Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.6122
Peer-review started: June 19, 2020
First decision: September 23, 2020
Revised: October 2, 2020
Accepted: November 2, 2020
Article in press: November 2, 2020
Published online: December 6, 2020
Processing time: 165 Days and 5.8 Hours
Neuronal intranuclear inclusion disease (NIID) is an unusual autosomal dominant, chronic progressive neurodegenerative disease. The clinical manifestations of NIID are complex and varied, complicating its clinical diagnosis. To the best of our knowledge, this report is the first to document sporadic adult-onset NIID mimicking acute cerebellitis (AC) that was finally diagnosed by imaging studies, skin biopsy, and genetic testing.
A 63-year-old man presented with fever, gait unsteadiness, dysarthria, and an episode of convulsion. His serum levels of white blood cells and C-reactive protein were significantly elevated. T2-weighted brain magnetic resonance imaging and fluid attenuation inversion recovery sequences showed bilateral high-intensity signals in the medial part of the cerebellar hemisphere beside the vermis. While we initially considered a diagnosis of AC, the patient’s symptoms improved significantly without special treatment, prompting our consideration of NIID. Diffusion-weighted imaging showed hyperintensity in the corticomedullary junction. Skin biopsy revealed eosinophilic inclusions positive for anti-p62 in epithelial sweat-gland cells. GGC repeat expansions in the Notch 2 N-terminal like C gene confirmed the diagnosis of NIID.
For patients with clinical manifestations mimicking AC, the possibility of underlying NIID should be considered along with prompt rigorous examinations.
Core Tip: While the clinical manifestations of neuronal intranuclear inclusion disease (NIID) are highly variable, a patient with NIID whose primary symptoms indicated acute cerebellitis has hitherto never been reported. We document the rare case of a patient with NIID who did not receive any special treatment and eventually recovered completely. Our report provides evidence of acute cerebellar ataxia and bilateral symmetric cerebellar high-intensity signal as clinical and imaging features of NIID, respectively.
- Citation: Guo JJ, Wang ZY, Wang M, Jiang ZZ, Yu XF. Neuronal intranuclear inclusion disease mimicking acute cerebellitis: A case report. World J Clin Cases 2020; 8(23): 6122-6129
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/6122.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.6122
Neuronal intranuclear inclusion disease (NIID) is a progressive neurodegenerative disease characterized by the presence of eosinophilic hyaline intranuclear inclusions in the central, peripheral, and autonomic nervous systems, as well as visceral organs[1]. NIID is classified according to the age of onset and its genetic etiology: Infantile, adolescent, and adult types; and sporadic and familial types; respectively[2]. NIID induces varying clinical manifestations, including pyramidal and extrapyramidal symptoms, dementia, convulsions, syncope, tremor, and autonomic dysfunction[3]. While a high-intensity signal along the cortical medullary junction on diffusion-weighted imaging (DWI) is a classic imaging feature of NIID[4], the condition has also been associated with cerebellar abnormalities on magnetic resonance imaging (MRI). The poor evaluation of these cerebellar abnormalities often results in misdiagnosis. NIID has recently been attributed to the repeated amplification of GGC sequences of the Notch 2 N-terminal like C (NOTCH2NLC) gene, providing a basis for genetic diagnosis[5].
We herein report the case of a patient with sporadic adult-onset NIID. The patient’s clinical course began with his manifestation of rare symptoms, and a diagnosis of NIID was finally confirmed by pathology and genetic studies. This case report recommends that a diagnosis of NIID be considered when patients present with clinical manifestations resembling acute cerebellitis (AC).
A 63-year-old Chinese man was admitted to the hospital complaining of fever, worsened gait unsteadiness, dysarthria, and vomiting for one day. His wife stated that he experienced a transient episodic convulsion. He was conscious during the convulsion, which had alleviated after 20 s.
During the 4 mo prior to his presentation at the hospital, the patient exhibited gradual deterioration of his memory and attention, as well as the onset of mild depressive symptoms; he would occasionally experience difficulty expressing himself, was unwilling to talk to others, and exhibited indifference during this period. His wife stressed that they did not consider her husband’s condition seriously, as his clinical symptoms were not persistent.
The patient had a 5-year medical history of hypertension and hyperlipidemia. He had been sweating excessively and experienced constipation for about 1 year without any recent weight loss.
The patient denied any history of viral infection or autoimmune disease. He had no notable family history and consumed a moderate amount of alcohol for 30 years.
His vital signs were as follows: Temperature of 38 ˚C, pulse rate of 66 beats/min with a regular rhythm, and blood pressure of 150/90 mmHg. His respiratory rate, oxygen saturation, and chest and abdominal examinations were all normal.
The patient had poor consciousness, dysarthria, and a drawling speech. His pupils were equal in size, round, but abnormally small in diameter (d = 1.5 mm); they were sensitive to direct and indirect light stimulation. He had mild horizontal nystagmus. His muscle strength and tone were normal. His tendon reflexes had diminished. The patient had obvious ataxia and no meningeal irritation. The findings of the other neurological examinations were unremarkable.
The lumbar puncture revealed the following: Intracranial pressure, 140 mmH2O; protein level, 0.72 g/L (normal, 0.15-0.45 g/L); immunoglobulin G, 38 mg/L (normal, 0-34 mg/L); and glucose, ion, and cell number, normal. Routine blood tests revealed that the patient's leukocyte count was high at 13.5 × 109/L (normal, 4-9 × 109/L), with a neutrophilic percentage of 57%. C-reactive protein was found to be 28 mg/L (normal, 0-10 × 109/L). No viruses or bacteria were found in the serum or cerebrospinal fluid. The results of the other laboratory tests were unremarkable.
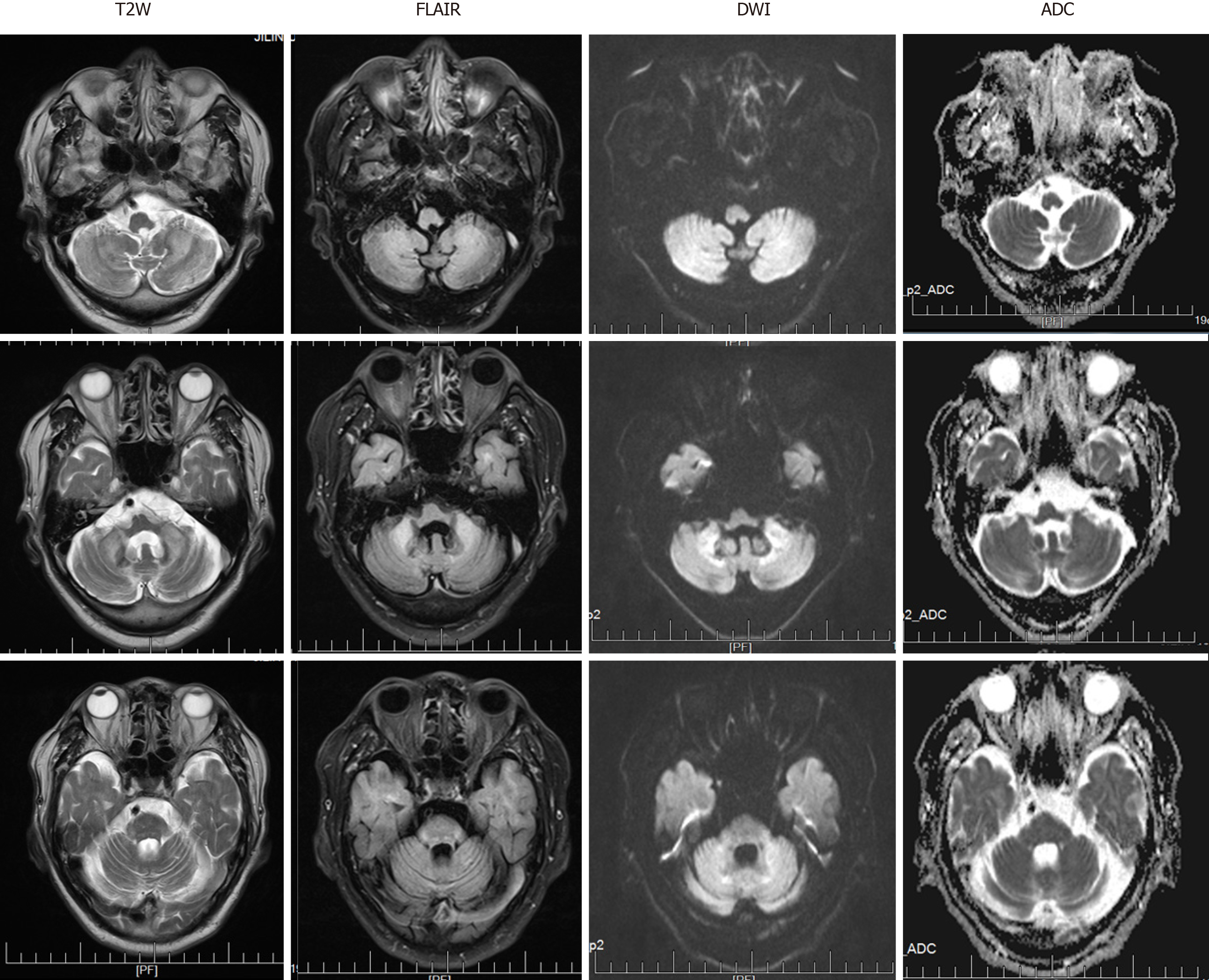
At the level of the cerebellum, MRI revealed symmetrical slightly high-intensity signals in the bilateral cerebral hemispheres, middle cerebellar peduncle, and paravermal regions. These findings were associated with atrophy of the vermis on T2-weighted (T2W) and fluid attenuation inversion recovery (FLAIR) sequences (Figure 1). The corresponding lesions were characterized by high signal intensity on DWI sequences (Figure 1).
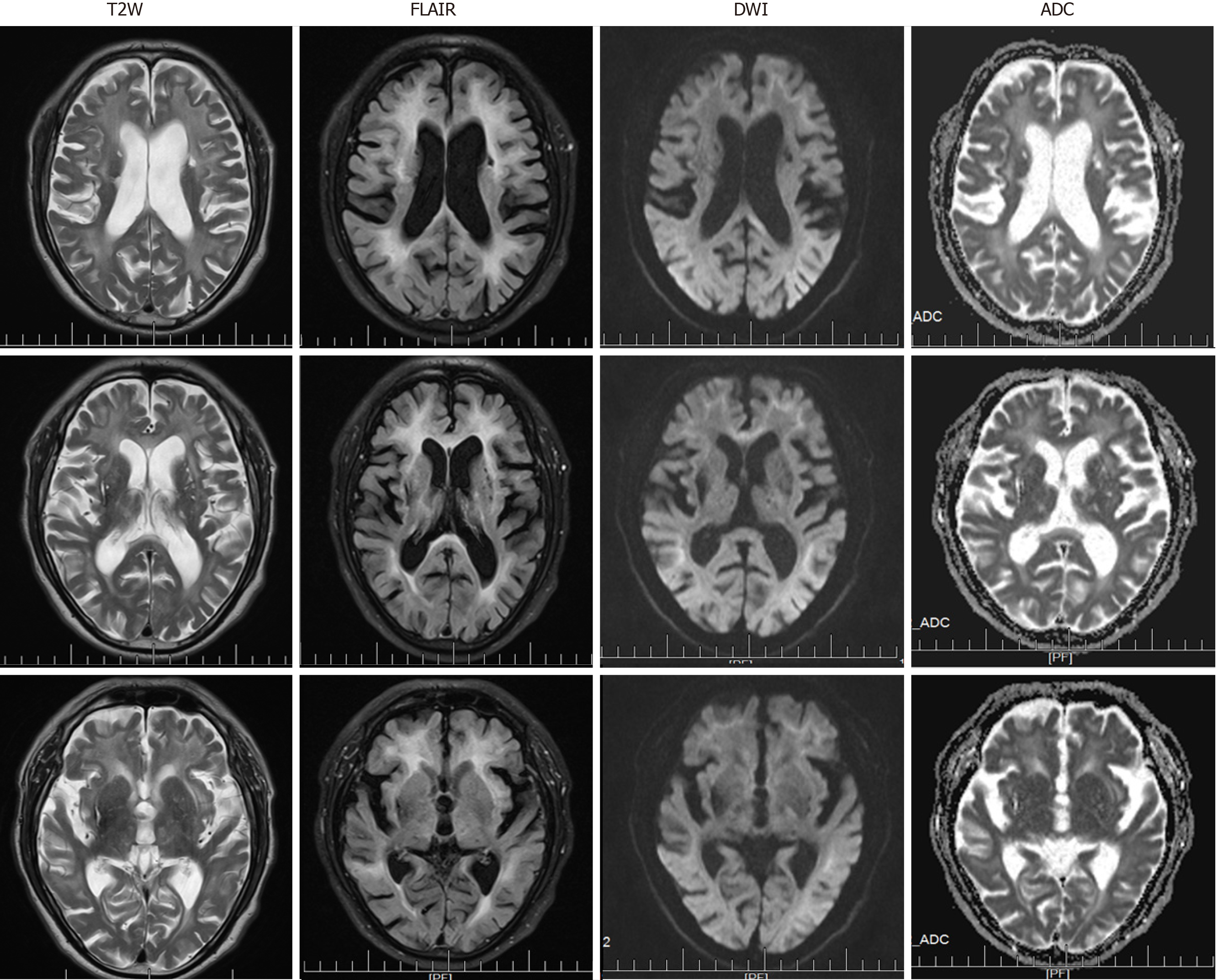
At the level of the cerebrum, T2W images demonstrated multiple old lacunar infarcts in the bilateral basal ganglia and cerebral atrophy (Figure 2). The DWI showed high-intensity signals that extended along the corticomedullary junction but not into the deep white matter (Figure 2). Furthermore, the T2W and FLAIR sequences revealed significant leukoencephalopathy around the paraventricular region of the bilateral lateral ventricles. The corresponding leukoencephalopathy was associated with slightly hyperintense signals on the apparent diffusion coefficient sequence (Figure 2).
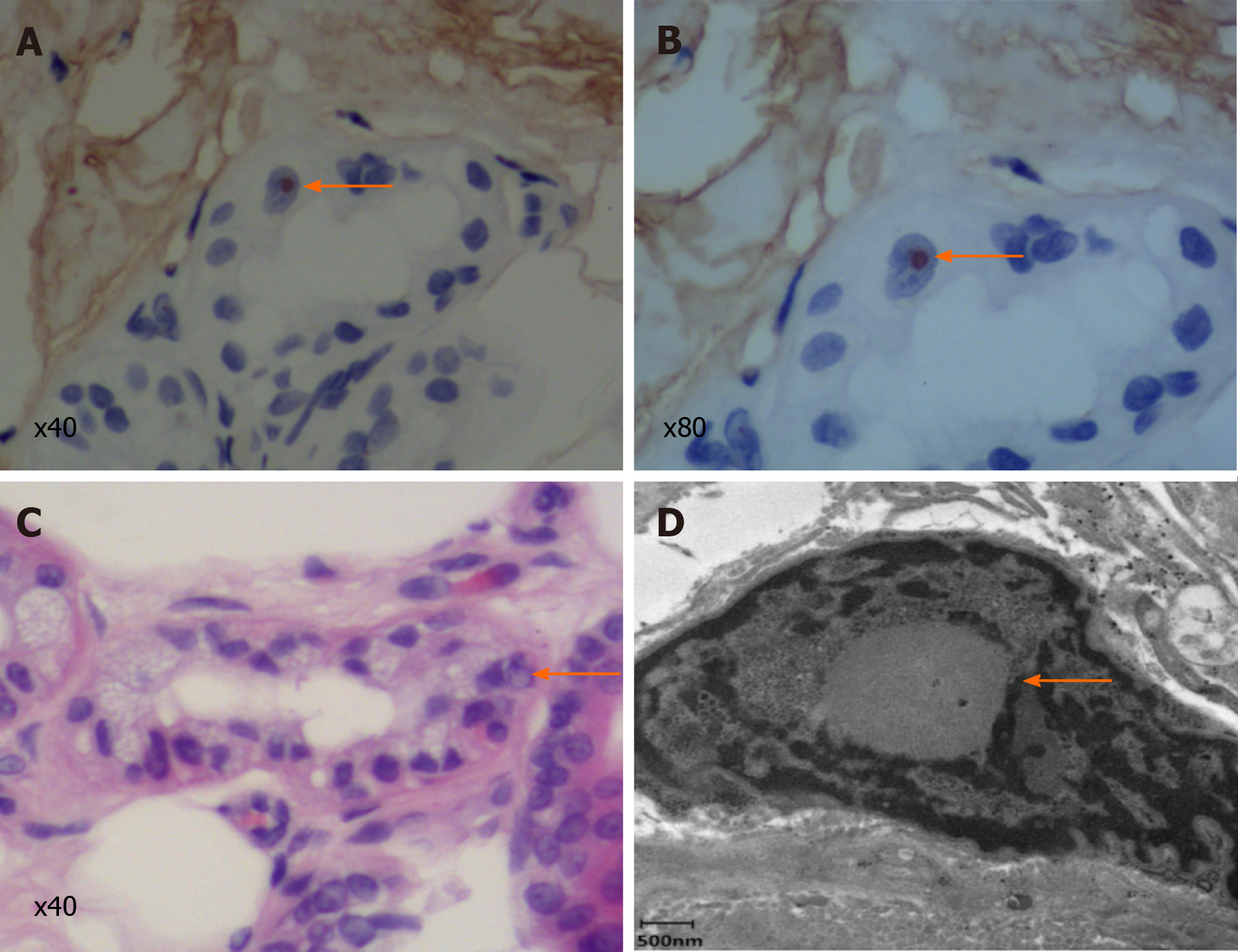
A biopsy specimen of 6 mm in diameter was obtained from 10 cm above the lateral malleolus after the administration of local anesthesia. Hematoxylin and eosin staining revealed eosinophilic intranuclear inclusions in the nucleus of sweat duct epithelium under a light microscope, while immunohistochemical staining showed that the inclusion bodies were positive for anti-p62 (Figure 3). Under an electron microscope, spherical inclusion bodies composed of fibrous substances without membranes were noted (Figure 3).
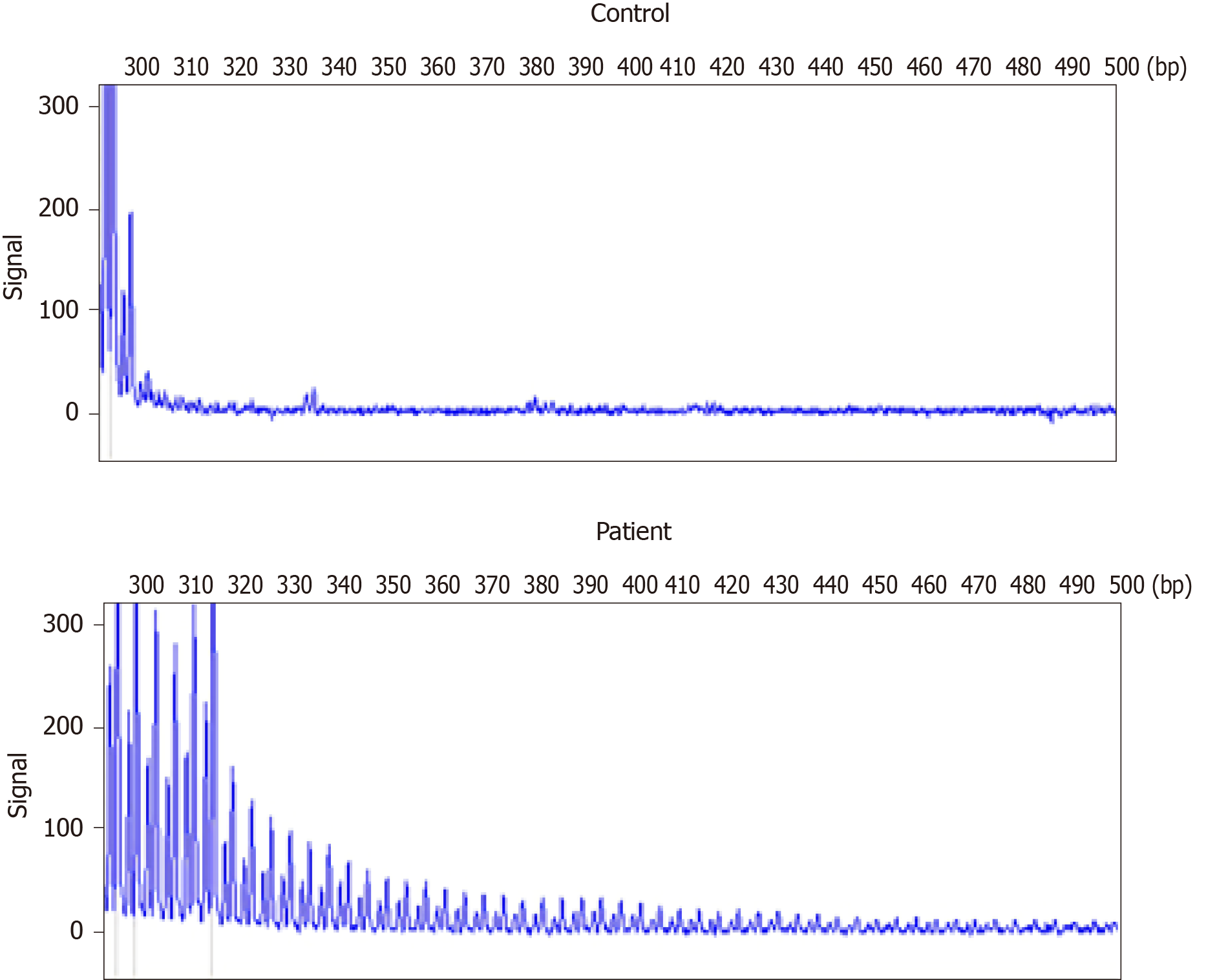
A repeat-primed polymerase chain reaction assay revealed 72 GGC repeats in the 5′ untranslated region of NOTCH2NLC (Figure 4). Screening for the FMR1 gene mutation excluded a diagnosis of fragile X–associated tremor ataxia syndrome.
We excluded infectious, immune-mediated, metabolic, and acute cerebellar ataxia (ACA) with a genetic etiology. The patient was eventually diagnosed with sporadic adult-onset NIID based on the clinical presentation and his imaging, pathology, and genetic testing results.
After the diagnosis of NIID, the patient was not treated with any pharmacotherapy, but rather, with physical cooling, rehydration, and nutritional support therapy.
The patient’s temperature returned to normal on the second day of admission. On the sixth day, he could speak freely with others and walk normally. Dysarthria and nystagmus improved within 2 wk, and his ataxia gradually disappeared. By the 2-mo follow-up, the patient had become able to take care of himself, and there was no change in his score on the neuropsychological rating scale.
The patient considered in the present report presented only with fever and ACA, a common clinical manifestation of AC. Serological examination suggested a high probability of infection. Brain MRI showed symmetrical abnormal signals in the bilateral cerebellum. These findings informed an initial diagnosis of AC, but the absence of pathogens in the patient's serum and cerebrospinal fluid and self-limiting nature of the patient’s symptoms ruled out this diagnosis. This unusual clinical presentation prompted our consideration of rare diseases.
Patients with NIID may reportedly develop either subacute or chronic cerebellar ataxia without any outbreaks. Moreover, the literature also features a report of NIID mimicking an encephalitis attack without cerebellar involvement[6]. In addition to presenting with acute symptoms, the patient also had chronic symptoms, such as dementia and peripheral and autonomic nerve damage that suggested a diagnosis of NIID. Observed in 94.7% of cases of sporadic NIID, dementia is the most common clinical symptom of the disorder and was the patient’s main reason for seeking neurological consultation[3,7]. Peripheral nerve involvement includes sensory disturbance, including the loss of slight and sense of vibration, as well as peripheral limb numbness[3]. A nerve conduction study should be performed in the case without high sign on DWI because over 90% of patients with NIID present with subclinical neuropathy[3,8]. Autonomic involvement includes narrowing of the pupils, dysuria, and constipation[9]. Acute symptoms such as convulsions, syncope, and vomiting tend to be intermittent in patients with NIID[3]. The reversibility of these acute symptoms can help inform a diagnosis of NIID.
In the present case, linear DWI signs and abnormal signals in the bilateral cerebellar hemispheres were accompanied by significant leukoencephalopathy and different degrees of atrophy in the cerebrum and cerebellum. In cases of NIID, abnormal signals observed along the corticomedullary junction on DWI and diffuse white matter hyperintensities on FLAIR have been considered as characteristics of the disease[4,10]. Studies have found that hyperintensity in the white matter is associated with pathological spongiform changes and diffuse non-spongiform myelin pallor[11]. It is worth noting, however, that the latter findings are also associated with diseases of other etiologies, including inflammatory (especially AC), neoplastic, neurodegenerative, metabolic, and demyelinating diseases. Furthermore, symmetrical cerebellar lesions and cerebellar atrophy can also indicate NIID[12,13]. Therefore, NIID can be misdiagnosed as cerebellitis with abnormal signals in the cerebellum or as a multisystem neurodegenerative disease with white matter damage. A recent study showed that only approximately 37.5% of affected familial individuals exhibit typical NIID radiological manifestations[14], which means that histopathological and genetic diagnosis are particularly important.
Skin biopsy is an effective and a less invasive diagnostic tool than autopsy or rectal biopsy with which to diagnose NIID[15]. Under electron microscopy, the intranuclear inclusions are circular, non-membrane bound structures composed of filamentous material. These eosinophilic inclusions are immunopositive for ubiquitin and p62, meaning that they are closely related to the inactivation of the proteolytic enzyme system[16]. However, as similar inclusion bodies have also been found in association with polyglutamine disease, skin biopsy is not a specific diagnostic tool for NIID[17]. Although the accumulation of abnormal proteins or the dysfunction of the nuclear protein degradation system may be the primary pathological cause underlying NIID, other specific etiologies or pathogenetic mechanisms remain obscure.
NIID lacks complete diagnostic criteria. Previously proposed diagnostic criteria include symptomatic and pathological evidence but not genetic findings[3]. Sone et al[5] recently demonstrated that the repeated amplification of the GGC sequence in the non-coding region 5′-untranslated region of human specific NOTCH2NLC gene, also called NBPF1, can cause NIID[5]. This finding refutes previous theories that have attributed NIID to polyglutamine disease. Furthermore, other research studies have implicated the GGC repeat amplification of NOTCH2NLC gene in the pathophysiological processes underlying various neurodegenerative diseases including multiple system atrophy, AD, PD, essential tremor, and leukoencephalopathy[5,18,19]. This literature suggests that NIID-related disorders encompass a spectrum of diseases caused by the expanded GGC repeat within NOTCH2NLC gene[14]. The present report supports this concept by demonstrating that extended mutations in non-coding regions involving the same repeat sequences can indicate overlapping diseases or induce clinical manifestations suggestive of several diseases. It also underscores the possibility that the same genetic cause can underlie various diseases[14,20]. We successfully detected the presence of GGC repeat amplification in our patient and confirmed the diagnosis of NIID. Furthermore, considering the wide distribution of intranuclear inclusions in the patient’s nervous system and other organs, our report supports the possibility of additional phenotypes of NIID. However, the mechanism by which abnormal GGC repeat amplification in the NOTCH2NLC gene leads to these different phenotypes remains unclear.
We document the rare case of a patient with NIID mimicking AC that was eventually diagnosed by imaging, pathology, and genetic evaluations. The wide range of clinical phenotypes of NIID complicates its diagnosis, especially in cases of adult-onset NIID. This report underscores the need to consider the possibility of NIID when patients exhibit fever and ACA with MRI findings of high-intensity signals in the cerebellum.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Park SB S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Deng J, Gu M, Miao Y, Yao S, Zhu M, Fang P, Yu X, Li P, Su Y, Huang J, Zhang J, Yu J, Li F, Bai J, Sun W, Huang Y, Yuan Y, Hong D, Wang Z. Long-read sequencing identified repeat expansions in the 5'UTR of the NOTCH2NLCgene from Chinese patients with neuronal intranuclear inclusion disease. J Med Genet. 2019;56:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Takahashi-Fujigasaki J. Neuronal intranuclear hyaline inclusion disease. Neuropathology. 2003;23:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Sone J, Mori K, Inagaki T, Katsumata R, Takagi S, Yokoi S, Araki K, Kato T, Nakamura T, Koike H, Takashima H, Hashiguchi A, Kohno Y, Kurashige T, Kuriyama M, Takiyama Y, Tsuchiya M, Kitagawa N, Kawamoto M, Yoshimura H, Suto Y, Nakayasu H, Uehara N, Sugiyama H, Takahashi M, Kokubun N, Konno T, Katsuno M, Tanaka F, Iwasaki Y, Yoshida M, Sobue G. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain. 2016;139:3170-3186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 289] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 4. | Han X, Han M, Liu N, Xu J, Zhang Y, Zhang Y, Hong D, Zhang W. Adult-onset neuronal intranuclear inclusion disease presenting with typical MRI changes. Brain Behav. 2019;9:e01477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Sone J, Mitsuhashi S, Fujita A, Mizuguchi T, Hamanaka K, Mori K, Koike H, Hashiguchi A, Takashima H, Sugiyama H, Kohno Y, Takiyama Y, Maeda K, Doi H, Koyano S, Takeuchi H, Kawamoto M, Kohara N, Ando T, Ieda T, Kita Y, Kokubun N, Tsuboi Y, Katoh K, Kino Y, Katsuno M, Iwasaki Y, Yoshida M, Tanaka F, Suzuki IK, Frith MC, Matsumoto N, Sobue G. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet. 2019;51:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 340] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 6. | Li M, Li K, Li X, Tian Y, Shen L, Wu G, Zhang Z, Chen W. Multiple reversible encephalitic attacks: a rare manifestation of neuronal intranuclear inclusion disease. BMC Neurol. 2020;20:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Cupidi C, Dijkstra AA, Melhem S, Vernooij MW, Severijnen LA, Hukema RK, Rozemuller AJM, Neumann M, van Swieten JC, Seelaar H. Refining the Spectrum of Neuronal Intranuclear Inclusion Disease: A Case Report. J Neuropathol Exp Neurol. 2019;78:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Dong H, Ji G, Liu P, Li Y, Tian Y, Shen L, Liu Y, Song X. A case of adult-onset neuronal intranuclear inclusion disease without abnormal high-intensity signal in the corticomedullary junction in diffusion-weighted imaging. Neurol Sci. 2020;41:2653-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Vermilion J, Johnson M, Srinivasan J, Mink JW. Neuronal Intranuclear Inclusion Disease: Longitudinal Case Report of Motor and Nonmotor Symptoms. J Child Neurol. 2019;34:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yu WY, Xu Z, Lee HY, Tokumaru A, Tan JMM, Ng A, Murayama S, Lim CCT. Identifying patients with neuronal intranuclear inclusion disease in Singapore using characteristic diffusion-weighted MR images. Neuroradiology. 2019;61:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Yokoi S, Yasui K, Hasegawa Y, Niwa K, Noguchi Y, Tsuzuki T, Mimuro M, Sone J, Watanabe H, Katsuno M, Yoshida M, Sobue G. Pathological background of subcortical hyperintensities on diffusion-weighted images in a case of neuronal intranuclear inclusion disease. Clin Neuropathol. 2016;35:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Sugiyama A, Sato N, Kimura Y, Maekawa T, Enokizono M, Saito Y, Takahashi Y, Matsuda H, Kuwabara S. MR Imaging Features of the Cerebellum in Adult-Onset Neuronal Intranuclear Inclusion Disease: 8 Cases. AJNR Am J Neuroradiol. 2017;38:2100-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Pedroso JL, Vale TC, Braga-Neto P, Dutra LA, França MC Jr, Teive HAG, Barsottini OGP. Acute cerebellar ataxia: differential diagnosis and clinical approach. Arq Neuropsiquiatr. 2019;77:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Tian Y, Wang JL, Huang W, Zeng S, Jiao B, Liu Z, Chen Z, Li Y, Wang Y, Min HX, Wang XJ, You Y, Zhang RX, Chen XY, Yi F, Zhou YF, Long HY, Zhou CJ, Hou X, Wang JP, Xie B, Liang F, Yang ZY, Sun QY, Allen EG, Shafik AM, Kong HE, Guo JF, Yan XX, Hu ZM, Xia K, Jiang H, Xu HW, Duan RH, Jin P, Tang BS, Shen L. Expansion of Human-Specific GGC Repeat in Neuronal Intranuclear Inclusion Disease-Related Disorders. Am J Hum Genet. 2019;105:166-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 15. | Sone J, Tanaka F, Koike H, Inukai A, Katsuno M, Yoshida M, Watanabe H, Sobue G. Skin biopsy is useful for the antemortem diagnosis of neuronal intranuclear inclusion disease. Neurology. 2011;76:1372-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Nakano Y, Takahashi-Fujigasaki J, Sengoku R, Kanemaru K, Arai T, Kanda T, Murayama S. PML Nuclear Bodies Are Altered in Adult-Onset Neuronal Intranuclear Hyaline Inclusion Disease. J Neuropathol Exp Neurol. 2017;76:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Mackenzie IR, Butland SL, Devon RS, Dwosh E, Feldman H, Lindholm C, Neal SJ, Ouellette BF, Leavitt BR. Familial frontotemporal dementia with neuronal intranuclear inclusions is not a polyglutamine expansion disease. BMC Neurol. 2006;6:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Okubo M, Doi H, Fukai R, Fujita A, Mitsuhashi S, Hashiguchi S, Kishida H, Ueda N, Morihara K, Ogasawara A, Kawamoto Y, Takahashi T, Takahashi K, Nakamura H, Kunii M, Tada M, Katsumoto A, Fukuda H, Mizuguchi T, Miyatake S, Miyake N, Suzuki J, Ito Y, Sone J, Sobue G, Takeuchi H, Matsumoto N, Tanaka F. GGC Repeat Expansion of NOTCH2NLC in Adult Patients with Leukoencephalopathy. Ann Neurol. 2019;86:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Fang P, Yu Y, Yao S, Chen S, Zhu M, Chen Y, Zou K, Wang L, Wang H, Xin L, Hong T, Hong D. Repeat expansion scanning of the NOTCH2NLC gene in patients with multiple system atrophy. Ann Clin Transl Neurol. 2020;7:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 20. | Jiao B, Zhou L, Zhou Y, Weng L, Liao X, Tian Y, Guo L, Liu X, Yuan Z, Xiao X, Jiang Y, Wang X, Yang Q, Li C, Zhu Y, Zhou L, Zhang W, Wang J, Li Y, Gu W, Yang J, Xia J, Huang Q, Yin J, Xue J, Duan R, Tang B, Shen L. Identification of expanded repeats in NOTCH2NLC in neurodegenerative dementias. Neurobiol Aging 2020; 89: 142.e1-142. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |