Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.6095
Peer-review started: July 12, 2020
First decision: September 24, 2020
Revised: October 2, 2020
Accepted: October 27, 2020
Article in press: October 27, 2020
Published online: December 6, 2020
Processing time: 145 Days and 1.8 Hours
Small-cell neuroendocrine carcinoma (SNEC) of the rectum is a rare tumor associated with poor prognosis.
We report a case of a 77-year-old male who came into our hospital because of blood with his stool. An endoscopy revealed a cauliflower-like neoplasm in his rectum. Imaging examination showed that the lesion in the upper rectum was likely rectal cancer, and there was no evidence of metastasis. The patient was treated with surgery. Pathological examination confirmed SNEC of the rectum and an R0 resection was achieved. However, 1 mo after the operation, the patient developed intestinal and ureteral obstructions due to peritoneal metastases. Finally, the patient died from renal failure.
SNEC of the rectum is a high-grade carcinoma with an aggressive phenotype, and surgery should be cautiously considered.
Core Tip: Total mesorectal excision was performed according to pathological analysis, and R0 resection was achieved. However, this patient had tumor recurrence only 1 mo after surgery. Therefore, small-cell neuroendocrine carcinoma of the rectum is a high-grade carcinoma with an aggressive phenotype.
- Citation: Chen ZZ, Huang W, Wei ZQ. Small-cell neuroendocrine carcinoma of the rectum — a rare tumor type with poor prognosis: A case report and review of literature. World J Clin Cases 2020; 8(23): 6095-6102
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/6095.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.6095
Small-cell neuroendocrine carcinomas (SNECs) are malignancies derived from the cells of the neuroendocrine system. Only 2%-2.5% of small-cell carcinomas (SCCs) arise from extra-pulmonary tissues with colorectal SCC accounting for 0.3% of all SCCs and 5.5% of extra-pulmonary SCCs[1]. Most patients with SNEC of the rectum experience symptoms such as defecation difficulties and anal discomfort, similar to those for rectal adenocarcinomas. Only a small number of patients experience carcinoid syndromes, such as hypotension, increased heart rate, and so on. The optimal treatment for rectal SNEC remains a topic of debate. Surgery is still considered the main treatment, and chemotherapy also plays a significant role for localized tumors[2]. The prognosis for extra-pulmonary SCC is poor, and tumor recurrence and metastasis often occur in the short term.
A 77-year-old male was admitted to our hospital with blood with his stool for over 1 mo and a change in bowel habits for the 15 d prior.
The patient noted alteration of blood with his stool a month ago, a change in bowel habit with increased stool frequency (6-8 times a day), a progressive diminution in size of stools, anal discomfort and a weight of loss of 4 kg. There was no abdominal pain, bloating, nausea and vomiting.
The patient’s history included hypertension for over 10 years and early-stage hypertensive nephropathy and atrial fibrillation for 2 years. The noted conditions had been controlled with daily medications, including amlodipine (2.5 mg), valsartan (80 mg), metoprolol (47.5 mg) and aspirin (40 mg).
The patient’s family history was ordinary, and there was no history of genetic heritability of colorectal cancer. The patient had no prior endoscopic examinations.
There were no pertinent findings upon physical examination, and the digital rectal examination did not detect the tumor.
Laboratory tests revealed mild renal insufficiency. Creatinine levels were 113 µmol/L (normal range: 54-106 µmol/L), and uric acid levels were 445 µmol/L (normal range: 149-416 µmol/L). Neuron-specific enolase (NSE) levels were 20.2 U/mL (normal range: < 12.5 U/mL). There were no other hematological or biochemical abnormalities.
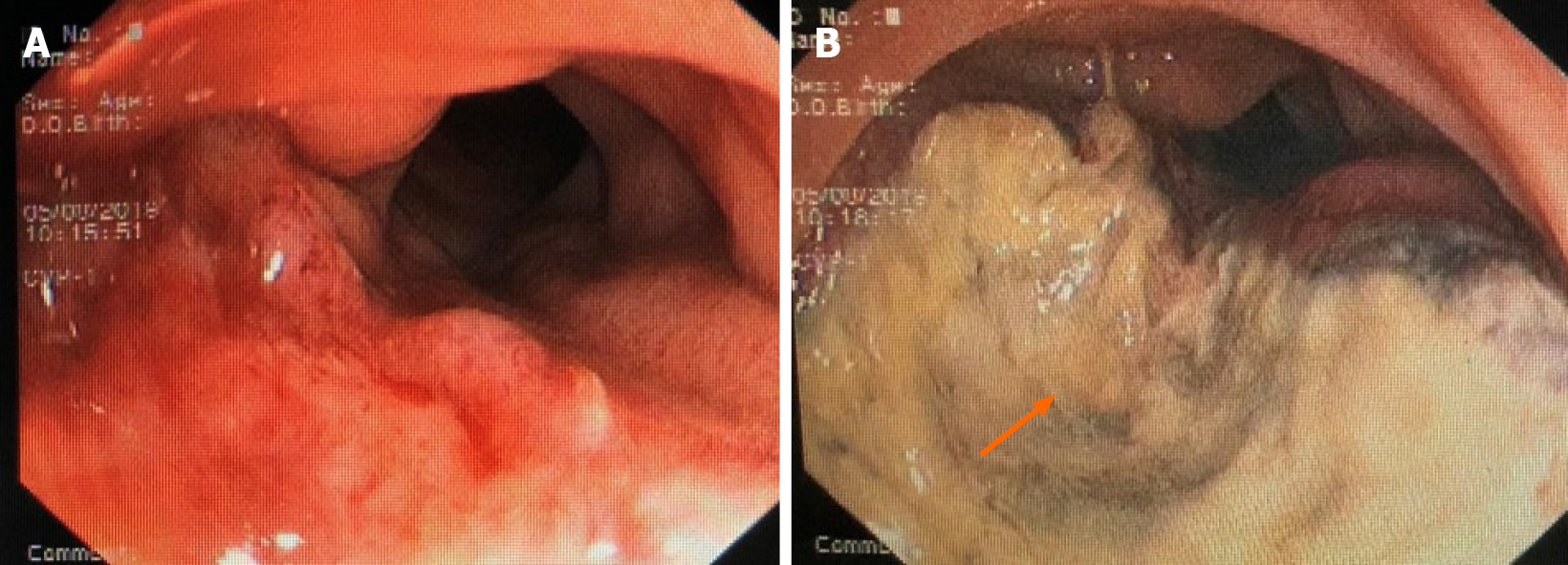
A colonoscopy revealed a cauliflower-like neoplasm located in the upper rectum, which invaded most of the intestinal wall. The intestinal surface also displayed a bleeding lesion and abundant necrotic tissue (Figure 1). The colonoscopy with biopsy determined that the tissue was a malignant tumor. The expression of multiple immunohistochemical markers used to determine the cells of origin, including LCA (-), CK (±), EMA (-), TIF (-), Syn (-), CgA (-), CD56 (+), Ki-67 70% (+),CKL (-), CKH (-), CD68 (-), TIA (-),GRB (-), CD3 (-), CD5 (-), CD7 (-), CD2 (-), CD20 (-), CD79a (-), PAX-5 (+), S100 (-), SOX (-), MelanA (-), MUM-1 (-), CD138 (+), HMB45 (-), Myogenin (-), ERG (-), CD31 (+) and CD34 (-), were negative. Therefore, the tumor type could not be determined.
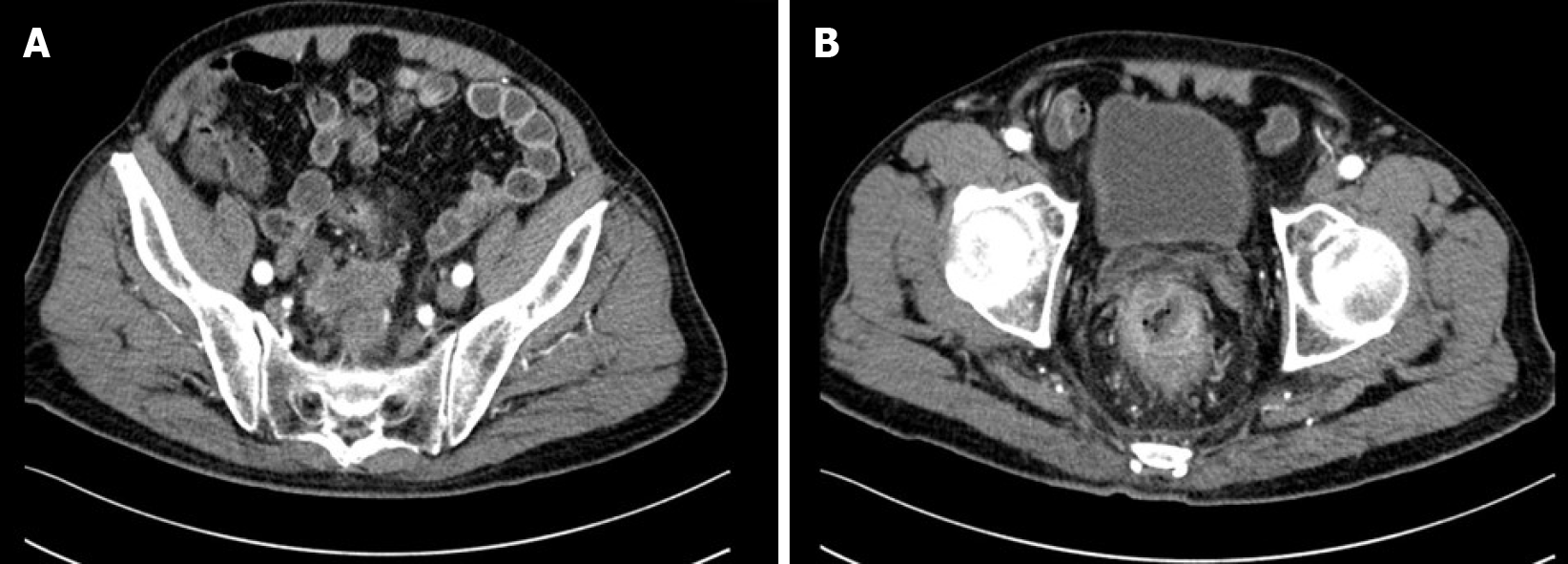
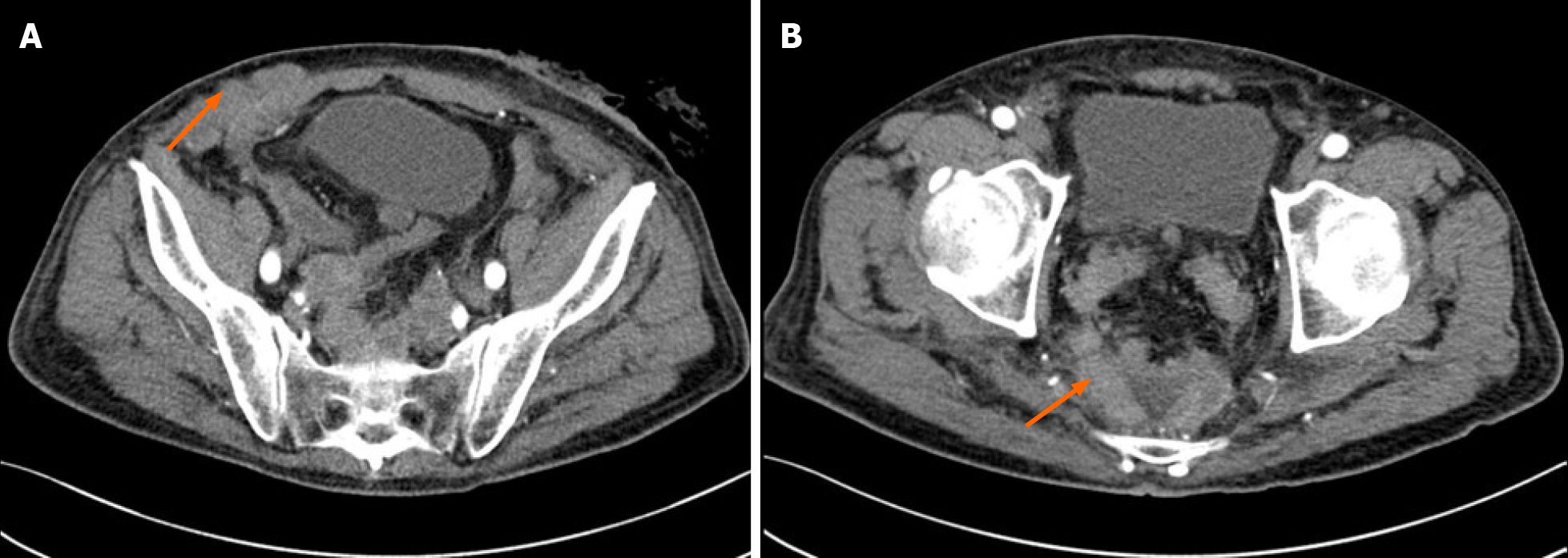
Contrast-enhanced computed tomography scans of the chest, abdomen and pelvis showed that the wall of the upper rectum was unevenly thickened. The lesion was very likely a rectal carcinoma, which broke through the adventitia and involved the mesentery. Multiple surrounding lymph nodes and the adjacent superior rectal artery were positive (Figure 2).
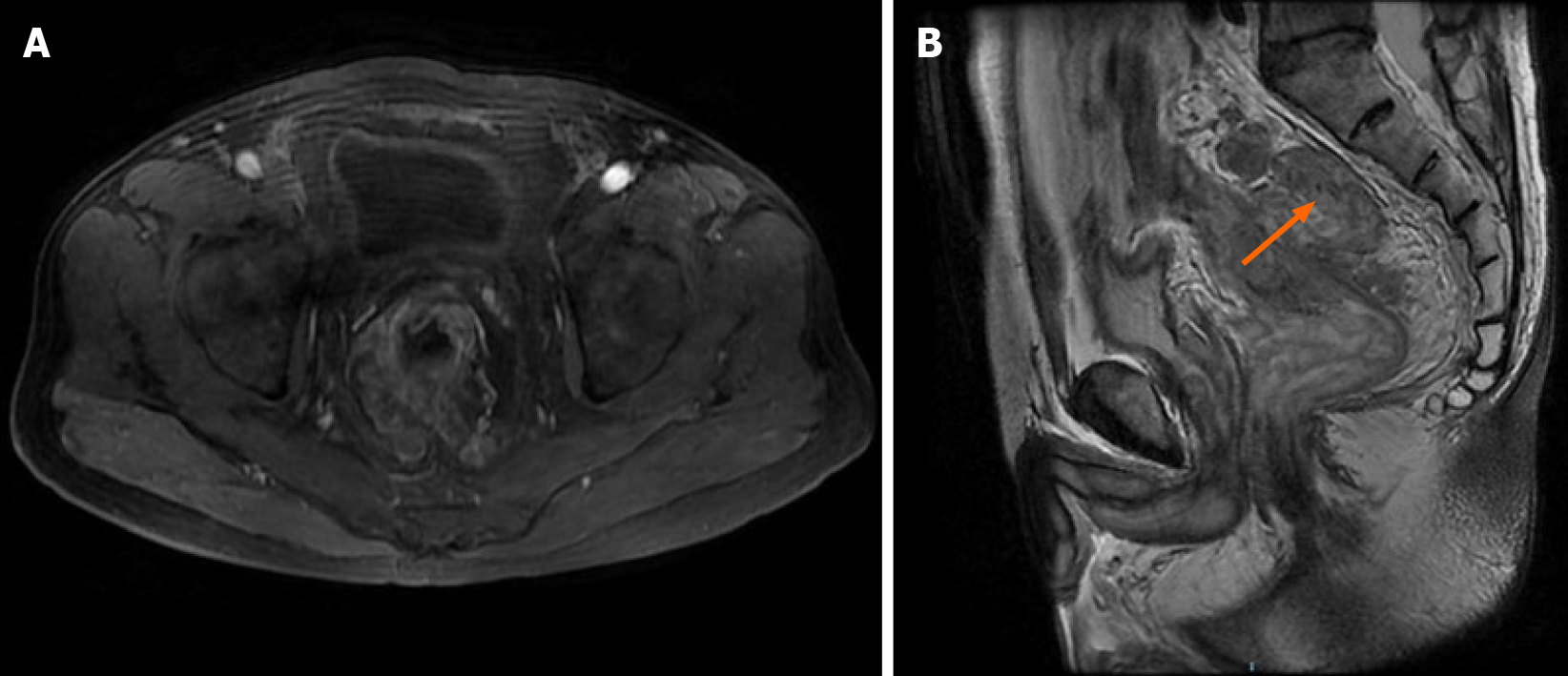
A magnetic resonance image (MRI) of the pelvis revealed the lesion in the upper rectum. The MR stage was T3N2, the circumferential resection margin was positive, and the extramural venous invasion was positive (Figure 3).
A final diagnosis of rectal SCC pT4aN2aM0 IIIC was made.
According to the computed tomography scans, the digital rectal examination did not detect the tumor located in the upper rectum. We did not recommend for this patient to have radiotherapy prior to radical surgery. Because the patient was 77-years-old and had previous underlying diseases including hypertension, atrial fibrillation and hypertensive nephropathy, the patient was treated with radical and elective surgery (Hartmann’s procedure), including proximal fistulation and distal closure and regional lymph node dissection. During the operation, the lesion was found to be located in the upper rectum and was clearly separated from the surrounding tissues. We did not find any metastases in the abdominal cavity. The tumor was completely resected.
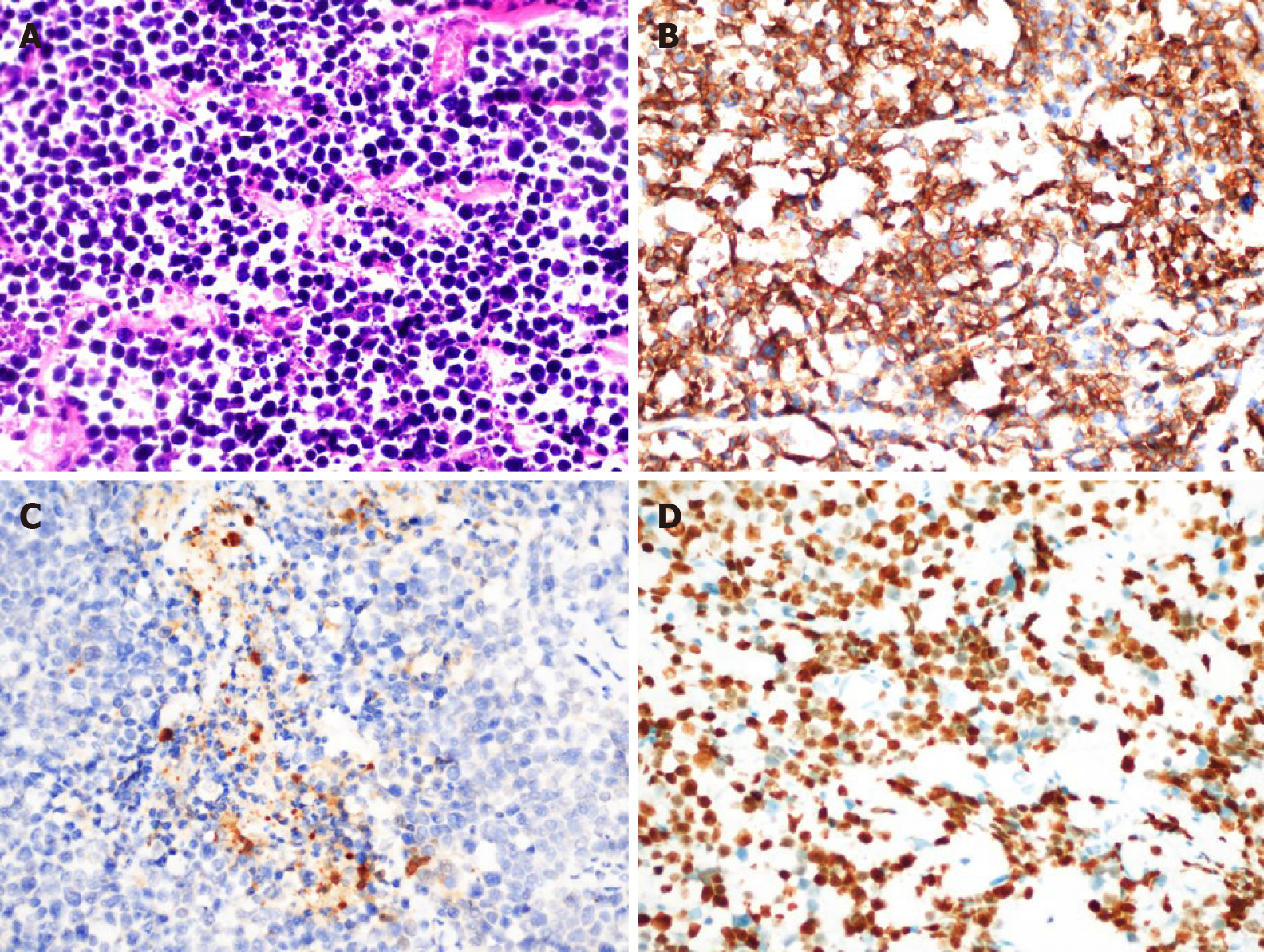
The resected lesion was ulcerated with a size of approximately 4 cm × 3 cm × 0.5 cm. Histopathological analysis revealed a poorly differentiated small-cell tumor. A total mesorectal excision was performed. According to the pathological results after surgery, the tumor was 3 cm from the distal margin, and the distance to the tumor from the far margin of the mesentery was 6 cm. At the same time, the excised mesorectum was intact. The R0 resection for the patient was considered successful because we did not observe tumor infiltration into the surgical margin, including the lateral margin, the distal margin and radial margin by microscopy. However, the lesion infiltrated the visceral peritoneum and peri-intestinal adipose tissue, which did not infiltrate the surrounding organs, and tumor metastases were observed in the peri-intestinal lymph nodes (6/9) (pT4aN2aM0 IIIC). Immunohistochemistry showed CD56, NSE and Ki-67 (80%) positivity (Figure 4), while CD20, myeloperoxidase and S100 were negative.
The patient recovered quickly after surgery and left the hospital within a few days. The patient was later referred to our hospital because of abdominal distension, cessation of defecation and exhaustion at the stoma 1 mo after surgery. Physical examination found that there were multiple nodules in the right lower abdomen. These nodules had a hard texture and unclear borders with sizes of approximately 1.5 cm × 1.5 cm. Contrast-enhanced computed tomography scans of the abdomen and pelvis showed intestinal and ureteral obstructions caused by peritoneal metastases (Figure 5).
In 2020, the World Health Organization subdivided neuroendocrine tumors into three categories: well-differentiated, poorly differentiated (consisting of small-cell and large-cell neuroendocrine carcinoma) and well or poorly differentiated (mixed neuro-endocrine non-neuroendocrine neoplasm)[3].
SNEC of the rectum is a rare tumor[3-5] that accounts for 1% of rectal malignancies[6]; rectal SNECs are derived from enterochromaffin (Kulchitsky) cells[7-9]. Most rectal SNECs produce symptoms similar to those for rectal adenocarcinomas, including defecation difficulties, anal discomfort and blood with the stool[10,11]. These nonspecific symptoms make clinical diagnosis difficult. Therefore, the diagnosis of rectal SNEC depends on pathological assessments, which reveal a morphology of mainly small cells with a thick chromatin layer, scarce cytoplasm, no obvious nucleoli and an increase in mitotic figures[12]. Most rectal SNECs are negative for immunohistochemical markers[13]. Some studies have shown that SCCs are negative for synaptophysin and chromogranin A[14]. Other neuroendocrine markers, such as NSE or CD56, are less specific and must be used with caution[15]. Close attention should be paid to the important role of cell morphology in the diagnosis of rectal SNEC. At the same time, immunohistochemical markers provide us with reference information about rectal SNEC. Recently, some studies proposed to classify neuroendocrine carcinomas by TP53 or RB1 mutations[2].
The prognosis of colorectal SCC is generally poor. The rate of lymph node and liver metastases in colorectal SCC patients are 60%-89% and 20%-71%, respectively[16]. Kumarasinghe et al[17] reported that 55% of patients have metastases at the time of diagnosis with a median survival time of only 10.4 mo. In a study of ten colorectal SCC patients, the median survival time was 5 mo, and all patients died within 11 mo[16]. A study by Burke et al[18] showed that 64% of patients with colorectal SCC die within 5 mo. In our case, the patient passed away 2 mo after surgery.
MRI plays an important role in SCC diagnostics, whereby it can be used for the assessment of the preoperative staging of the tumor. For the patient in this case report, the pathological results showed that the tumor invaded peri-intestinal fat tissue (pT4), which is consistent with a T3 diagnosis by MRI imaging[19,20]. Therefore, the postoperative pathological results were consistent with the preoperative MRI scans.
The optimal treatment for SCC remains a topic of debate. Radical resection is considered the main treatment method with chemotherapy for rectal SNEC helping to control SCC arising from pulmonary tissue[21-23]. Conclusive benefits of radiotherapy for rectal SNEC have yet to be reported[24,25]. There is still controversy in the field over the efficacy of surgery for colorectal SCC. Smith et al[26] demonstrated that resection of primary colorectal neuroendocrine neoplasms does not result in improved prognosis, which is in contrast to adenocarcinoma but consistent with small-cell lung cancer. The prognosis in the Smith et al[26] study was consistent with a study by Palvio et al[27]. However, some studies revealed that there were significantly improved survival rates in gastrointestinal SCC after radical surgery[28,29]. Some studies have demonstrated that surgery may cause damage to the vascular lining by causing the release of reactive oxygen species produced by macrophages leading to exposure of the extracellular matrix and allowing circulating tumor cells to bind[30]. For the patient reported here, there were two main reasons to support performing surgery: (1) The patient did not have metastases; and (2) It was predicted that the lesion could be completely resected. However, surgery did not bring a survival benefit to the patient reported here. The patient’s disease progressed 1 mo following surgery. The time to tumor relapse was short, suggesting that the tumor was an aggressive phenotype.
In summary, this case highlights that SNEC of the rectum is a high-grade carcinoma with an aggressive phenotype and has a poor prognosis, even if the primary resection is radical in nature.
We would like to express our gratitude to all those who contributed to the study. This work was supported by The First Affiliated Hospital of Chongqing Medical University.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Tonelli F, Wani I S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Grossman RA, Pedroso FE, Byrne MM, Koniaris LG, Misra S. Does surgery or radiation therapy impact survival for patients with extrapulmonary small cell cancers? J Surg Oncol. 2011;104:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Shah MH, Goldner WS, Halfdanarson TR, Bergsland E, Berlin JD, Halperin D, Chan J, Kulke MH, Benson AB, Blaszkowsky LS, Eads J, Engstrom PF, Fanta P, Giordano T, He J, Heslin MJ, Kalemkerian GP, Kandeel F, Khan SA, Kidwai WZ, Kunz PL, Kuvshinoff BW, Lieu C, Pillarisetty VG, Saltz L, Sosa JA, Strosberg JR, Sussman CA, Trikalinos NA, Uboha NA, Whisenant J, Wong T, Yao JC, Burns JL, Ogba N, Zuccarino-Catania G. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw. 2018;16:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 3. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington MK, Carneiro F, Cree IA. WHO classification of tumors of the digestive system. Lyon: IARC Press, 2020. |
| 4. | Wang ZJ, An K, Li R, Shen W, Bao MD, Tao JH, Chen JN, Mei SW, Shen HY, Ma YB, Zhao FQ, Wei FZ, Liu Q. Analysis of 72 patients with colorectal high-grade neuroendocrine neoplasms from three Chinese hospitals. World J Gastroenterol. 2019;25:5197-5209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Conte B, George B, Overman M, Estrella J, Jiang ZQ, Mehrvarz Sarshekeh A, Ferrarotto R, Hoff PM, Rashid A, Yao JC, Kopetz S, Dasari A. High-Grade Neuroendocrine Colorectal Carcinomas: A Retrospective Study of 100 Patients. Clin Colorectal Cancer. 2016;15:e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Okuyama T, Korenaga D, Tamura S, Yao T, Maekawa S, Watanabe A, Ikeda T, Sugimachi K. The effectiveness of chemotherapy with cisplatin and 5-fluorouracil for recurrent small cell neuroendocrine carcinoma of the rectum: report of a case. Surg Today. 1999;29:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Kim DH, Lee WY, Yun HR, Choi YC, Cho YB, Yun SH, Kim HC, Chun HK. Neuroendocrine carcinoma of the colon and rectum. J Korean Soc Coloproctol. 2009;25:46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1852] [Article Influence: 84.2] [Reference Citation Analysis (1)] |
| 9. | van der Heijden HF, Heijdra YF. Extrapulmonary small cell carcinoma. South Med J. 2005;98:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Chang S, Choi D, Lee SJ, Lee WJ, Park MH, Kim SW, Lee DK, Jang KT. Neuroendocrine neoplasms of the gastrointestinal tract: classification, pathologic basis, and imaging features. Radiographics. 2007;27:1667-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Levy AD, Sobin LH. From the archives of the AFIP: Gastrointestinal carcinoids: imaging features with clinicopathologic comparison. Radiographics. 2007;27:237-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Sarsfield P, Anthony PP. Small cell undifferentiated ('neuroendocrine') carcinoma of the colon. Histopathology. 1990;16:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 714] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 14. | Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B; all other Frascati Consensus Conference participants; European Neuroendocrine Tumor Society (ENETS). TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1087] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 15. | Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, Caplin M, O'Toole D, Perren A; Vienna Consensus Conference participants. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 421] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 16. | Wick MR, Weatherby RP, Weiland LH. Small cell neuroendocrine carcinoma of the colon and rectum: clinical, histologic, and ultrastructural study and immunohistochemical comparison with cloacogenic carcinoma. Hum Pathol. 1987;18:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Kumarasinghe MP, Weng EK. Pathological features and their prognostic implications in colorectal endocrine cell tumours: a long-term follow-up study. Pathology. 2005;37:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Burke AB, Shekitka KM, Sobin LH. Small cell carcinomas of the large intestine. Am J Clin Pathol. 1991;95:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Beets-Tan RG, Beets GL. Local staging of rectal cancer: a review of imaging. J Magn Reson Imaging. 2011;33:1012-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Heijnen LA, Lambregts DM, Mondal D, Martens MH, Riedl RG, Beets GL, Beets-Tan RG. Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol. 2013;23:3354-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 22. | Wu Z, Yu D, Zhao S, Gao P, Song Y, Sun Y, Chen X, Wang Z. The efficacy of chemotherapy and operation in patients with colorectal neuroendocrine carcinoma. J Surg Res. 2018;225:54-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Mao R, Li K, Cai JQ, Luo S, Turner M, Blazer D 3rd, Zhao H. Adjuvant Chemotherapy Versus Observation Following Resection for Patients With Nonmetastatic Poorly Differentiated Colorectal Neuroendocrine Carcinomas. Ann Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Aytac E, Ozdemir Y, Ozuner G. Long term outcomes of neuroendocrine carcinomas (high-grade neuroendocrine tumors) of the colon, rectum, and anal canal. J Visc Surg. 2014;151:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Modrek AS, Hsu HC, Leichman CG, Du KL. Radiation therapy improves survival in rectal small cell cancer - Analysis of Surveillance Epidemiology and End Results (SEER) data. Radiat Oncol. 2015;10:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Smith JD, Reidy DL, Goodman KA, Shia J, Nash GM. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann Surg Oncol. 2014;21:2956-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Palvio DH, Sørensen FB, Kløve-Mogensen M. Stem cell carcinoma of the colon and rectum. Report of two cases and review of the literature. Dis Colon Rectum. 1985;28:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Fields AC, Lu P, Vierra BM, Hu F, Irani J, Bleday R, Goldberg JE, Nash GM, Melnitchouk N. Survival in Patients with High-Grade Colorectal Neuroendocrine Carcinomas: The Role of Surgery and Chemotherapy. Ann Surg Oncol. 2019;26:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Shafqat H, Ali S, Salhab M, Olszewski AJ. Survival of patients with neuroendocrine carcinoma of the colon and rectum: a population-based analysis. Dis Colon Rectum. 2015;58:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Gül N, Bögels M, Grewal S, van der Meer AJ, Rojas LB, Fluitsma DM, van den Tol MP, Hoeben KA, van Marle J, de Vries HE, Beelen RH, van Egmond M. Surgery-induced reactive oxygen species enhance colon carcinoma cell binding by disrupting the liver endothelial cell lining. Gut. 2011;60:1076-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |