Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.6158
Peer-review started: July 11, 2020
First decision: September 24, 2020
Revised: September 30, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: December 6, 2020
Processing time: 146 Days and 2.6 Hours
Some patients present to the intensive care unit due to noninfectious pathologies resulting in fever, especially acute neurological injuries, including brain trauma and intracranial haemorrhage. The cause has been identified to be central hyperthermia characterized by a high core temperature and a poor response to antipyretics and antibiotics. However, no proper guidelines on how to treat central hyperthermia have been developed for clinical practice.
A 63-year-old woman was transferred to our hospital due to injury after a traffic accident. Eight hours after admission, her pupils enlarged bilaterally from 2.5 mm to 4.0 mm. She developed severe coma and underwent decompressive craniectomy. She was diagnosed with central hyperthermia after surgery and was prescribed bromocriptine. The standard dose of bromocriptine could not control her hyperpyrexia, and we prescribed 30 mg a day to control her temperature.
Bromocriptine may be effective in controlling central hyperthermia and have a dosage effect.
Core Tip: Central hyperthermia is characterized by a high core temperature and a poor response to antipyretics and antibiotics. No guidelines on how to treat central hyperthermia have been developed for clinical practice, but bromocriptine has been reported to control the condition effectively. A unique female patient who had central hyperthermia that could not be controlled by a standard dose of bromocriptine was treated in our department. We prescribed 30 mg bromocriptine per day to control her hyperpyrexia.
- Citation: Ge X, Luan X. Uncontrolled central hyperthermia by standard dose of bromocriptine: A case report. World J Clin Cases 2020; 8(23): 6158-6163
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/6158.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.6158
Fever has been reported to be a frequent complication in intensive care unit patients and associated with high mortality and unfavourable prognoses[1]. Noninfectious pathologies resulting in fever, especially acute neurological injuries such as brain trauma and intracranial haemorrhage, affect some patients. The cause has been identified as central hyperthermia, which is characterized by a high core temperature and a poor response to antipyretics and antibiotics[2]. Currently, no guidelines on how to treat central hyperthermia have been developed for clinical practice. However, a multimodal approach that includes the administration of additional medications, such as morphine, fentanyl, surface or intravascular cooling devices, and especially bromocriptine, has been reported[3]. A unique female patient who had central hyperthermia uncontrollable by a standard dose of bromocriptine was treated in our department. We prescribed 30 mg bromocriptine per day to control her hyperpyrexia. The results of a literature search suggest that a high dose of bromocriptine of 30 mg per day to treat central hyperthermia has not been previously reported.
A 63-year-old woman was transferred to our hospital due to injury after a traffic accident.
She was hit broadside, rolled off the car’s roof, and fell to the ground.
In the emergency room, the patient was in a moderate coma [Glasgow Coma Scale (GCS) score 9, E2V2M5]. However, 8 h later, her pupils enlarged bilaterally from 2.5 mm to 4.0 mm. The patient developed a coma (GCS: 6, E1V1M4) and received a computed tomography (CT) scan immediately. Unfortunately, an intracranial pressure monitor was not available in our centre, so a standard fronto-temporo-parietal decompressive craniectomy was performed.
Most of laboratory examinations were normal.
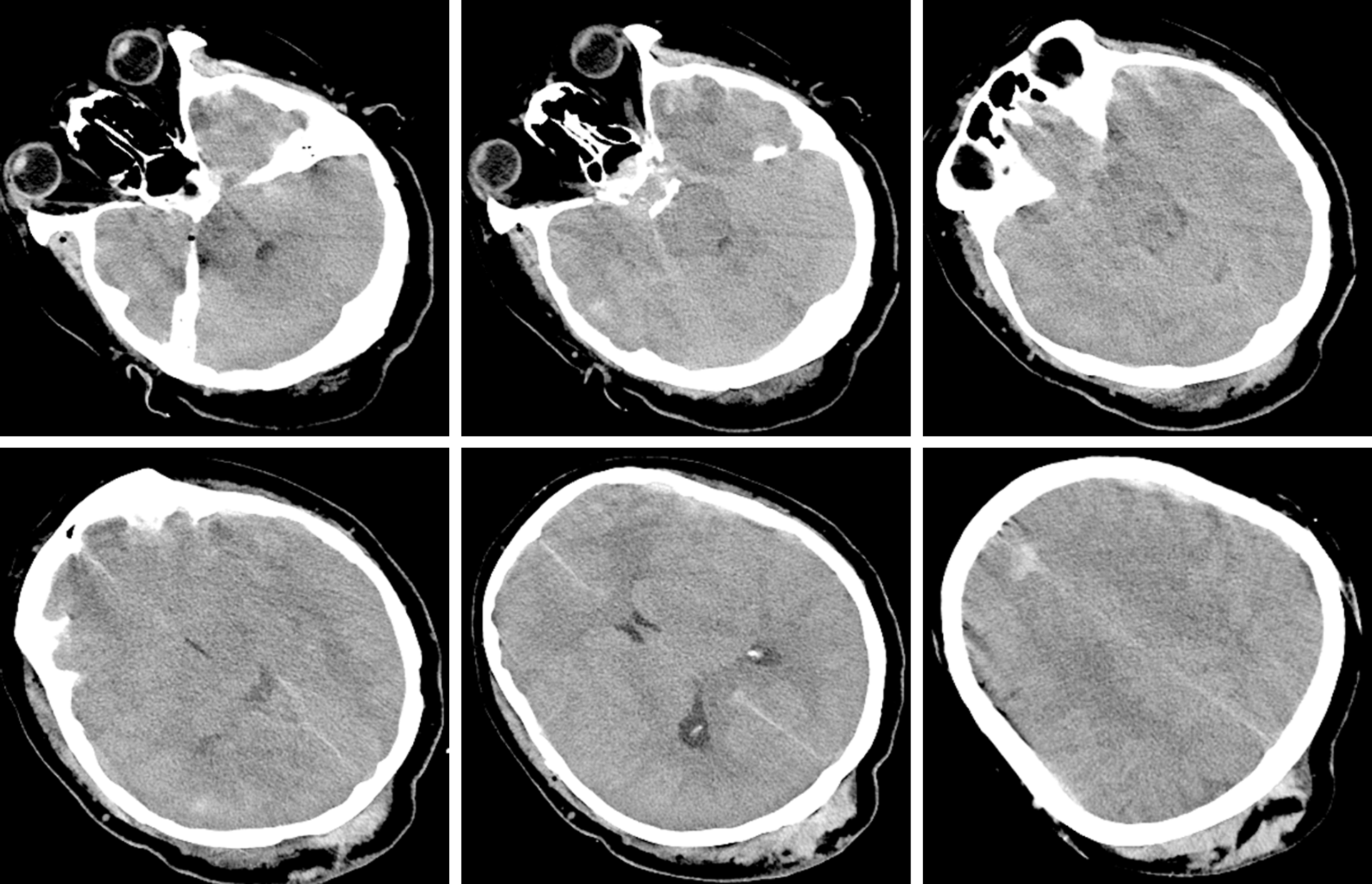
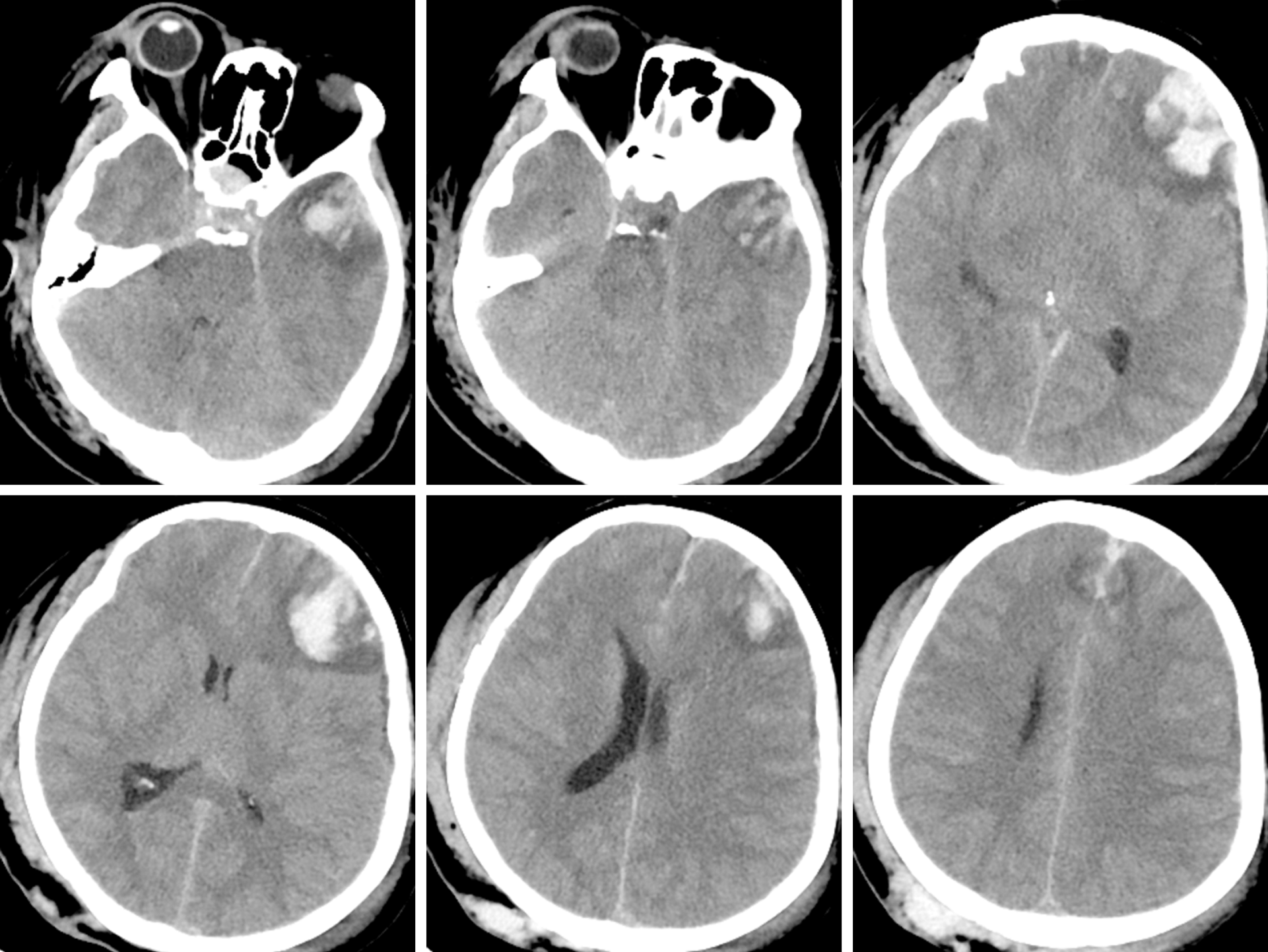
The CT scan in the emergency department illustrated that there were specks of blood in the left frontal and temporal lobes and there was no midline shift (Figure 1). Eight hours later, repeat CT showed that the specks of blood in the left frontal and temporal lobes had become haematomas causing a 9 mm midline shift (Figure 2).
Central hyperthermia.
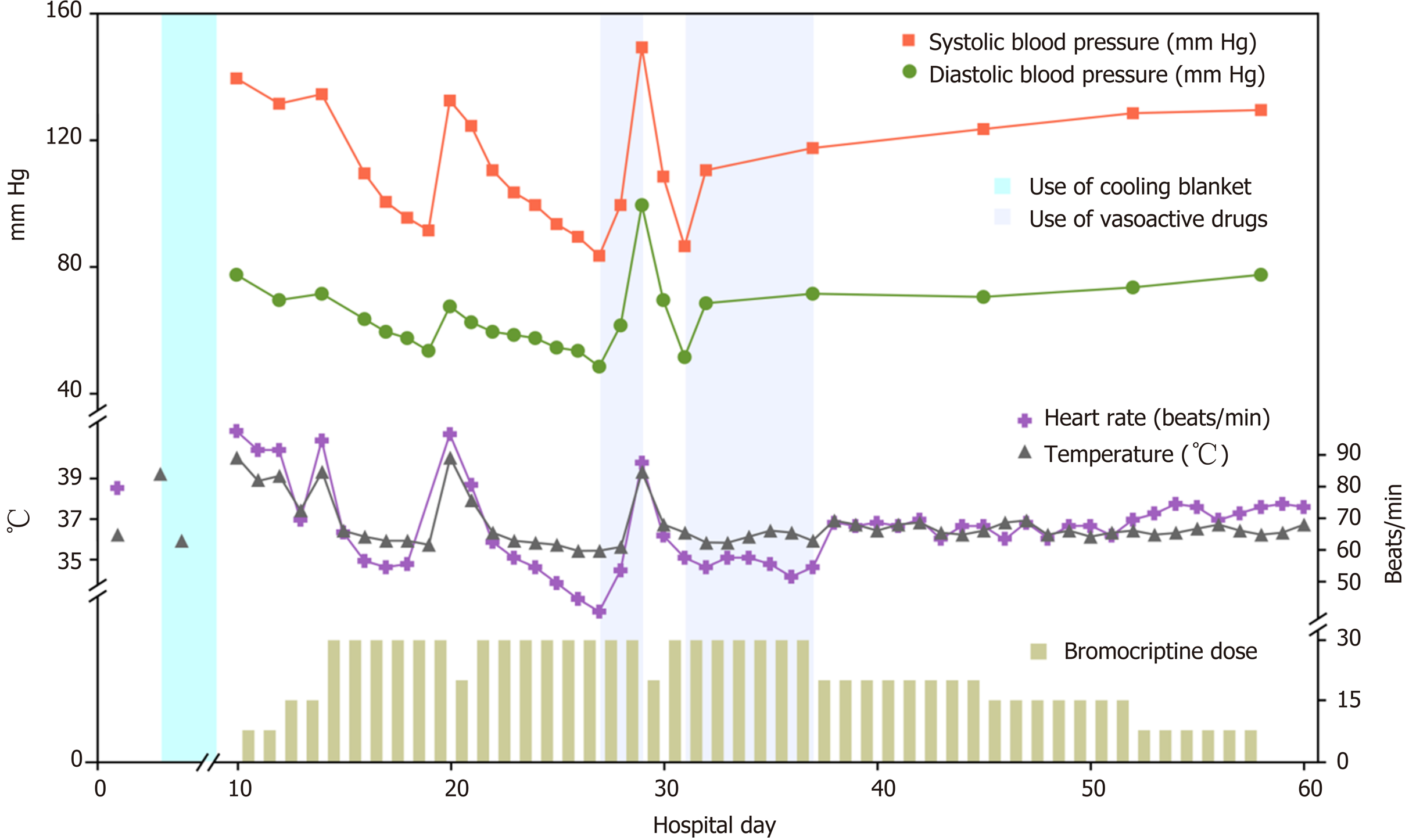
We decided to increase the dose of bromocriptine. Five milligrams of bromocriptine three times a day was prescribed. Sixteen hours later, her temperature dropped to 37.5 °C and remained at this level for a short time; 3 h later, her temperature increased again. This phenomenon was observed three times during the following day. The next day, after obtaining written informed consent from the patient's legally authorized representative, we prescribed 5 mg bromocriptine six times a day. As expected, her temperature dropped to 36.5 °C and remained at this temperature, but her heart rate declined from 95 to 57 per min, and her blood pressure decreased. Five days after her temperature was controlled, we attempted to decrease the dose of bromocriptine to 5 mg four times a day but failed. Her temperature rose again to 40.1 °C and dropped to 38.0 °C for 3 to 4 h. We resumed the previous dose, and her temperature became stable. The side effects of the drug included declines in her heart rate and blood pressure. Her heart rate had decreased to 41 per min but remained stable with vasoactive drugs. Seven days later, we tried to decrease the dose of bromocriptine and failed again. Six weeks after surgery, when bromocriptine had been administered for 34 d, we decreased the dose and managed to successfully stop administration within 2 wk (Figure 3).
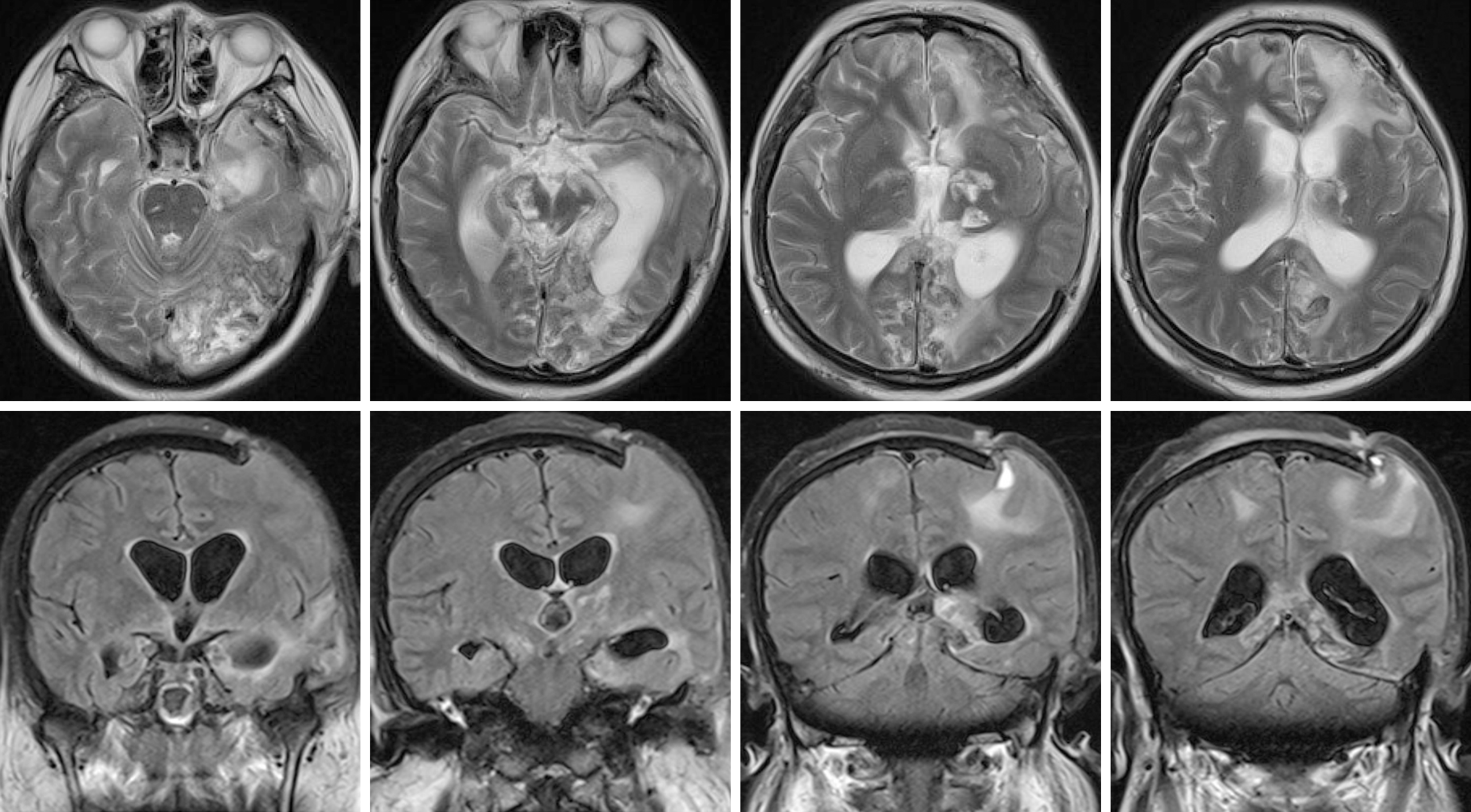
Eight weeks after surgery, her GCS score was 5 (E1VTM3). She underwent a magnetic resonance imaging (MRI) scan, which illustrated speck signals in the midbrain, pontine, and pituitarium and multiple damage signals in the left frontal, temporal, and occipital lobes, basal ganglia, thalamus, and corpus callosum (Figure 4). The MRI findings were supportive of the diagnosis of diffuse axonal injury (DAI). She was sent to rehabilitation center. The GCS score was 8 (E3VTM4) 6 mo later.
An elevated temperature affects not only the brain but also other organs and may increase the risk of mortality[4]. A noninfectious pathology resulting in fever in a patient with severe TBI is not uncommon, and it is known as neurogenic fever[5]. Neurogenic fever is characterized by symptoms of hyperthermia, tachycardia, hyperhidrosis, hypertension, and sometimes seizures, which are referred to as dysautonomia or paroxysmal sympathetic hyperactivity or diencephalic syndrome[6]. However, the symptoms of tachycardia, hyperhidrosis, and hypertension were not found in this patient. A previous study indicated that the characteristics of central hyperthermia may be due to the compression of hypothalamic and brainstem thermoregulatory centres[7]. Some areas, including the preoptic area of the hypothalamus, inputs from spinothalamocortical relay pathways, and the lateral parabrachial nucleus at the junction of the pons and medulla, may regulate the thermo-regulating centres[8].
In this case study, the MRI findings confirmed the diagnosis of DAI. DAI is a rotational acceleration-deceleration traumatic brain injury due to strain or shearing forces. DAI has three distinctive structural features[9]: (1) Diffuse supratentorial damage to axons (grade I); (2) A focal lesion in the corpus callosum (grade II); and (3) A focal lesion or lesions in the rostral brain stem (grade III). Therefore, this patient represents a severe case because the MRI scan showed all of these features. Hypothalamus injuries have been associated with autonomic dysfunction symptoms observed in DAI cases[9]. The compression of the hypothalamus and brainstem may result in the deregulation of the selective loss of warm-sensitive neurons, progesterone or prostaglandin hormonal changes leading to modifications in the firing rate of heat-sensitive neurons in the medial preoptic nucleus, or osmotic changes detected by the organum vasculosum laminae terminalis[10]. Bromocriptine is a dopamine D2 agonist drug that acts on the hypothalamus and the corpus striatum. Its efficacy in the treatment of central hyperthermia[3] and paroxysmal sympathetic hyperactivity[11] has been reported in previous studies because of its effects on dopaminergic transmission. A dose of 7.5 mg per day has been reported and indicated to be effective[3]. We tried to prescribe the same dose to control central hyperthermia but failed. On the basis of the clinical findings, we increased the dose to 30 mg per day, which was successful. The side effects of bromocriptine at high doses during treatment were bradycardia and hypotension. It was found that the higher the dosage of bromocriptine, the worse the bradycardia and hypotension. We used vasoactive drugs such as atropine and norepinephrine to stabilize hemodynamics in patients and succeeded. We attempted to decrease the dose of bromocriptine twice but failed due to severe side effects. Therefore, we conclude that both the effect of bromocriptine in controlling hyperpyrexia and the dosage effect were demonstrated in this study.
This case report demonstrates that bromocriptine may be used to control central hyperthermia, and the dose may affect the clinical effect. Central hyperthermia caused by severe DAI can be treated with high doses of bromocriptine. Side effects should be considered and, if required, managed with vasoactive drugs.
The authors would like to thank the patient and her family.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Smyrniotis V S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Niven DJ, Laupland KB. Pyrexia: aetiology in the ICU. Crit Care. 2016;20:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Yu KW, Huang YH, Lin CL, Hong CZ, Chou LW. Effectively managing intractable central hyperthermia in a stroke patient by bromocriptine: a case report. Neuropsychiatr Dis Treat. 2013;9:605-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Kang SH, Kim MJ, Shin IY, Park DW, Sohn JW, Yoon YK. Bromocriptine for control of hyperthermia in a patient with mixed autonomic hyperactivity after neurosurgery: a case report. J Korean Med Sci. 2012;27:965-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Lee BH, Inui D, Suh GY, Kim JY, Kwon JY, Park J, Tada K, Tanaka K, Ietsugu K, Uehara K, Dote K, Tajimi K, Morita K, Matsuo K, Hoshino K, Hosokawa K, Lee KH, Lee KM, Takatori M, Nishimura M, Sanui M, Ito M, Egi M, Honda N, Okayama N, Shime N, Tsuruta R, Nogami S, Yoon SH, Fujitani S, Koh SO, Takeda S, Saito S, Hong SJ, Yamamoto T, Yokoyama T, Yamaguchi T, Nishiyama T, Igarashi T, Kakihana Y, Koh Y; Fever and Antipyretic in Critically ill patients Evaluation (FACE) Study Group. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care. 2012;16:R33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Meier K, Lee K. Neurogenic Fever. J Intensive Care Med. 2017;32:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Meyfroidt G, Baguley IJ, Menon DK. Paroxysmal sympathetic hyperactivity: the storm after acute brain injury. Lancet Neurol. 2017;16:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 7. | Deogaonkar A, De Georgia M, Bae C, Abou-Chebl A, Andrefsky J. Fever is associated with third ventricular shift after intracerebral hemorrhage: pathophysiologic implications. Neurol India. 2005;53:202-206; discussion 206-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Morrison SF, Nakamura K. Central Mechanisms for Thermoregulation. Annu Rev Physiol. 2019;81:285-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 9. | Frati A, Cerretani D, Fiaschi AI, Frati P, Gatto V, La Russa R, Pesce A, Pinchi E, Santurro A, Fraschetti F, Fineschi V. Diffuse Axonal Injury and Oxidative Stress: A Comprehensive Review. Int J Mol Sci. 2017;18:2600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Rango M, Arighi A, Airaghi L, Bresolin N. Central hyperthermia, brain hyperthermia and low hypothalamus temperature. ClinAuton Res. 2012;22:299-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Deepika A, Mathew MJ, Kumar SA, Devi BI, Shukla D. Paroxysmal sympathetic hyperactivity in pediatric traumatic brain injury: A case series of four patients. AutonNeurosci. 2015;193:149-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |