Published online Apr 26, 2020. doi: 10.12998/wjcc.v8.i8.1507
Peer-review started: January 18, 2020
First decision: February 26, 2020
Revised: April 3, 2020
Accepted: April 8, 2020
Article in press: April 8, 2020
Published online: April 26, 2020
Processing time: 97 Days and 3.9 Hours
We describe the case of a 74-year-old man diagnosed with primary cutaneous mantle cell lymphoma (MCL), an extremely rare and controversial condition that is not included in the World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.
The patient presented diffuse cutaneous erythematous plaques and nodules throughout the body. Skin lesions were biopsied and histopathological examination showed diffuse monomorphic lymphocyte infiltration in the dermal and subcutaneous layers, sparing the epidermis. Immunohistochemical staining revealed CD20, cyclin-D1, CD5, and SOX-11 expression. Fluorescence in situ hybridization showed CCND1/IGH gene rearrangement. Correct diagnosis of primary cutaneous MCL requires ensuring that no other parts are involved; these cases require close follow-up to monitor their possible progression to systemic disease and for treating relapsed cutaneous disease. In this case, positron emission tomography scanning and clinical staging revealed no systemic involvement, and follow-up examination at 20 mo after diagnosis showed no evidence of systemic disease. The prognosis of primary cutaneous MCL is relatively good. Our patient received six cycles of chemotherapy, and the cutaneous manifestations presented almost complete remission.
Primary cutaneous MCL is rare, and its prognosis is relatively favorable. However, correct diagnosis is a prerequisite for proper treatment.
Core tip: Primary cutaneous mantle cell lymphoma (MCL) is rare and has been the subject of isolated case reports. To correctly diagnose primary cutaneous MCL, it must be ensured that no other parts are involved, and these cases require close follow-up to monitor the possible progression to systemic disease and treat relapsed cutaneous disease. Correct diagnosis is crucial for clinical treatment and prognosis. The prognosis of primary cutaneous MCL is relatively favorable, and treatment options should comply with the clinical condition of the patient.
- Citation: Zheng XD, Zhang YL, Xie JL, Zhou XG. Primary cutaneous mantle cell lymphoma: Report of a rare case. World J Clin Cases 2020; 8(8): 1507-1514
- URL: https://www.wjgnet.com/2307-8960/full/v8/i8/1507.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i8.1507
Mantle cell lymphoma (MCL) is a mature B-cell neoplasm characterized by uncontrolled proliferation of small to medium-sized lymphoid cells within the mantle zone[1]. More than 95% of cases of MCL show the CCND1 chromosomal translocation t(11;14)(q13;q32), which triggers cyclin D1 overexpression[2,3]. Lymph nodes are the most frequently involved sites. The spleen and bone marrow, with or without peripheral blood involvement, are also important sites of the disease. Other frequently involved extra-nodal sites include the gastrointestinal tract and Waldeyer’s ring. However, skin involvement in MCL is rare, and most cases occur due to secondary cutaneous dissemination by widespread disease[4-8]. Primary cutaneous MCL is even rarer, and mostly isolated cases have been documented previously and the majority of such patients typically present with systemic lymphoma in later stages[4,9-13]. The World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EORTC) does not classify primary cutaneous MCL as a cutaneous lymphoma[14]. Here, we report a rare case of primary cutaneous MCL and describe its clinical, histopathological, immunohistochemical, and genetic features, as well as its treatment and prognosis.
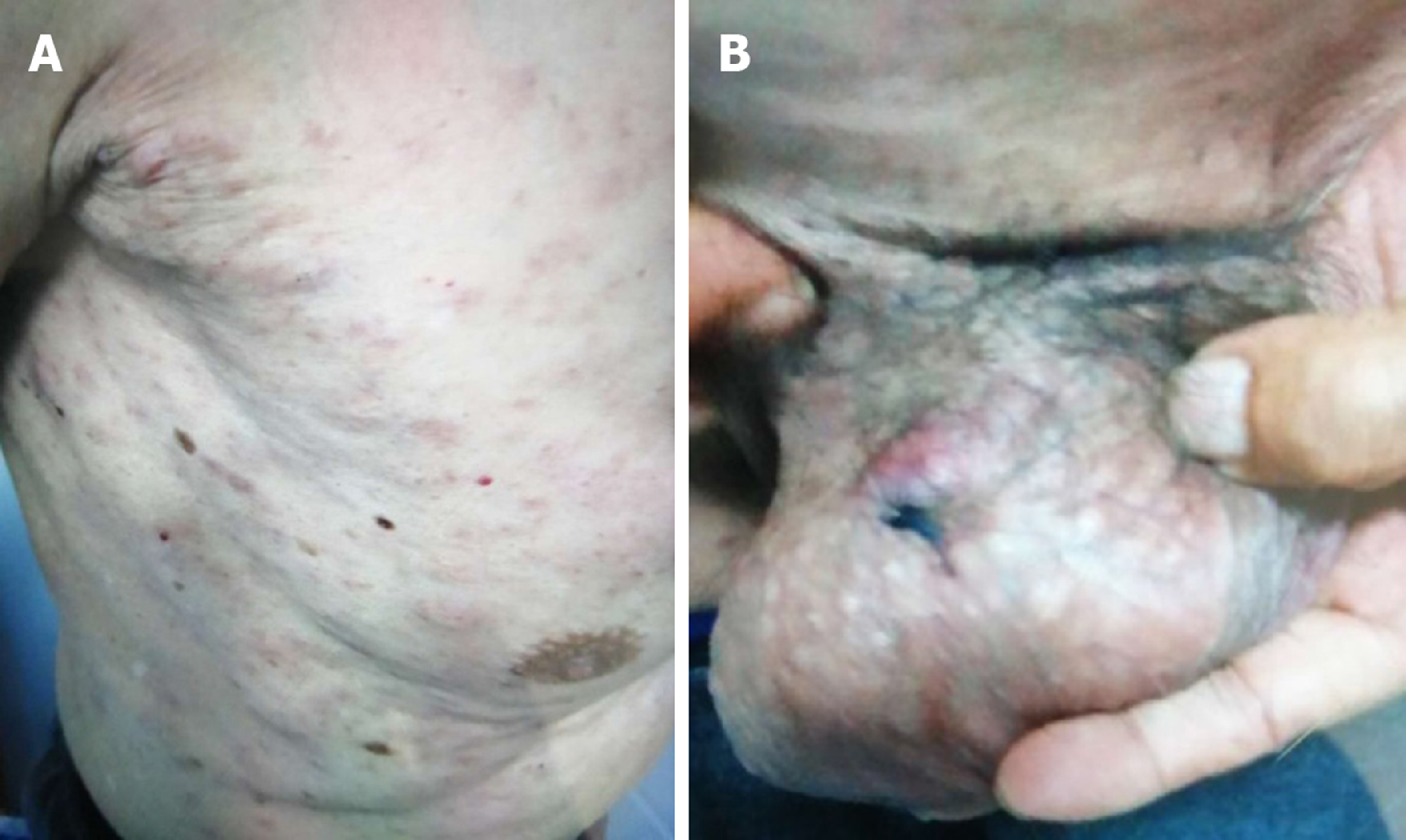
Diffuse cutaneous erythematous plaques and nodules (Figure 1).
In May 2018, a 74-year-old man showed diffuse cutaneous erythematous plaques and nodules with irregular borders on the chest; the effect of anti-allergy treatment was not prominent.
The patient’s history of past illness was not remarkable.
The patient’s father had a history of hypertension, and his mother had a history of breast cancer.
After the first examination, the number of cutaneous lesions increased progressively and involved the entire body, with diameters ranging from 0.5 to 1 cm. The patient’s overall physical condition was good, without B symptoms.
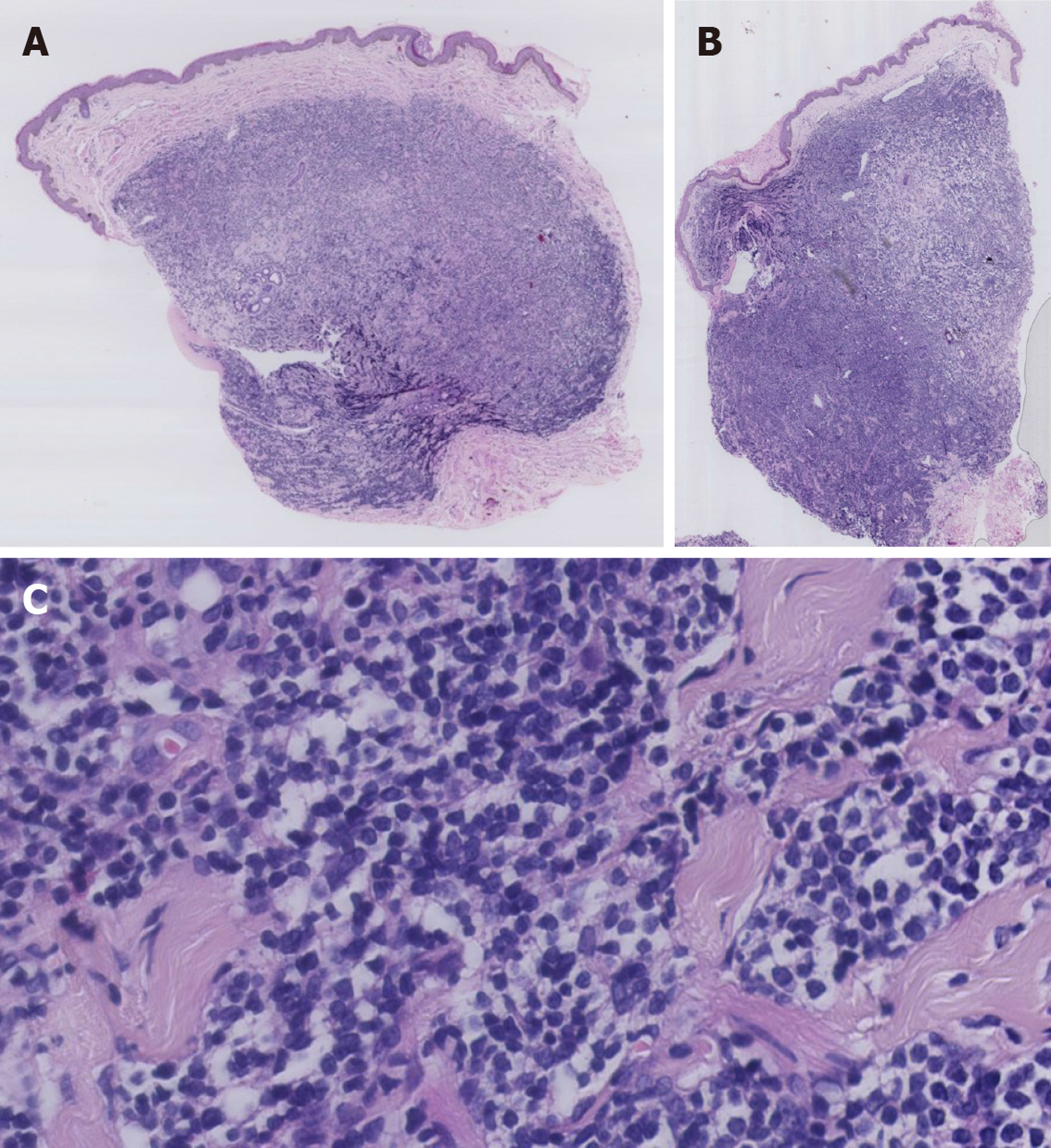
Skin lesions on the trunk and scrotum were biopsied. Histopathological examination showed diffuse monomorphic lymphocyte infiltration in the dermal and subcutaneous layers, but not in the epidermis (Figure 2A and B). The lymphoid cells were mostly small to medium in size, with irregular nuclear contours (Figure 2C).
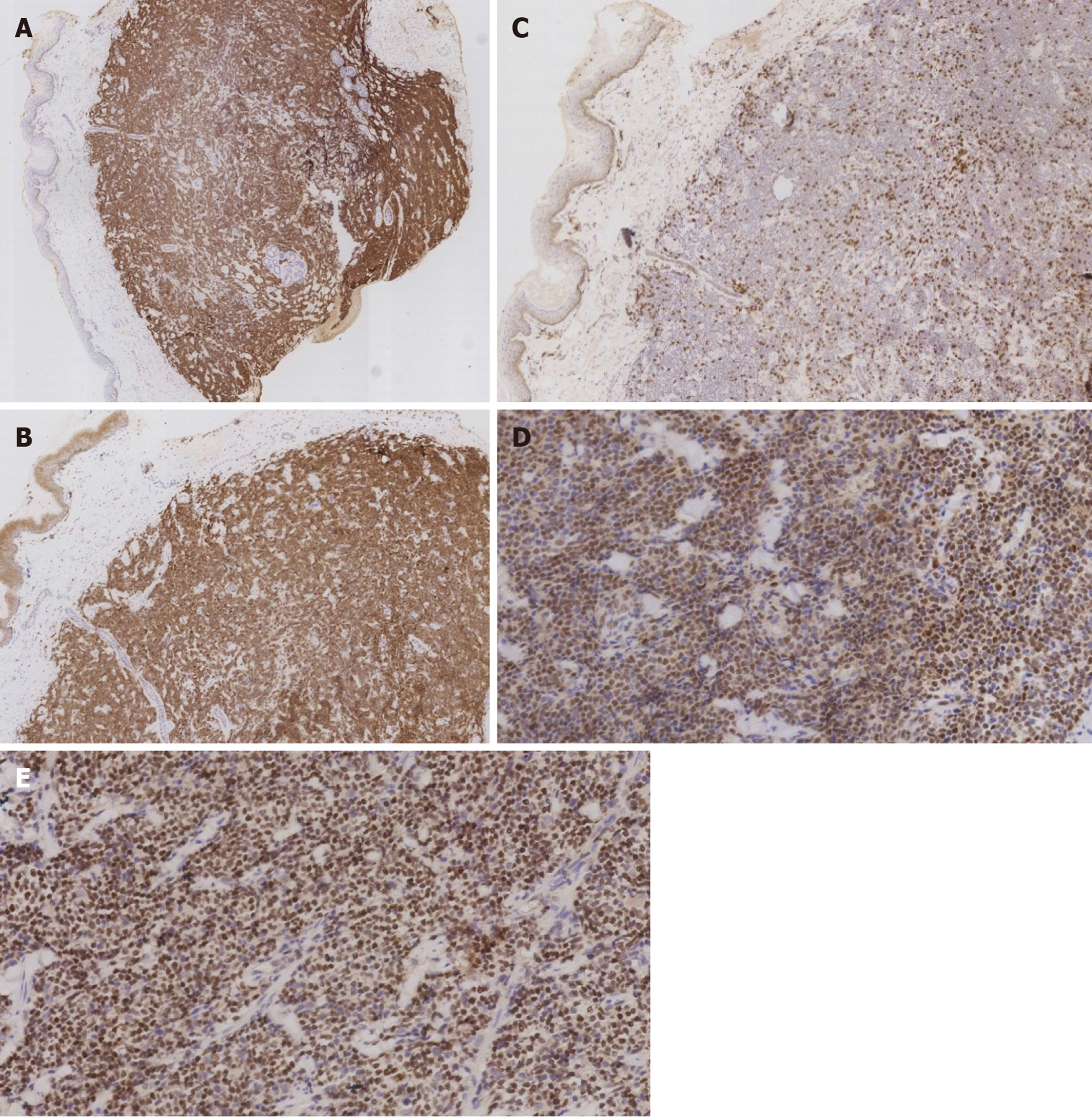
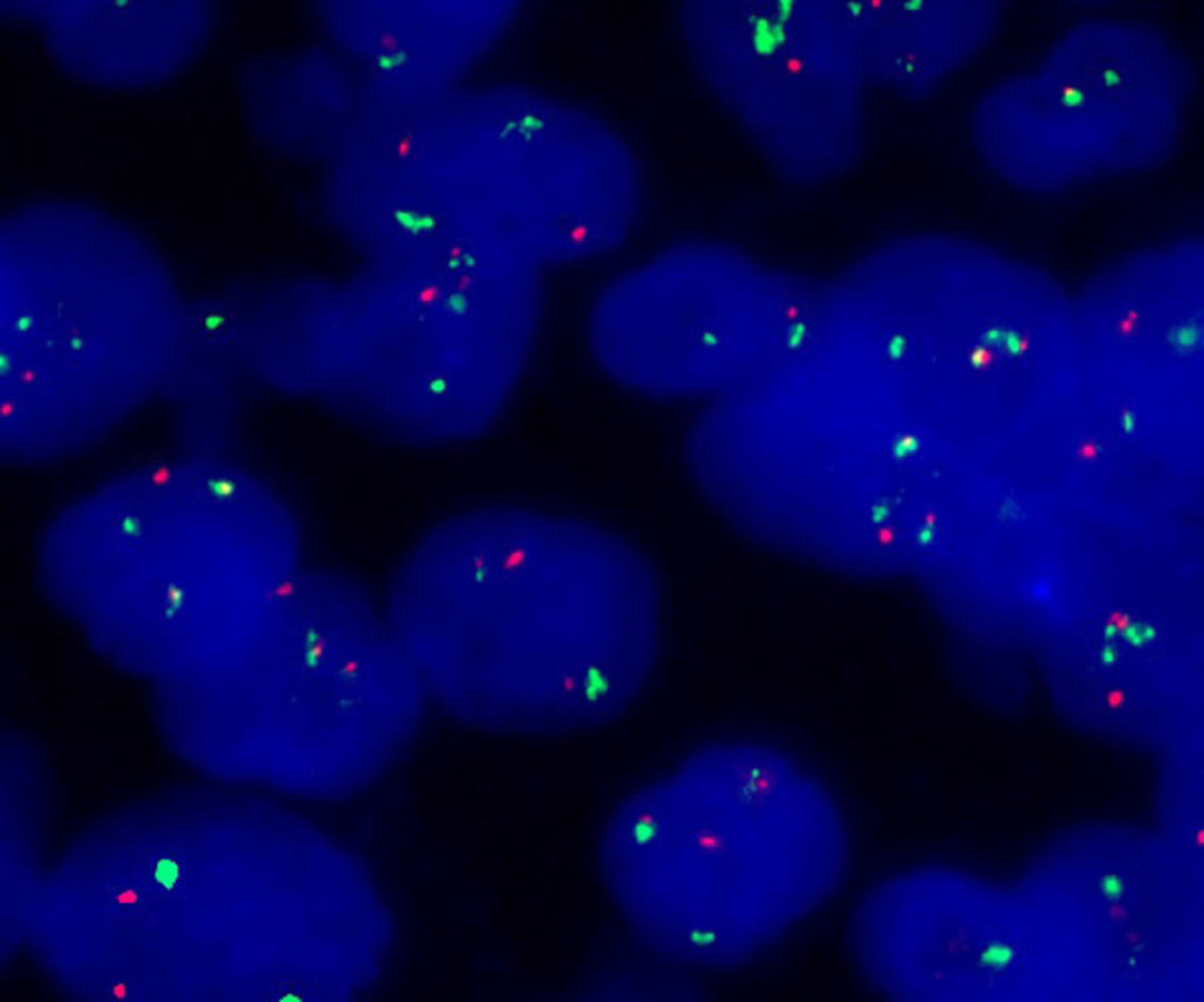
Immunohistochemistry (Figure 3) showed the following phenotypic characteristics: CD21 and CD23 staining displayed a broken follicular dendritic cell network; staining for other markers was as follows: Cyclin D1+, SOX11+, CD5+, CD20+, CD43+, BCL2+, BCL-6 scattered+, CD10-, LEF1-, CD56-, CD30-, CD2-, TIA1-, CD4-, CD8-, and Ki-67+. Tumor cells showed a proliferation index of approximately 20%. In situ hybridization revealed the absence of EBER+ tumor cells. Fluorescence in situ hybridization (FISH) revealed CCND1/IGH gene rearrangement, a distinctive feature of the t(11;14)(q13;q32) chromosomal translocation (Figure 4). Altogether, these clinical phenotypes were consistent with the diagnosis of cutaneous MCL. The clinical data revealed no lymphadenopathy, bone marrow biopsy and peripheral blood examination were normal, and there was no spleen or liver involvement.
Positron emission tomography did not reveal systemic involvement.
Primary cutaneous MCL was the ultimate diagnosis.
The patient received six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) chemotherapy.
The cutaneous manifestations presented almost complete remission without recurrence and progression.
The patient underwent a follow-up examination in January 2020, 20 mo after diagnosis, and no evidence of systemic disease was observed. The peripheral blood test results and bone marrow biopsy at this time were normal.
Skin involvement in MCL is very uncommon, accounting for 2%-6% of all cases[6]. Cutaneous manifestations in MCL occur primarily in cases with systemic disease. Secondary skin involvement in MCL has been described in several studies, but primary cutaneous MCL has seldom been reported[4-13]. Indeed, the presence of primary cutaneous MCL is controversial. It is not included in the WHO-EORTC classification[14] and in the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues[2] for cutaneous lymphomas. A primary cutaneous lymphoma following the WHO-EORTC definition[14], refers to a lymphoma that presents in the skin without extracutaneous disease during the process of diagnosis. Some studies[11,15] have pointed out that a conclusive diagnosis of primary cutaneous MCL can only be achieved if there are no extracutaneous lesions during 6 mo of clinical follow-up with adequate staging. Our patient showed no signs of extracutaneous lesions during the process of diagnosis and up to 20 mo after diagnosis.
A review of the literature reveals less than 50 reported cases of cutaneous MCL. Sen et al[4] reported five cases of cutaneous MCL in 2002, Motegi et al[5] reviewed 17 cases of skin involvement in MCL in 2006, Wehkamp et al[6] reported 14 cases in 2015, and Gru et al[7] reviewed ten cases in 2016. In addition, several case reports are available in literature[9,12,13]. Of these cases, only eight were thought to be primary cutaneous MCL, of which only five were found as clear cases of this condition[4,10-13]. The clinical features and follow-up information of these cases are summarized in Table 1. The lesions of the described cases were limited to the skin without systemic involvement. Our 74-year-old male patient presented diffuse cutaneous erythematous plaques and nodules.
| Case | Ref. | Age (yr) | Sex | Site | Clinical presentation | Treatment | Outcome (mo) |
| 1 | Sen et al[4], 2004 | 76 | Male | Thigh | A single nodule | Hyper-CVAD | 30 |
| 2 | Estrozi et al[11], 2009 | 72 | Male | Temporal region | Cutaneous nodule | Surgical resection and radiotherapy | 6 |
| 3 | Zattra et al[10], 2010 | 77 | Male | The whole body | Diffuse erythematous nodules and plaques | Chemotherapy (refer to note) | 28 |
| 4 | Mazzuoccolo et al[12], 2013 | 72 | Female | Back | Erythematous nodule | Surgical resection | 24 |
| 5 | Hamad et al[13], 2015 | 68 | Female | Legs | Cutaneous erythema | Chemotherapy (R-CHOP) | 31 |
Gru et al[7] reported that among the cases studied, they observed tumor cell infiltration into the dermis and subcutaneous tissue, with cell-free infiltration zone between the dermis and epidermis. The lymphocytes were mostly small to intermediate in size, with two of ten cases belonging to the blastoid variant. A previous study by Sen et al[4] reported that most cases of cutaneous MCL had blastoid morphology and should be classified as blastoid variants. Recently, the largest case study on cutaneous MCL was conducted by Wehkamp et al[6], who also found that all of their analyzed cases, except two, had blastoid morphology, and this type was considered extensively aggressive. The median survival of patients with classic histological features was 50 mo, whereas that for patients with blastoid morphology was 18 mo[16]. The five previously reported cases of primary cutaneous MCL showed that the tumor cell sizes were predominantly intermediate to large. However, our case showed a diffuse monomorphic small to medium-sized lymphocyte infiltration, which did not belong to the blastoid variant.
MCL refers to a B-cell lymphoma with characteristic immunohistochemical and molecular features such as the expression of cyclin D1 and t(11;14)(q13;q32) translocation. More than 95% of MCL cases express cyclin D1, whereas only a few cases are negative[17]. In MCL cases, SOX11 has emerged as an effective marker for diagnosis, with approximately 93%-95% of MCL cases being SOX11-positive. Combined immunohistochemistry tests for cyclin D1 and SOX11 exhibited a specificity and sensitivity of both 100% for diagnosing MCL[18]. Accurate differentiation of MCL from other cutaneous lymphomas is thus possible by using the characteristic immunohistochemical and genetic features. Our case was positive for both cyclin D1 and SOX11 and showed CCND1 translocation.
Skin involvement in MCL mainly appears in cases with systemic disease, which show invasive clinical progression and poor prognosis because of a high recurrence rate. According to previous studies[19,20], systemic chemotherapy is the standard treatment for MCL. However, the therapy of choice for primary cutaneous MCL is difficult to confirm as this condition has been not reported frequently; it is thus unclear whether the standard treatment for systemic disease can be applicable to a localized manifestation. Of the five previously reported cases of primary cutaneous MCL in addition to the current case, four patients received chemotherapy, whereas the remaining two showed isolated skin nodules and were treated with local radiotherapy or surgery. The prognoses of all six patients were relatively good, with nearly complete remission of the skin lesions after 6–31 mo of follow-up.
In conclusion, primary cutaneous MCL is rare and has been the subject of isolated case reports. Correct diagnosis of primary cutaneous MCL requires ensuring that no other parts are involved, and these cases require close follow-up to monitor the possible progression to systemic disease and for the treatment of relapsed cutaneous disease. The prognosis of primary cutaneous MCL is relatively favorable, provided that the diagnosis is conclusive, and treatment options should comply with the clinical condition of the patient. For localized disease, radiotherapy or surgical resection followed by frequent observation should be the treatment of choice; however, chemotherapy should be considered efficient for prolonged survival.
The authors would like to thank Lan Sun from our department for performing and providing the FISH results and corresponding images.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cerwenka H, Saito M S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Banks PM, Chan J, Cleary ML, Delsol G, De Wolf-Peeters C, Gatter K, Grogan TM, Harris NL, Isaacson PG, Jaffe ES. Mantle cell lymphoma. A proposal for unification of morphologic, immunologic, and molecular data. Am J Surg Pathol. 1992;16:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 309] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | WHO. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon: IARC, 2017: 586. |
| 3. | Pileri SA, Falini B. Mantle cell lymphoma. Haematologica. 2009;94:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Sen F, Medeiros LJ, Lu D, Jones D, Lai R, Katz R, Abruzzo LV. Mantle cell lymphoma involving skin: cutaneous lesions may be the first manifestation of disease and tumors often have blastoid cytologic features. Am J Surg Pathol. 2002;26:1312-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Motegi S, Okada E, Nagai Y, Tamura A, Ishikawa O. Skin manifestation of mantle cell lymphoma. Eur J Dermatol. 2006;16:435-438. [PubMed] |
| 6. | Wehkamp U, Pott C, Unterhalt M, Koch K, Weichenthal M, Klapper W, Oschlies I. Skin Involvement of Mantle Cell Lymphoma May Mimic Primary Cutaneous Diffuse Large B-cell Lymphoma, Leg Type. Am J Surg Pathol. 2015;39:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Gru AA, Hurley MY, Salavaggione AL, Brodell L, Sheinbein D, Anadkat M, Porcu P, Frater JL. Cutaneous mantle cell lymphoma: a clinicopathologic review of 10 cases. J Cutan Pathol. 2016;43:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Canpolat F, Taş E, Albayrak Sönmez A, Oktay M, Eskioğlu F, Alper M. Cutaneous presentation of mantle cell lymphoma. Acta Derm Venereol. 2010;90:548-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Lynch DW, Verma R, Larson E, Geis MC, Jassim AD. Primary cutaneous mantle cell lymphoma with blastic features: report of a rare case with special reference to staging and effectiveness of chemotherapy. J Cutan Pathol. 2012;39:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Zattra E, Zambello R, Marino F, Bordignon M, Alaibac M. Primary cutaneous mantle cell lymphoma. Acta Derm Venereol. 2011;91:474-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Estrozi B, Sanches JA, Varela PC, Bacchi CE. Primary cutaneous blastoid mantle cell lymphoma-case report. Am J Dermatopathol. 2009;31:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Mazzuoccolo LD, Castro Perez GA, Sorin I, Bravo AI. Primary cutaneous mantle cell lymphoma: a case report. Case Rep Dermatol Med. 2013;2013:394596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Hamad N, Armytage T, McIlroy K, Singh N, Ward C. Primary Cutaneous Mantle-Cell Lymphoma: A Case Report and Literature Review. J Clin Oncol. 2015;33:e104-e108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2714] [Cited by in RCA: 2576] [Article Influence: 128.8] [Reference Citation Analysis (2)] |
| 15. | Salama S. Primary cutaneous B-cell lymphoma and lymphoproliferative disorders of skin: current status of pathology and classification. Am J Clin Pathol. 2000;114 Suppl:S104-S128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Bosch F, López-Guillermo A, Campo E, Ribera JM, Conde E, Piris MA, Vallespí T, Woessner S, Montserrat E. Mantle cell lymphoma: presenting features, response to therapy, and prognostic factors. Cancer. 1998;82:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Fu K, Weisenburger DD, Greiner TC, Dave S, Wright G, Rosenwald A, Chiorazzi M, Iqbal J, Gesk S, Siebert R, De Jong D, Jaffe ES, Wilson WH, Delabie J, Ott G, Dave BJ, Sanger WG, Smith LM, Rimsza L, Braziel RM, Müller-Hermelink HK, Campo E, Gascoyne RD, Staudt LM, Chan WC; Lymphoma/Leukemia Molecular Profiling Project. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106:4315-4321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 18. | Hsi AC, Hurley MY, Lee SJ, Rosman IS, Pang X, Gru A, Schaffer A. Diagnostic utility of SOX11 immunohistochemistry in differentiating cutaneous spread of mantle cell lymphoma from primary cutaneous B-cell lymphomas. J Cutan Pathol. 2016;43:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Delarue R, Haioun C, Ribrag V, Brice P, Delmer A, Tilly H, Salles G, Van Hoof A, Casasnovas O, Brousse N, Lefrere F, Hermine O; Groupe d'Etude des Lymphomes de l'Adulte (GELA). CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d'Etude des Lymphomes de l'Adulte. Blood. 2013;121:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 20. | Witzens-Harig M, Hess G, Atta J, Zaiss M, Lenz G, Scholz C, Repp R, Reiser M, Pott C, Pelz H, La Rosée P, Kirchner H, Kiewe P, Keller U, Buske C, Viardot A, Dreyling M. Current treatment of mantle cell lymphoma: results of a national survey and consensus meeting. Ann Hematol. 2012;91:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |