Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6380
Peer-review started: August 6, 2020
First decision: September 13, 2020
Revised: September 28, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: December 26, 2020
Processing time: 135 Days and 7.6 Hours
45,X/46,XY mosaicism is a rare chromosomal abnormality with a wide range of phenotypes in both males and females, from normal individuals with different degrees of genital ambiguity to those who show signs of Turner’s syndrome. More rarely, cases of 45,X/46,XY mosaicism with a normal-appearing male phenotype are not found until a chromosome test is performed to investigate the cause of male infertility.
In this study, a 29-year-old male patient with complete azoospermia is reported. Chromosomal analyses of his lymphocytes revealed the karyotype 45,X[93%]/46,X,+mar(Y)[7%]. In addition, Y chromosome-specific markers, such as SRY, ZFY, AZFa, AZFb and AZFc, were not observed in his blood DNA according to multiplex polymerase chain reaction test. A literature review identified several 45,X/46,XY cases with a normal-appearing male phenotype, most of whom were diagnosed during infertility investigation. However, the present case is the first SRY-negative 45,X/46,XY male case diagnosed during a premarital medical examination.
This finding further suggests that sex determination is a complex process regulated by multiple genetic and environmental factors.
Core Tip: A rare chromosomal abnormality is 45,X/46,XY mosaicism. Here, we describe the diagnosis of a rare case of a 45,X/46,XY SRY-negative man with complete virilization and infertility as the main anomaly.
- Citation: Wu YH, Sun KN, Bao H, Chen YJ. SRY-negative 45,X/46,XY adult male with complete masculinization and infertility: A case report and review of literature. World J Clin Cases 2020; 8(24): 6380-6388
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6380.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6380
As a rare complement, the chromosomal abnormality of 45,X/46,XY mosaicism is found in 1.7 cases among 10000 newborns[1]. The spectrum of observed phenotypes ranges continuously from normal individuals with varying degrees of genital ambiguity to Turner’s syndrome[2]. Most normal-appearing male phenotype cases are diagnosed during the prenatal period, and cases with genital/gonadal anomalies are usually diagnosed after birth[3]. More rarely, cases of 45,X/46,XY mosaicism with a normal-appearing male phenotype are not found until a chromosome test is performed to investigate the cause of male infertility.
Because the Y chromosome carries testis-determining factor, which is a genetically predominant locus, under normal circumstances, the bipotent gonadal primordium can be triggered and testes formation can be processed, which makes the Y chromosome a key factor in human sex determination. The SRY gene located in Yp11.2 was found to be cytogenetic and confirmed to play a critical role in the complex and tightly regulated processes of testis development[4] and sex differentiation[5]. However, more and more SRY-negative male cases with various karyotypes have been reported. In addition, increasing studies have shown that other factors, including both genetic and environmental factors, may regulate gender determination and differentiation through a multi-target approach.
Key genetic factors are known to regulate spermatogenesis on Yq, namely azoospermia factors (AZFs), including AZFa, AZFb and AZFc. Spermatogenetic failure caused by AZF microdeletions is a common cause of male infertility. Studies have shown that AZF microdeletions can be detected in approximately 10%-15% of azoospermia patients in China[6].
In the present report, we describe the diagnosis of a rare SRY-negative male case with 45,X/46,XY mosaicism. In addition, we review the 45,X/46,XY male phenotype cases reported in the literature to date to provide a more comprehensive description of the genetic and pathological features of this subgroup.
A 29-year-old man visited our urology clinic for a premarital medical examination, with complaints of occasional scrotal pain.
For the previous month, the patient had experienced occasional minor pain in the testicles.
The patient had no notable previous medical history.
He denied any family history and had no specific past history.
His height was 167 cm, and his weight was 57.9 kg. After physical examination, we found that he had no dysmorphisms and had a normal distribution of pubic hair and body hair. His external urethral meatus was in a normal position, and his penis had a normal appearance and size (5.7 cm, non-erectile).
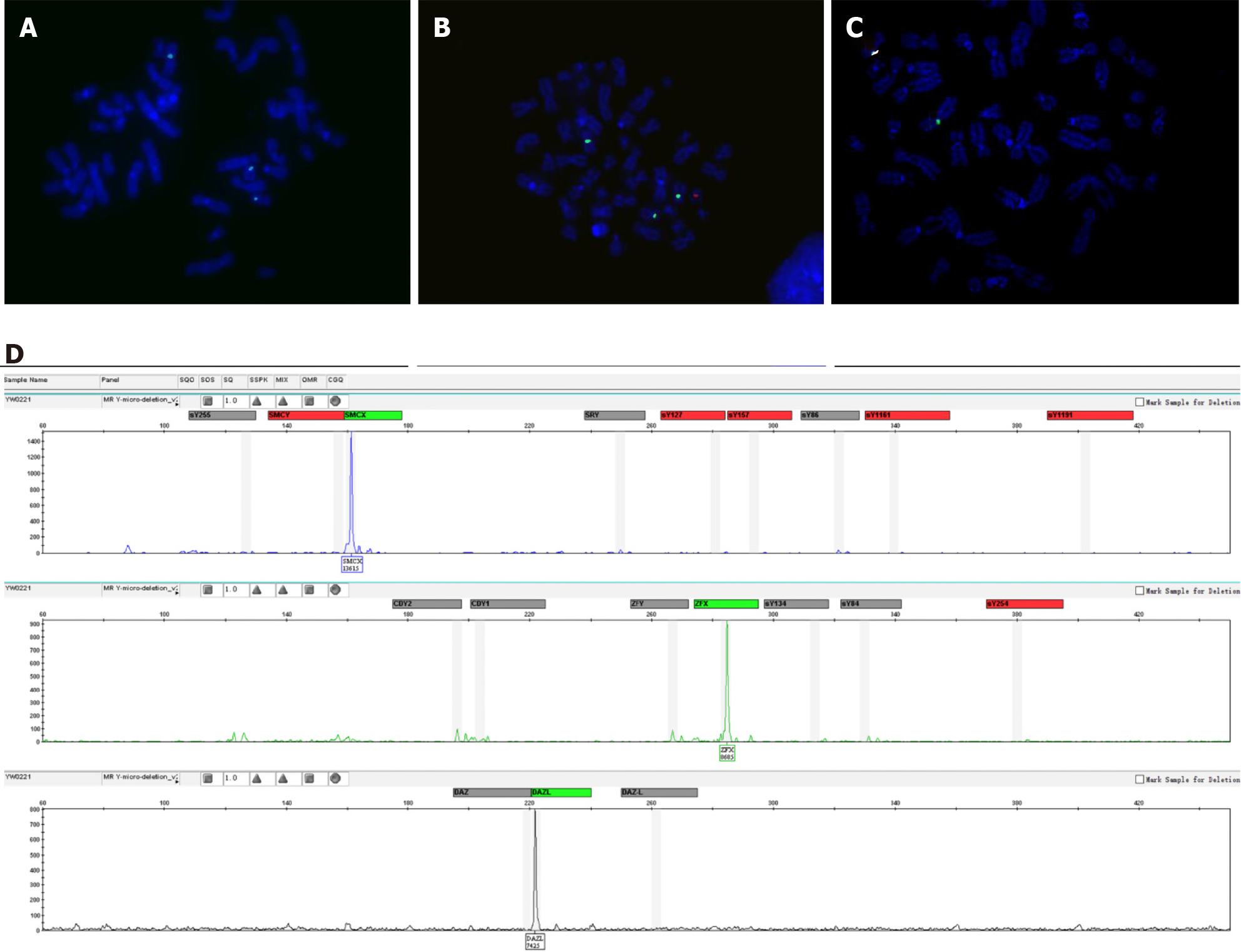
The results of the patient's serum test revealed that the luteinizing hormone (LH) concentration was elevated at 15.73 IU/L (normal range: 1.7-8.6 IU/L), and the follicle-stimulating hormone (FSH) concentration was elevated at 14.13 IU/L (normal range: 1.5-12.4 IU/L). However, the serum testosterone hormone concentration was 3.22 μg/L, which was in the normal range for adult males of 2.49-8.36 μg/L. Azoospermia was determined after repeated seminal analysis. Chromosomal analysis was performed twice on samples collected at different times, and 100 metaphases were analyzed in each analysis. Two different cell lines with the karyotype 45,X[93%] /46,X,+mar(Y)[7%] were observed by GTG banding. Fluorescence in situ hybridization analysis with screening of metaphase and interphase lymphocytes was carried out to confirm the result of the karyotype analysis. Two cell lines, one with one green signal for Xcen (182/200) and the other with one green signal and one red signal for Xcen and Ycen (18/200), respectively, were observed according to fluorescence in situ hybridization (Figure 1A and B). All the metaphase and interphase lymphocytes showed one signal for Xcen but no SRY signal, except for cell lines containing SRY (Figure 1C). Polymerase chain reaction amplification of 16 Y-STS gene loci (SRY, ZFY, sY86, sY84, CDY2, SMCY, sY127, sY134, sY1161, sY1191, sY254, sY255, DAZ, sY157, CDY1,
Ultrasound scanning of the scrotum showed that both testicles were located in the scrotum, but the volumes (6.6 mL and 6.8 mL, respectively) were significantly smaller than the normal adult male testicle size (range: 15-23 mL). In addition, a normal-sized prostate and seminal vesicles were observed by internal genitalia ultrasound analysis.
Azoospermia.
The recommended treatments were hormone replacement therapy, including oral testosterone undecanoate to maintain sexual function, and sperm donation and assisted reproductive technology to solve fertility problems.
Follow-up found that the patient had a normal penile erection. He was married 1 year later, and the couple decided to adopt a child after marriage.
Stability of the number and structure of chromosomes is the basic requirement for maintaining the normal sex differentiation process. Two decades ago, Telvi et al[3] found that 45,X/46,XY mosaicism can manifest as a normal male phenotype and can also cause some abnormal clinical phenotypes, including Turner’s syndrome, pseudohermaphroditism, and mixed gonad dysplasia. The subgroup of 45,X/46,XY mosaicism with normal adult male phenotype is usually diagnosed during infertility investigation. Lashkari et al[7] reported that the occurrence rate of 45,X/46,XY mosaicism in azoospermic and oligozoospermic patients was 0.78%. Among the 49 infertile adult male patients with the 45,X/46,XY mosaicism karyotype that they evaluated, 21 showed azoospermia, 24 had sperm abnormalities, and four displayed a normal spermogram.
We reviewed additional literature regarding 45,X/46,XY adult male cases (Table 1)[8-18]. Among the 34 cases reviewed, 96.5% (28/29) showed azoospermia or oligozoospermia. The 45,X/46,XY mosaicism rates ranged from 6/93.3 to 94/6.7. There was no relationship between the mosaicism rate in peripheral blood lymphocyte and the phenotype, which was consistent with previous results[19,20]. The mosaicism ratio in different tissues may explain the variety of phenotypes in mixed gonadal dysgenesis[21]. Moreover, these individuals showed short or normal stature, with height ranged from 148-181 cm (data from 24 cases). Most of the patients had small testicles (92%, 23/25), elevated LH concentration (65.2%, 15/23), elevated FSH concentration (80.9%, 17/21), and normal testosterone concentration (95.2%, 20/21). All patients had the SRY gene, while 62.1% (18/29) had the AZF microdeletion. The most common AZF microdeletions were AZF(b+c) (66.7%, 12/18) followed by AZFc (22.2%, 4/12), AZFb (5.5%, 1/18), and AZF(a+b+c) (5.5%, 1/18). In our study, the man with the 45,X/46,XY mosaic karyotype also showed complete masculinization and azoospermia, with short stature, small testicles, elevated LH and FSH concentrations, and a normal T concentration. However, the SRY gene, ZFY gene, and AZF(a+b+c) regions of blood DNA were missing in this case, and this is the first male with the SRY-negative 45,X/46,XY mosaic karyotype according to our literature search results.
| Ref. | No. | Age in yr | Karyotype[%] | Reason for examination | Semen analysis | Height in cm | Genital/gonads | LH in U/L | FSH in U/L | T in ng/mL | E2 in pg/mL | AZF | SRY |
| Wu et al[8], 2017 | 1 | 22 | 45,X[93.3]/46,XY[6.7] | Primary sterility | AZO | 148 | Male/left and right TV: 4.4 mL, 1.6 mL dysplasia of epididymis | 17.07 | 52.78 | 3.94 | 13.58 | No missing | + |
| 2 | 23 | 45,X[36.7]/46,X,del(Y)(q11.223)[63.3] | Primary sterility | AZO | 159 | Male/left and right TV: 8.7 mL, 8.7 mL | 9.98 | 16.22 | 4.32 | 37.38 | AZF(b+c) | + | |
| 3 | 23 | 45,X[65]/46,XY[35] | Primary sterility | AZO | 173 | Male/left and right TV: 4 mL, 5.4 mL | 9.10 | 15.33 | 2.52 | < 5.00 | AZF(b+c) | + | |
| 4 | 26 | 45,X[6]/46,XY[94] | Primary sterility | AZO | 170 | Male/left and right TV: 3.8 mL, 4.5 mL | 9.32 | 17.04 | 4.06 | 47.79 | AZF(b+c) | + | |
| 5 | 26 | 45,X[83.3]/46,X,Yqh–[16.7] | Primary sterility | AZO | 165 | Male/left and right TV: 6 mL, 6 mL | 10.44 | 28.20 | 7.12 | 33.26 | AZF(b+c) | + | |
| 6 | 29 | 45,X[45]/46,X,Yqh–[55] | Primary sterility | AZO | 160 | Male/left and right TV: 5.4 mL, 6.2 mL | 13.58 | 22.00 | 3.49 | 40.95 | AZF(b+c) | + | |
| 7 | 29 | 45,X[28.3]/46,XY[71.7] | Primary sterility | AZO | 165 | Male/left and right TV: 6 mL, 3.2 mL | 14.26 | 31.31 | 3.26 | 20.64 | AZF(b+c) | + | |
| Akinsal et al[9], 2018 | 8 | 24 | 45,X[66]/46,XY[34] | Primary sterility | AZO | 158 | Male/left and right TV: 18 mL, 12 mL | 19.7 | 15.15 | 3.83 | AZFc | + | |
| 9 | 26 | 45,X[70]/46,XY[30] | Primary sterility | AZO | 178 | Male/left and right TV: 9 mL, 9 mL | 7.5 | 9.8 | 6.74 | 16.14 | No missing | + | |
| 10 | 29 | 45,X[40]/46,XY[60] | Primary sterility | AZO | 156 | Male/left and right TV: 7 mL, 7 mL | 7.97 | 20.4 | 7.44 | 48.6 | AZFc | + | |
| 11 | 40 | 45,X[30]/46,XY[70] | Primary sterility | AZO | 165 | Male/left and right TV:14 mL, 14 mL | 9.96 | 22.29 | 3.78 | No missing | + | ||
| 12 | 26 | 45,X[55]/46,XY[45] | Primary sterility | AZO | 165 | Male/left and right TV: 12 mL, 14 mL | 7.96 | 6.91 | 3.96 | 19.01 | No missing | + | |
| 13 | 29 | 45,X[66]/46,XY[34] | Primary sterility | AZO | 164 | Male/left and right TV: 8 mL, 10 mL | 13.2 | 28 | 4.00 | 40.8 | AZF(b+c) | + | |
| 14 | 33 | 45,X[73]/46,XY[27] | Primary sterility | AZO | 160 | Male/left and right TV: 18 mL, 18 mL | 3.8 | 11.12 | 2.52 | 18.63 | No missing | + | |
| 15 | 41 | 45,X[45]/46,XY[55] | Primary sterility | AZO | 155 | male/left and right TV: 8 mL, 7 mL | 19.25 | 25.16 | 1.68 | 72.06 | No missing | + | |
| Lindhardt et al[10], 2012 | 16 | 20 | 45,X[63]/46,Xdel(Y)(q12)[37] | Delayed puberty | Male/left and right TV:12 mL, 15 mL | AZF(b+c) | + | ||||||
| 17 | 28 | 45,X[20]/46,XY[80] | Infertility | Male/left and right TV: 2 mL, 0 mL | No missing | + | |||||||
| 18 | 33 | 45,X[55.6]/46,XY[44.4] | Infertility | Male/left and right TV: 12 mL, 6 mL | AZF(b+c) | + | |||||||
| 19 | 49 | 45,X[33.3]/46,Xidic(Y)(p)[66.7] | Infertility | Male/left and right TV: 4 mL, 4 mL | AZF(a+b+c) | + | |||||||
| Ren et al[11], 2015 | 20 | 27 | 45,X[50]/46,XY[50] | Primary infertility | N | 14 IU/L | N | N | No missing | + | |||
| Rosa et al[12], 2014 | 21 | 24 | 45,X[33]/46,XY[67] | Primary infertility | AZO | EMS 12 | N | ||||||
| Ketheeswaran et al[13], 2019 | 22 | 39 | 45,X[6]/46,XY[94], peripheral blood; 46,XY, buccal mucosal cells | Primary infertility | N | 181 | Male/left and right TV: 20 mL, 18 mL | 1.8 IU/L | 1.3 IU/L | 10.2 nmol/L | 0.06 nmol/L | ||
| Reddy et al[14], 1998 | 23 | 37 | 45,X[47]/46,XY[53], peripheral blood; 45,X[43]/46,XY[57], testis | Infertility | AZO | Male/atrophic testis | |||||||
| Gassó-Matoses et al[15], 1992 | 24 | 33 | 45,X[85]/46,XY[15], testis | Infertility | AZO | 157 | Bilateral small testis | 10 | 17.2 | 430 ng/dL | |||
| Li et al[16], 2013 | 25 | 23 | 45,X[19]/46,XY[81] | Primary infertility | AZO | 162 | N | AZFc | + | ||||
| 26 | 25 | 45,X[15]/46,XY,Yqh-[85] | Primary infertility | OLIGO | 158 | N | No missing | + | |||||
| 27 | 30 | 45,X[15]/46,XY[85] | Primary infertility | AZO | 163 | N | AZF(b+c) | + | |||||
| 28 | 24 | 45,X[56]/46,X,dic(Y)[44] | Primary infertility | AZO | 157 | N | AZFc | + | |||||
| 29 | 36 | 45,X[55]/46,XY,Yp+[45] | Primary infertility | AZO | 155 | N | No missing | + | |||||
| 30 | 26 | 45,X[22]/46,X,dic(Y)[78] | Primary infertility | AZO | 168 | N | No missing | + | |||||
| Kilic et al[17], 2010 | 31 | 28 | 45,X[5]/46,XY[95] | Infertility | AZO | Testicular diameters: 3 cm × 3.5 cm | 16 | 21 | 2.21 | AZFb | + | ||
| 32 | 25 | 45,X[20]/46,XY[80] | Primary infertility | OLIGO | Testicular diameters: 3.2 cm × 2.5 cm | 18 | 26 | 2.8 | |||||
| 33 | 32 | 45,X[45]/46,XY[55] | Primary infertility | AZO | Testicular diameters: 4.0 cm × 2.8 cm | 17 | 23 | 2.77 | AZF(b+c) | + | |||
| Valetto et al[18], 2004 | 34 | 41 | 45,X[71]/46,X,idic(Yp)[26]/46,XY[3] | Infertility | AZO | 155 | N | AZF(b+c) | + | ||||
The process of sexual differentiation begins in the early stages of human embryo development. After a series of complex and orderly procedures, bipotential gonads eventually develop into testes or ovaries. The SRY gene plays a critical role in the cascade of events of sexual differentiation. The histogenesis of testis is initiated by the SRY gene, beginning at about 6 wk post-implantation[21,22]. On the other hand, a deletion mutant of the SRY gene can affect masculinization and may cause 46,XY female sex reversal[23]. Even though the SRY gene is critical in the initiation of testis determination, some of the SRY-negative phenotype may show the typical male phenotype, as seen in the present patient. Our findings further suggest that testicular formation and development occurs via a comprehensive process jointly regulated by other key genetic factors or environmental factors in addition to the SRY gene. Several hypotheses have attempted to explain rationally the formation of testicles in SRY-negative males, such as the possible predominance of the 46,XY cell line in the gonads[20], hidden mosaicism for a Y-derivative material, or mutation of an autosomal or X-chromosomal gene downstream from SRY. In addition, studies have shown that overexpression of the SOX9 gene can initiate testis differentiation when the SRY gene is silenced. This result indicates that the SOX9 gene, as downstream factor of SRY, plays an important role in the sex determination process[24-26]. In the present study, the patient refused to undergo genetic analysis of genital skin and gonadal fibroblasts as well as further examinations, and thus, the exact mechanism of his gender development could not be explained.
The AZF gene is located on the long arm of the Y chromosome, and its locus contains protein-coding genes essential for spermatogenesis[27]. Y chromosome microdeletion, which might result from Y-chromosome instability and lead to 45,X karyotype, is one of the key causes of severe male infertility. Clinical statistics indicate that 10%-15% of azoospermic patients and 5%-10% of severe oligospermia patients have Y chromosome microdeletion[6,28]. Studies have reported that deletion of large and submicroscopic Y chromosome may lead to an increased proportion of 45,X abnormal karyotype cells among sperm cells and lymphocytes[29]. In the present case, the patient had deletions of AZF(a+b+c) regions in addition to 45,X/46, XY mosaicism, which may explain the high percentage of 45,X cells and azoospermia.
From the perspective of oncology, gonadal germ cell tumors are detected at an elevated frequency among patients with a 45,X/46,XY karyotype and malformations of the external genitalia[30]. The risk for malignant transformation is reportedly about 10% and increases with age in patients with 45,X/46,XY gonadal dysgenesis[31,32]. It is worth noting that the prognoses of 45,X/46,XY patients with an apparently normal male phenotype until adulthood and patients who are born with severe genital anomalies show no statistical difference[33]. Therefore, adult patients with the 45,X/46,XY mosaic karyotype must be followed up for life, with particular focus on testicular function and testicular tumor screening.
In conclusion, we have described the clinical and genetic findings for a male with complete virilization in SRY-negative 45,X/46,XY mosaicism. We believe that a perfect karyotype analysis and Y-microdeletion analysis could not only reveal the cause of male infertility, in order to facilitate reproductive counseling, but also provide prognostic information for patients with specific karyotypes.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carloni R S-Editor: Chen XF L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Chang HJ, Clark RD, Bachman H. The phenotype of 45,X/46,XY mosaicism: an analysis of 92 prenatally diagnosed cases. Am J Hum Genet. 1990;46:156-167. [PubMed] |
| 2. | Layman LC, Tho SP, Clark AD, Kulharya A, McDonough PG. Phenotypic spectrum of 45,X/46,XY males with a ring Y chromosome and bilaterally descended testes. Fertil Steril. 2009;91:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Telvi L, Lebbar A, Del Pino O, Barbet JP, Chaussain JL. 45,X/46,XY mosaicism: report of 27 cases. Pediatrics. 1999;104:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Zeng YT, Ren ZR, Zhang ML, Huang Y, Zeng FY, Huang SZ. A new de novo mutation (A113T) in HMG box of the SRY gene leads to XY gonadal dysgenesis. J Med Genet. 1993;30:655-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Goodfellow PN, Lovell-Badge R. SRY and sex determination in mammals. Annu Rev Genet. 1993;27:71-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 263] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Zhang YS, Dai RL, Wang RX, Zhang HG, Chen S, Liu RZ. Analysis of Y chromosome microdeletion in 1738 infertile men from northeastern China. Urology. 2013;82:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Mohammadpour Lashkari F, Sadighi Gilani MA, Ghaheri A, Zamanian MR, Borjian Boroujeni P, Mohseni Meybodi A, Sabbaghian M. Clinical aspects of 49 infertile males with 45,X/46,XY mosaicism karyotype: A case series. Andrologia. 2018;50:e13009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Wu Q, Wang C, Shi H, Kong X, Ren S, Jiang M. The Clinical Manifestation and Genetic Evaluation in Patients with 45,X/46,XY Mosaicism. Sex Dev. 2017;11:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Akinsal EC, Baydilli N, Bayramov R, Ekmekcioglu O. A Rare Cause of Male Infertility: 45,X/46,XY Mosaicism. Urol Int. 2018;101:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Lindhardt Johansen M, Hagen CP, Rajpert-De Meyts E, Kjærgaard S, Petersen BL, Skakkebæk NE, Main KM, Juul A. 45,X/46,XY mosaicism: phenotypic characteristics, growth, and reproductive function--a retrospective longitudinal study. J Clin Endocrinol Metab. 2012;97:E1540-E1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Ren H, Chow V, Ma S. Meiotic behaviour and sperm aneuploidy in an infertile man with a mosaic 45,X/46,XY karyotype. Reprod Biomed Online. 2015;31:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Rosa RF, D'Ecclesiis WF, Dibbi RP, Rosa RC, Trevisan P, Graziadio C, Paskulin GA, Zen PR. 45,X/46,XY mosaicism: report on 14 patients from a Brazilian hospital. A retrospective study. Sao Paulo Med J. 2014;132:332-338. [PubMed] |
| 13. | Ketheeswaran S, Alsbjerg B, Christensen P, Gravholt CH, Humaidan P. 45,X/46,XY Mosaicism and Normozoospermia in a Patient with Male Phenotype. Case Rep Med. 2019;2019:2529080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Reddy KS, Sulcova V. Pathogenetics of 45,X/46,XY gonadal mosaicism. Cytogenet Cell Genet. 1998;82:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Gassó-Matoses M, Picó-Alfonso A, Fernández-García J, Lobato-Encinas J, Mira-Llinares A. 45,X/46,XY gonadal dysgenesis in an infertile adult male. Urol Int. 1992;48:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Li LL, Wu J, Dong Y, Zhu HB, Li LL, Liu RZ. [Clinical and cytogenetic analysis of 45,X/46,XY individuals]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013;30:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Kilic S, Yukse B, Tasdemir N, Dogan M, Ozdemir E, Yesilyurt A, Keskin I. Assisted reproductive treatment applications in men with normal phenotype but 45,X/46,XY mosaic karyotype: clinical and genetic perspectives. Taiwan J Obstet Gynecol. 2010;49:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Valetto A, Bertini V, Rapalini E, Baldinotti F, Di Martino D, Simi P. Molecular and cytogenetic characterization of a structural rearrangement of the Y chromosome in an azoospermic man. Fertil Steril. 2004;81:1388-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Martinerie L, Morel Y, Gay CL, Pienkowski C, de Kerdanet M, Cabrol S, Lecointre C, Coutant R, Baron S, Colle M, Brauner R, Thibaud E, Leger J, Nihoul-Fekete C, Bouvattier C. Impaired puberty, fertility, and final stature in 45,X/46,XY mixed gonadal dysgenetic patients raised as boys. Eur J Endocrinol. 2012;166:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Tosson H, Rose SR, Gartner LA. Description of children with 45,X/46,XY karyotype. Eur J Pediatr. 2012;171:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2257] [Cited by in RCA: 2109] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 22. | Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 1454] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 23. | Akinsal EC, Baydilli N, Demirtas A, Saatci C, Ekmekcioglu O. Ten cases with 46,XX testicular disorder of sex development: single center experience. Int Braz J Urol. 2017;43:770-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813-2822. [PubMed] |
| 25. | Huang B, Wang S, Ning Y, Lamb AN, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349-353. [PubMed] [DOI] [Full Text] |
| 26. | Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 460] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 27. | Vogt PH. AZF deletions and Y chromosomal haplogroups: history and update based on sequence. Hum Reprod Update. 2005;11:319-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Suganthi R, Vijesh VV, Vandana N, Fathima Ali Benazir J. Y choromosomal microdeletion screening in the workup of male infertility and its current status in India. Int J Fertil Steril. 2014;7:253-266. [PubMed] |
| 29. | Siffroi JP, Le Bourhis C, Krausz C, Barbaux S, Quintana-Murci L, Kanafani S, Rouba H, Bujan L, Bourrouillou G, Seifer I, Boucher D, Fellous M, McElreavey K, Dadoune JP. Sex chromosome mosaicism in males carrying Y chromosome long arm deletions. Hum Reprod. 2000;15:2559-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Müller J, Ritzén EM, Ivarsson SA, Rajpert-De Meyts E, Norjavaara E, Skakkebaek NE. Management of males with 45,X/46,XY gonadal dysgenesis. Horm Res. 1999;52:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Liu AX, Shi HY, Cai ZJ, Liu A, Zhang D, Huang HF, Jin HM. Increased risk of gonadal malignancy and prophylactic gonadectomy: a study of 102 phenotypic female patients with Y chromosome or Y-derived sequences. Hum Reprod. 2014;29:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Huang H, Wang C, Tian Q. Gonadal tumour risk in 292 phenotypic female patients with disorders of sex development containing Y chromosome or Y-derived sequence. Clin Endocrinol. 86:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Dumeige L, Chatelais L, Bouvattier C, De Kerdanet M, Hyon C, Esteva B, Samara-Boustani D, Zenaty D, Nicolino M, Baron S, Metz-Blond C, Naud-Saudreau C, Dupuis C, Léger J, Siffroi JP, Donadille B, Christin-Maitre S, Carel JC, Coutant R, Martinerie L. Should 45,X/46,XY boys with no or mild anomaly of external genitalia be investigated and followed up? Eur J Endocrinol. 2018;179:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |