Published online Apr 26, 2020. doi: 10.12998/wjcc.v8.i8.1385
Peer-review started: March 25, 2020
First decision: April 7, 2020
Revised: April 8, 2020
Accepted: April 11, 2020
Article in press: April 11, 2020
Published online: April 26, 2020
Processing time: 29 Days and 16.8 Hours
In patients infected with severe acute respiratory syndrome coronavirus 2, the respiratory symptoms, such as fever, cough and dyspnea, are the most frequent clinical manifestations. These patients may also present with less well-defined symptoms like diarrhea, nausea, vomiting and/or abdominal discomfort both at the time of diagnosis and during the clinical course. In a few cases, these symptoms may also present before the appearance of respiratory symptoms. To penetrate the body, Severe acute respiratory syndrome coronavirus 2 uses ACE2 receptors, which are present not only in respiratory epithelium but also in gastrointestinal mucosa and liver cholangiocytes. In several cases, viral RNA is detectable in the stool of patients with coronavirus disease 2019 (COVID-19). The liver damage seems to show a multifactorial origin. About 2%-11% of patients with COVID-19 have known underlying hepatic pathologies. In 14%-53% of COVID-19 cases, there is an alteration of the indices of liver cytolysis and is more frequently observed in severe forms of COVID-19, especially during hospitalization.
Core tip: Severe acute respiratory syndrome coronavirus 2 infection currently represents an emerging pandemic. More and more published papers, with constantly updated data, highlight a concomitant hepatic impairment, particularly, an hypertransaminasemia. In this mini-review, we will try to analyze the incidence and pathogenetic hypothesis of this phenomenon using the currently available data.
- Citation: Zippi M, Fiorino S, Occhigrossi G, Hong W. Hypertransaminasemia in the course of infection with SARS-CoV-2: Incidence and pathogenetic hypothesis. World J Clin Cases 2020; 8(8): 1385-1390
- URL: https://www.wjgnet.com/2307-8960/full/v8/i8/1385.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i8.1385
As of December 2019, a viral infection defined as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), caused by the pathogen of coronavirus disease 2019 (COVID-19), belonging to the Coronavirus family, has developed suddenly and has had and has a strong impact worldwide[1]. This virus is similar to other two preceding coronavirus sharing 79% of its genome with those of the 2003 severe acute respiratory syndrome (SARS-CoV) and 50% with those of the 2012 Middle Eastern respiratory syndrome (MERS-CoV)[2]. All these viruses are able to cause liver damage, in addition to the well-known respiratory manifestations[2]. In a recent Comment, Zhang et al[3] have analyzed the effects of the virus on liver and concluded that this pathogen can alter liver enzymes [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] in 14%-53% of cases, SARS-CoV-2 and as such an infection is present in 2%-11% of patients with known hepatic pathologies (chronic viral hepatitis, nonalcoholic fatty liver disease, alcoholic hepatitis, immune-mediated liver disease).
The aim of this mini-review is to identify those studies who have considered liver involvement, especially hypertransaminasemia that occurred during COVID-19 infection, by searching through MEDLINE/PubMed and Google Scholar. In particular, the following keywords were searched: “liver”, “SARS-CoV-2”, “COVID-19”, “transaminases”, “AST”, and “ALT”. We would like to emphasize that we used updated data at the time of submission of the present work, as these are continuously being updated.
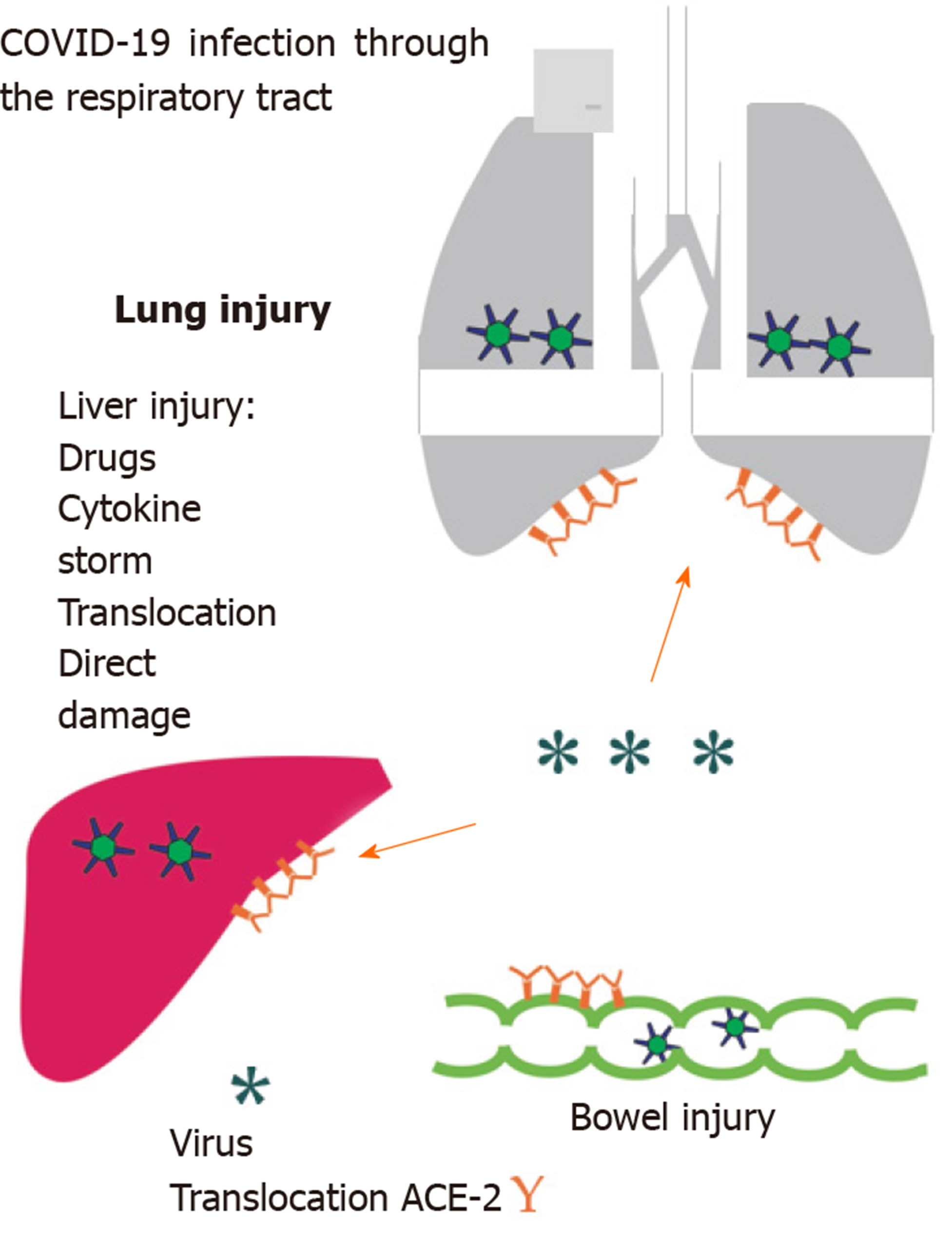
Hepatic involvement due to a predominant respiratory virosis is unclear. Liver damage appears to be of multifactorial origin. (1) Direct damage: Caused by the virus itself as it can bind to ACE-2 receptors, which are expressed in lung, kidney, and gastrointestinal tract[4,5]. The ACE-2 receptors are particularly present in endothelial cells of the liver[6]; (2) Intestinal translocation: It has been observed how about 2%-10% of affected patients have diarrhea, and SARS-CoV-2 RNA has been detected both in stool as well as blood samples. This could lead to a greater “translocation” from the intestinal lumen[3,7-9]. In fact, we know that gut integrity, especially during abdominal sepsis, may be altered, leading to increased cellular apoptosis with subsequent altered barrier permeability[10,11]; (3) Drug hepatotoxicity: In their study, Zhang et al[3] have observed that liver function tended to alter both during and after the ongoing infection (COVID-19); they hypothesized a “residual effect” on the liver due to the drugs taken to counteract the infection. As underlined by Rismanbaf et al[12], we must also consider the side effects of the therapies used in virosis on the liver. In their Letter, the authors emphasized how the currently used drugs, like Oseltamivir, Lopinavir, Ritonavir, Ribavirin, and Chloroquine Phosphate or Hydroxy Chloroquine Sulfate, are all metabolized in the liver[12]. In a recent multicenter retrospective study, Liu et al[13] analyzed 32 patients, of which 28 (87.5%) had a mild or moderate disease and 4 (12.5%) had severe one, observing how liver damage was prominent in severe patients under medical therapy; and (4) Immune-mediated inflammation: the hepatic involvement that occurs during this infection must be considered as it may cause a “cytokine storm”, especially, in severe forms of COVID-19[14]. In this patient cohort, increased levels of interleukin (IL)-2, IL-7, interferon-γ and tumor necrosis factor-α were observed[15]. Adhikari et al[16] reviewing 65 research articles found how a surgery history before admission for Covid-19 was one of the factors that make people more likely to contract the infection. In fact, surgical stress can exacerbate “cytokine storm” and the COVID-19 disease progression (Figure 1).
To date, looking in the available literature, we could identify 16 papers relating to the evaluation of hepatic impairment in SARS-CoV-2 patients. These results are available in Table 1.
| Ref. | Year 2020 (online date) | Number of patients with SARS-CoV-2 | Number of patients with known liver diseases (%) | Number of patients with transaminase increased (%) |
| Zhang et al[3] | Mar 4 | 56 | 2 (3.6) | 16 (28.6) |
| Guan et al[17] | Feb 28 | 1099 | 23 (2.3) | AST: 168/757 (22.2) |
| ALT: 158/741 (21.3) | ||||
| Huang et al[18] | Jan 24 | 41 | 1 (2.0) | 5 (31) |
| Chen et al[19] | Jan 30 | 99 | / | 43 (43.0) |
| Wang et al[20] | Feb 7 | 138 | 4 (2.9) | / |
| Shi et al[21] | Feb 24 | 81 | 7 (8.6) | 43 (53.1) |
| Xu et al[22] | Feb 19 | 62 | 7 (11.0) | 10 (16.1) |
| Yang et al[23] | Feb 24 | 52 | NA | 15 (29.0) |
| Cai et al[24] | Feb 19 | 298 | 8 (2.7) | 44 (14.8) |
| Fan et al[25] | Feb 28 | 148 | / | 75 (50.7) |
| Zhang et al[26] | Feb 27 | 82 | 2 (2.4) | 64 (78) |
| Huang et al[27] | Mar 5 | 36 | / | AST: 18/31 (58.06) |
| ALT: 4/30 (13.3) | ||||
| Ding et al[28]1 | Mar 20 | 5 | 2 (40) | 2 (40) |
| Wan et al[29] | Mar 21 | 135 | 2 (1.5) | AST: 33.4 (27.8-43.7) U/L (elevated) |
| ALT: 26 (12.9-33.15) U/L | ||||
| Jin et al[30] | Mar 24 | 74 (GI symptoms) vs 577 (No GI symptoms) | 8/74 (10.81) vs 17/577 (2.95) (P = 0.004) | AST: 29.35 (20.87-38.62) U/L vs 24.4 (19.0-32.0) U/L (P = 0.02) |
| ALT: 25.0 (15.75–38.47) U/L vs 21.5 (15.0–32.8) U/L (P = 0.203) | ||||
| Zhang et al[31] | Apr 2 | 115 | / | AST: 17/715 (9.6) |
| ALT: 11/115 (14.8) |
In a similar study, not included in Table 1, it is reported that out of 128 patients affected by COVID-19, there was an increase in transaminases only in severe cases[32]. Guan et al[17] further analyzed in a subgroup the increase in transaminases based on the degree of disease severity. They reported elevated levels of AST in 39.4% (56/142 patients) patients with severe disease compared with 18.2% (112/615 patients) patients with mild/moderate disease. Also, the levels of ALT were higher in the severe form (28.1% vs 19.8% of mild/moderate conditions)[17]. Huang et al[18] reported an increase in AST level in 62% of 13 patients recovered in Intensive Care Unit. A further confirmation comes from the work of Shi et al[21], in which patients with pulmonary involvement, assessed by computed tomography, showed a significant increase in AST level compared to those suffering from a subclinical form. A final consideration is given by Yang et al[23], who reported no significant difference in increased transaminase between survivors (30%) and non-survivors (28%).
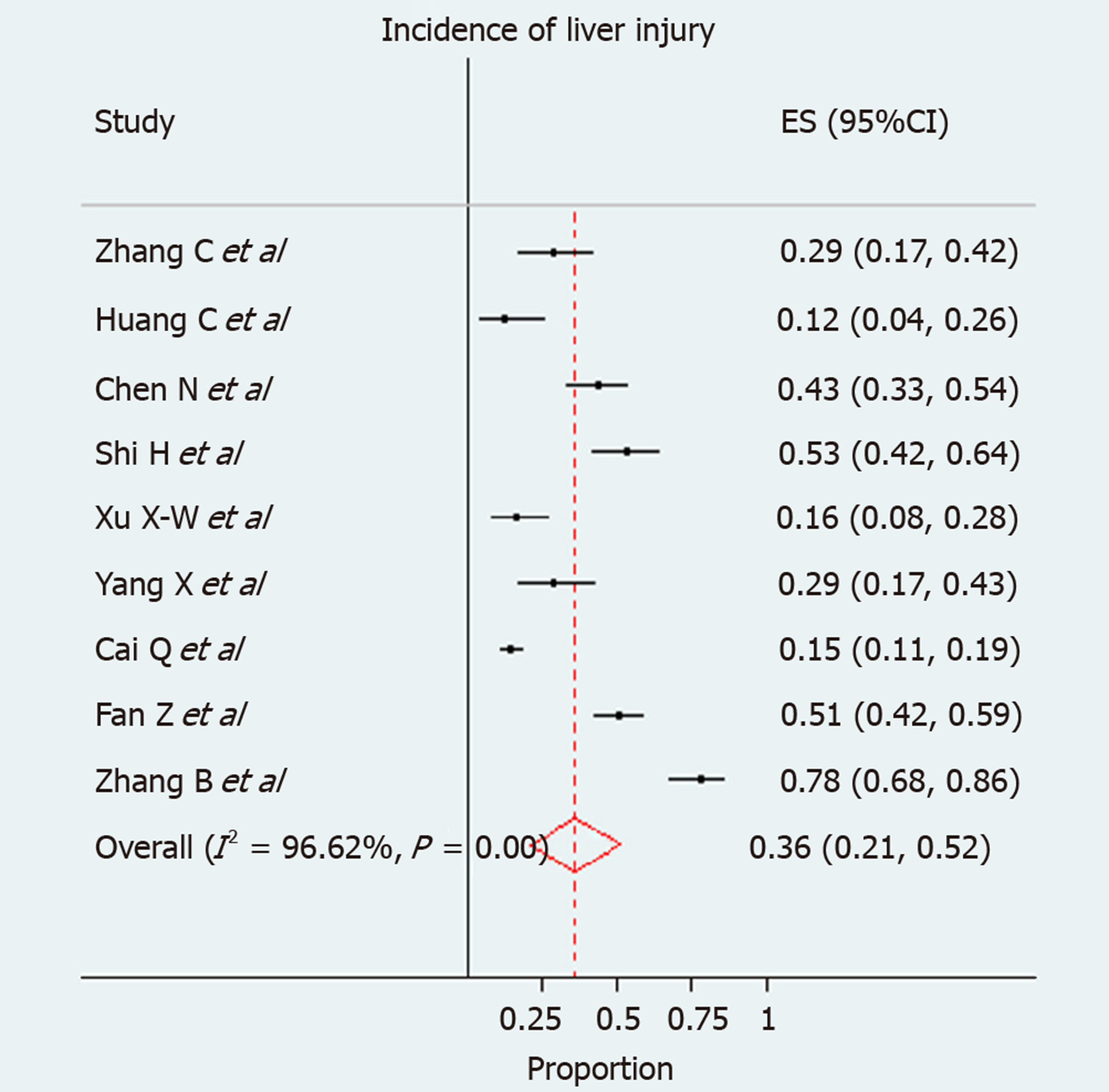
We used forest plots for estimating pooled effect sizes and the effect of each study, with 95%CI, to provide a visual summary of the data. Heterogeneity among studies was evaluated by Cochran Q-test and I-squared index, with I2 values above 75% as thresholds for high heterogeneity. When heterogeneity was present, we used a random effects model (DerSimonian–Laird model); otherwise, a fixed effects model (Mantel–Haenszel) used to compute overall effects[33]. A total of 9 studies were enrolled in the current pooled analysis. The incidence of liver injury in individual studies ranged from 12% to 78%. Due to extensive heterogeneity between studies (I-squared = 96.6%), a random effect model was used. The pooled incidence of liver injury in patients with COVID-29 infection was 0.36 (95%CI: 0.21-0.52) (P < 0.001) (Figure 2).
Hepatic involvement during SARS-CoV-2 infection is not uncommon. Altered transaminases are most frequently reported in the available works. This finding does not support the hypothesis of a hypoxic hepatitis, as their elevated levels are present also in not critically ill patients, especially in those ones without mechanical ventilation[34]. The increase in cytolysis indices may be due to several causes, including the antiviral drugs. In this regard, the histological description of liver biopsies performed on a 50-year-old subject who died owing to COVID-19 is interesting. It shows a mild lobular and portal activity associated with a moderate microvascular steatosis, as to indicate how liver damage may have been caused either directly by the virus or by the administered drugs[35]. The increase in transaminases observed in the reviewed works could also be partly due to the increased risk of toxicity when the optimal therapeutic dose is reached. As the etiology of this phenomenon can be traced back to various factors, currently there is no therapeutic approach or management for it. What can be deduced, from the available literature to date, is keeping monitoring the level of transaminases during hospitalization. As underlined by Al-Busafi et al[36], the serum alanine aminotransferase and aspartate aminotransferase are the best markers of hepatocellular injury and their highest levels tend to indicate a more severe liver damage. It’s known that drugs with their respective dosages play a fundamental role in hepatotoxicity, especially in patients undergoing polytherapy. In a “normal” situation, the European Association for the Study of the Liver recommends to discontinue the involved agent, but in this type of patient it is not possible to suspend the antiviral medications[37]. Limited data are available about liver involvement in infected children with COVID-19, especially for infected infants. Cui et al[38] described a case of a 55-d-old case with COVID-19 in China who presented with altered hepatic function during hospitalization. Recently, Sun et al[39] described the laboratory findings in 8 severe patients in the ages between 2 mo and 15 years, including 6 males and 2 females. Their results showed AST in norm and ALT increased in 4 cases (50%).
In conclusion, hypertransaminasemia present in these patients may be due to several reasons. Further studies will be needed to understand the main cause of this phenomenon in order to guide the best treatment.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Song B, Ulaşoğlu C, Xia ZY S-Editor: Dou Y L-Editor: A E-Editor: Liu JH
| 1. | Andrea G, Daniele D, Barbara A, Davide M, Laura A, Paolo R, Alessandra B, Giorgio R. Coronavirus Disease 2019 and Transplantation: a view from the inside. Am J Transplant. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J Gen Intern Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 869] [Cited by in RCA: 703] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 3. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 4. | Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ; SARS Working Group. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3147] [Cited by in RCA: 3072] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 5. | Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 6. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 7. | Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 8. | Tang A, Tong ZD, Wang HL, Dai YX, Li KF, Liu JN, Wu WJ, Yuan C, Yu ML, Li P, Yan JB. Detection of Novel Coronavirus by RT-PCR in Stool Specimen from Asymptomatic Child, China. Emerg Infect Dis. 2020;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 9. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 10. | Hu Q, Ren H, Li G, Wang D, Zhou Q, Wu J, Zheng J, Huang J, Slade DA, Wu X, Ren J. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. EBioMedicine. 2019;41:497-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 11. | Lima MT, dos Santos Pereira Andrade AC, Oliveira GP, Nicoli JR, dos Santos Martins F, Kroon EG, Abrahão JS. Virus and microbiota relationships in humans and other mammals: An evolutionary view. Human Microbiome. 2019;11:100050. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Rismanbaf A, Zarei S. Liver and Kidney Injuries in COVID-19 and Their Effects on Drug Therapy; a Letter to Editor. Arch Acad Emerg Med. 2020;8:e17. [PubMed] |
| 13. | Liu C, Jiang ZC, Shao CX, Zhang HG, Yue HM, Chen ZH, Ma BY, Liu WY, Huang HH, Yang J, Wang Y, Liu HY, Xu D, Wang JT, Yang JY, Pan HQ, Zou SQ, Li FJ, Lei JQ, Li X, He Q, Gu Y, Qi XL. [Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 14. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6751] [Article Influence: 1350.2] [Reference Citation Analysis (0)] |
| 15. | Zhou Y, Fu B, Zheng X, Wang D, Zhao C, qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 714] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 16. | Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H, Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1089] [Article Influence: 217.8] [Reference Citation Analysis (0)] |
| 17. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18876] [Article Influence: 3775.2] [Reference Citation Analysis (7)] |
| 18. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30114] [Article Influence: 6022.8] [Reference Citation Analysis (3)] |
| 19. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12975] [Article Influence: 2595.0] [Reference Citation Analysis (1)] |
| 20. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 21. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2310] [Article Influence: 462.0] [Reference Citation Analysis (0)] |
| 22. | Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1263] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 23. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6659] [Article Influence: 1331.8] [Reference Citation Analysis (0)] |
| 24. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Fu Y, Liu L, Chen J. COVID-19 in a Designated Infectious Diseases Hospital Outside Hubei Province, China. medRxiv. 2020;. [DOI] [Full Text] |
| 25. | Fan Z, Chen L, Li J, Cheng Tian, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Damage. medRxiv. 2020;. [DOI] [Full Text] |
| 26. | Zhang B, Zhou X, Qiu Y, Feng F, Feng J, Jia Y, Zhu H, Hu K, Liu J, Liu Z, Wang S, Gong Y, Zhou C, Zhu T, Cheng Y, Liu Z, Deng H, Tao F, Ren Y, Cheng B, Gao L, Wu X, Yu L, Huang Z, Mao Z, Song Q, Zhu B, Wang J. Clinical characteristics of 82 death cases with COVID-19. medRxiv. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Huang Y, Zhou H, Yang R, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv. 2020;. [DOI] [Full Text] |
| 28. | Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 29. | Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, Huang X, Lv J, Luo Y, Shen L, Yang H, Huang G, Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 643] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 30. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 870] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 32. | Cao W. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei. medRxiv. 2020;. [DOI] [Full Text] |
| 33. | Lashaki EK, Teshnizi SH, Gholami S, Fakhar M, Brant SV, Dodangeh S. Global prevalence status of avian schistosomes: A systematic review with meta-analysis. Parasite Epidemiol Control. 2020;9:e00142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 35. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5782] [Article Influence: 1156.4] [Reference Citation Analysis (2)] |
| 36. | Al-Busafi SA, Hilzenrat N. Mild Hypertransaminasemia in Primary Care. ISRN Hepatol. 2013;2013:256426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | European Association for the Study of the Liver; Clinical Practice Guideline Panel: Chair, Panel members; EASL Governing Board representative. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70:1222-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 669] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 38. | Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, Wang L, Chen Y, Liu W, Zhang K, Wu Y, Yang Z, Tao J, Feng J, Liu K, Ye X, Wang R, Zhang X, Zha Y. A 55-Day-Old Female Infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 39. | Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, Liu ZS. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |