma. All five of them had severe respiratory failure and were on a ventilator; two were accompanied by bacterial and/or fungal pneumonia; and one was under extracorporeal membrane lung oxygenation treatment. The four patients with no comorbid disease received plasma therapy on day 20 of hospitalization, and one patient with hypertension and mitral valve insufficiency received plasma therapy on day 10 of hospitalization. These patients showed improvement approximately 1 wk after infusion of plasma. At the same time, these patients were also receiving antiviral drugs, including lopinavir/ritonavir and interferon[12,13]. The study was not a randomized clinical study, and there was no control group that did not receive plasma therapy. The patients also received many other treatments, such as hormones and antibiotics. Therefore, it is difficult to determine the exact role of plasma therapy, the best timing for plasma therapy, and whether the earlier application of plasma therapy can lead to different clinical results. By contrast, the treatment plan for our patient at the early stage after the infection was detected was the effective maintenance of nutritional and supportive treatment and did not include antiviral and antibiotic treatment, while convalescent plasma was infused on day 7 after admission. Although the novel coronavirus nucleic acid test was still positive on the second day after infusion, the cycle threshold values were significantly higher than those on day 5 of hospitalization. There were no adverse reactions after blood transfusion, and the vital signs were relatively stable. On day 11 of hospitalization (the third day after the first blood transfusion), the patient received the second round of convalescent plasma therapy, and there was no transfusion reaction during the blood transfusion. The red blood cell count and hemoglobin both improved and remained stable. Afterward, the results of the novel coronavirus nucleic acid test were negative for two consecutive days.
According to the clinical classification[14], although the clinical symptoms of this patient were moderate, clinical indications such as his high age and multiple high-risk factors, suggested a risk of critical illness. The treatment plan we adopted did not include antiviral treatment but used nutritional and supportive treatment to alleviate the patient's symptoms, and we promptly carried out the treatment plan of convalescent plasma transfusion. The patient’s immunity was significantly improved, and the patient became negative for the virus within 6 d and did not become severely ill.
Our experience treating this patient suggests that for the elderly and patients with underlying conditions, administering immune-enhancing treatment such as convalescent plasma transfusion while maintaining supportive treatment at the early phase after the beginning of symptoms can effectively reduce the number of patients who progress into critically illness. The success in plasma transfusion in this case also provides a reference for the selection of the optimal time point for plasma treatment. The successful treatment of this case also gave us increased confidence in curing elderly patients.
Most patients with COVID-19 have a good prognosis, a small percentage of the patients are critically ill, and critical illness and death occur mostly in the elderly. The elderly is the high-risk group of COVID-19 pneumonia patients, so improving the medical treatment of elderly patients with severe COVID-19 pneumonia is of great significance. Our experience suggests that for patients with high-risk factors for COVID-19 pneumonia, such as old age and underlying diseases, improving the patients’ immunity while maintaining supportive treatment in the early stages after they show symptoms can effectively reduce the number of patients who became critically ill. In addition, this patient was treated with convalescent plasma on days 7 and 11 of hospitalization and was successfully cured on day 12. This choice of time points provides a reference for choosing the best timing of convalescent plasma treatment and highlights the effectiveness of the clinical strategy of plasma treatment in the recovery period of patients with COVID-19 pneumonia. The results from this case report are encouraging, yet long-term data from more elderly COVID-19 patients with underlying diseases will be needed to draw definite conclusions and bring it into mainstream clinical practice.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciotti M, Frater JL S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LL
| 1. | CCTV. National Health Commission: men account for two-thirds of all deaths from pneumonia, and 80 percent of them are over the age of 60. 2020 [cited December 10, 2020]. Available from: http://m.news.cctv.com/2020/02/04/ARTICyA7Lj6qtB8hg78M7ayK200204.shtml. |
| 2. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12964] [Article Influence: 2592.8] [Reference Citation Analysis (1)] |
| 3. | Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P; PLACID Trial Collaborators. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 467] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 4. | Joob B, Wiwanitkit V. Convalescent plasma and covid-19 treatment. Minerva Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30069] [Article Influence: 6013.8] [Reference Citation Analysis (3)] |
| 6. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14751] [Article Influence: 2950.2] [Reference Citation Analysis (0)] |
| 7. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18848] [Article Influence: 3769.6] [Reference Citation Analysis (7)] |
| 8. | National Health Commission of the People's Republic of China. Convalescent plasma clinical therapy for COVID-19 patients (trial version 2): National Health Commission of the People's Republic of China, 2020. [cited December 10, 2020]. Available from: http://www.nhc.gov.cn/yzygj/s7658/202003/61d608a7e8bf49fca418a6074c2bf5a2.shtml. |
| 9. | Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 693] [Cited by in RCA: 686] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 10. | Kraft CS, Hewlett AL, Koepsell S, Winkler AM, Kratochvil CJ, Larson L, Varkey JB, Mehta AK, Lyon GM 3rd, Friedman-Moraco RJ, Marconi VC, Hill CE, Sullivan JN, Johnson DW, Lisco SJ, Mulligan MJ, Uyeki TM, McElroy AK, Sealy T, Campbell S, Spiropoulou C, Ströher U, Crozier I, Sacra R, Connor MJ Jr, Sueblinvong V, Franch HA, Smith PW, Ribner BS; Nebraska Biocontainment Unit and the Emory Serious Communicable Diseases Unit. The Use of TKM-100802 and Convalescent Plasma in 2 Patients With Ebola Virus Disease in the United States. Clin Infect Dis. 2015;61:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Dean CL, Hooper JW, Dye JM, Zak SE, Koepsell SA, Corash L, Benjamin RJ, Kwilas S, Bonds S, Winkler AM, Kraft CS. Characterization of Ebola convalescent plasma donor immune response and psoralen treated plasma in the United States. Transfusion. 2020;60:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323:1582-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1590] [Article Influence: 318.0] [Reference Citation Analysis (0)] |
| 13. | Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 652] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 14. | National Health Commission of the People's Republic of China. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7): National Health Commission of the People's Republic of China, 2020. [cited December10, 2020]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. |