Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.5944
Peer-review started: July 30, 2020
First decision: August 22, 2020
Revised: September 5, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: December 6, 2020
Processing time: 127 Days and 0 Hours
Hernia is a common condition requiring abdominal surgery. The current standard treatment for hernia is tension-free repair using meshes. Globally, more than 200 new types of meshes are licensed each year. However, their clinical applications are associated with a series of complications, such as recurrence (10% - 24%) and infection (0.5% - 9.0%). In contrast, 3D-printed meshes have significantly reduced the postoperative complications in patients. They have also shortened operating time and minimized the loss of mesh materials. In this study, we used the myopectineal orifice (MPO) data obtained from preoperative computer tomography (CT)-based 3D reconstruction for the production of 3D-printed biologic meshes.
To investigate the application of multislice spiral CT-based 3D reconstruction technique in 3D-printed biologic mesh for hernia repair surgery.
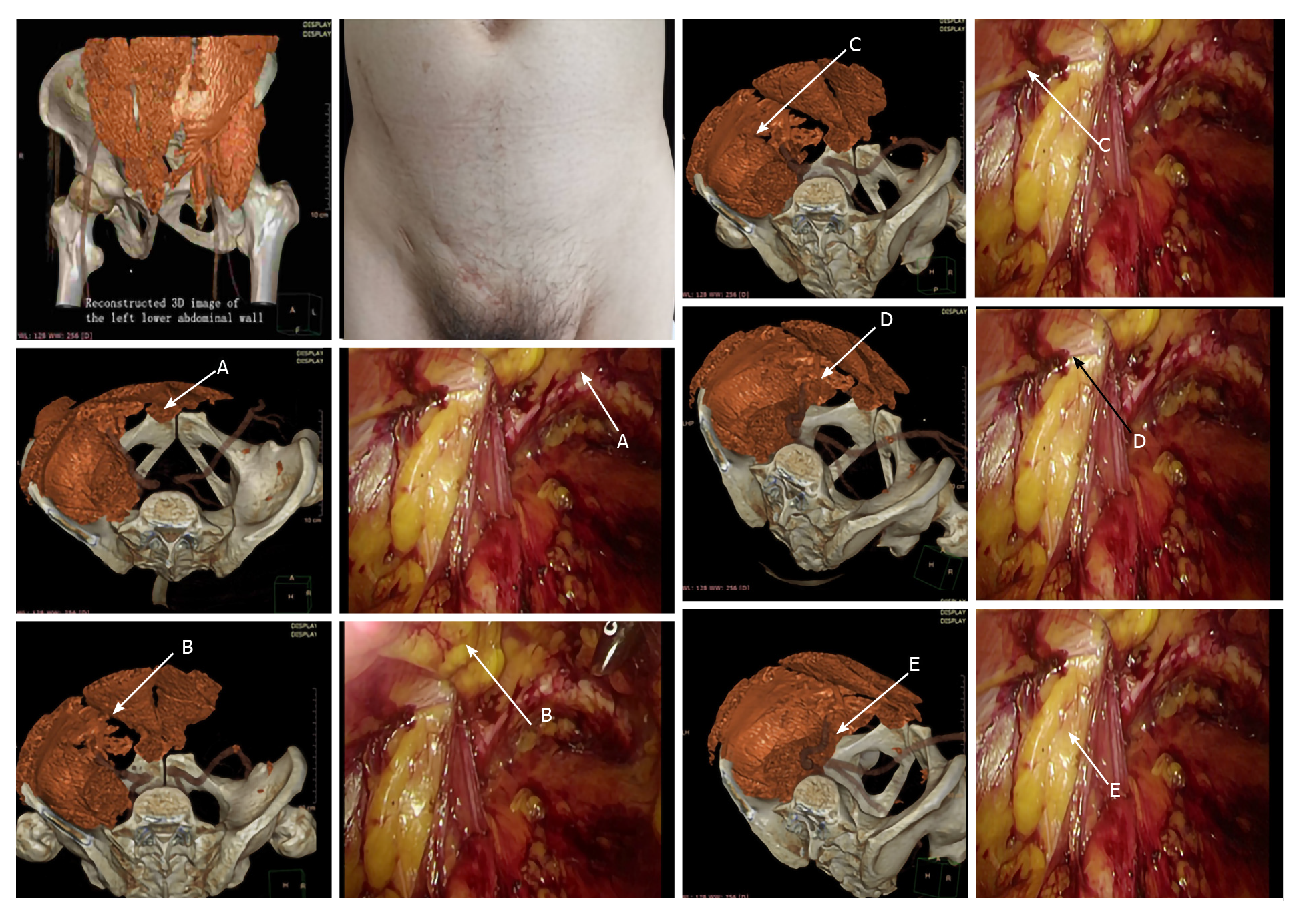
We retrospectively analyzed 60 patients who underwent laparoscopic tension-free repair for inguinal hernia in the Department of General Surgery of the First Hospital of Shanxi Medical University from September 2019 to December 2019. This study included 30 males and 30 females, with a mean age of 40 ± 5.6 years. Data on the MPO were obtained from preoperative CT-based 3D reconstruction as well as from real-world intraoperative measurements for all patients. Anatomic points were set for the purpose of measurement based on the definition of MPO: A: The pubic tubercle; B: Intersection of the horizontal line extending from the summit of the inferior edge of the internal oblique and transversus abdominis and the outer edge of the rectus abdominis, C: Intersection of the horizontal line extending from the summit of the inferior edge of the internal oblique and transversus abdominis and the inguinal ligament, D: Intersection of the iliopsoas muscle and the inguinal ligament, and E: Intersection of the iliopsoas muscle and the superior pubic ramus. The distance between the points was measured. All preoperative and intraoperative data were analyzed using the t test. Differences with P < 0.05 were considered significant in comparative analysis.
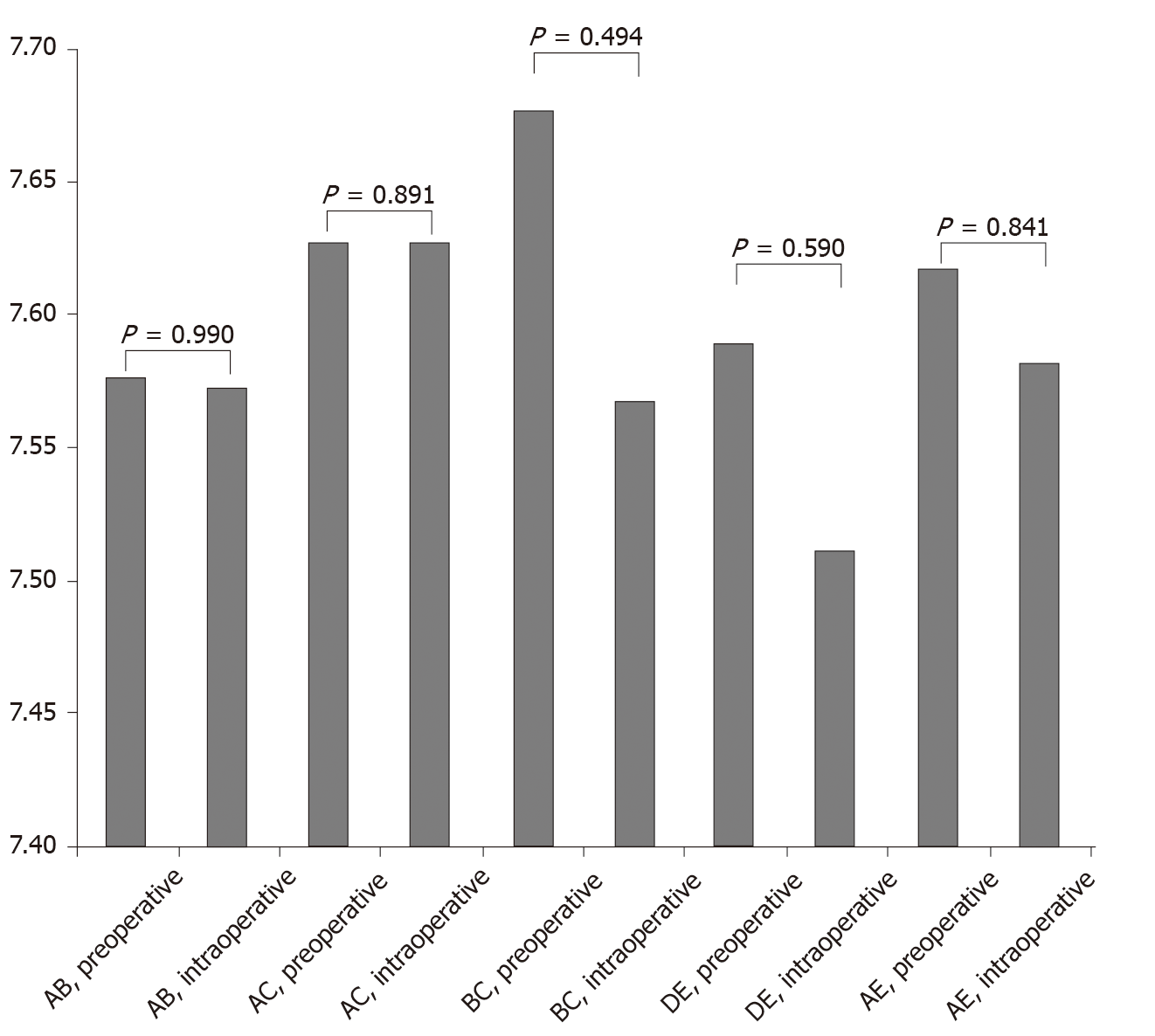
The distance between points AB, AC, BC, DE, and AE based on preoperative and intraoperative data was 7.576 ± 0.212 cm vs 7.573 ± 0.266 cm, 7.627 ± 0.212 cm vs 7.627 ± 0.212 cm, 7.677 ± 0.229 cm vs 7.567 ± 0.786 cm, 7.589 ± 0.204 cm vs 7.512 ± 0.21 cm, and 7.617 ± 0.231 cm vs 7.582 ± 0.189 cm, respectively. All differences were not statistically significant (P > 0.05).
The use of multislice spiral CT-based 3D reconstruction technique before hernia repair surgery allows accurate measurement of data and relationships of different anatomic sites in the MPO region. This technique can provide precise data for the production of 3D-printed biologic meshes.
Core Tip: We investigated the application of multislice spiral computer tomography (CT)-based 3D reconstruction technique in 3D-printed biologic mesh for hernia repair surgery. We retrospectively analyzed 60 patients who underwent laparoscopic tension-free repair for inguinal hernia. Data on the myopectineal orifice (MPO) were obtained from preoperative CT-based 3D reconstruction as well as from real-world intraoperative measurements for all patients. All preoperative and intraoperative data were analyzed using the t test. Their differences were not statistically significant. The use of multislice spiral CT-based 3D reconstruction technique before hernia repair surgery allows accurate measurement of data and relationships of different anatomic sites in the MPO region. This technique can provide precise data for the production of 3D-printed biologic meshes.
- Citation: Wang F, Yang XF. Application of computer tomography-based 3D reconstruction technique in hernia repair surgery. World J Clin Cases 2020; 8(23): 5944-5951
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/5944.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.5944
Hernia is a common condition requiring abdominal surgery. The current standard treatment for hernia is tension-free repair using meshes[1,2]. Globally, more than 200 new types of meshes are licensed each year. They are primarily divided into synthetic and biologic meshes, which are used to repair myopectineal orifice (MPO) defects with different mechanisms. However, their clinical applications are associated with a series of complications, such as recurrence and infection, leading to a bottleneck in the development of hernia repair techniques[3-6]. Statistics show that the recurrence rate after hernia repair surgery ranges from 10% to 24%[7], and postoperative mesh infection rate ranges from 0.5% to 9.0%[8]. This has led to the advent of 3D-printed meshes. Since their emergence, 3D-printed meshes have significantly reduced postoperative complications in patients. They have also shortened operating time and minimized the loss of mesh materials[9,10]. Physicians in Italy have conducted investigations with 3D-printed meshes and achieved considerable progress. However, there is no systematically formulated process of clinical application. When using 3D printing technology in hernia surgery, the first step is the generation of stereoscopic images of the MPO and defect sites for data analysis using computer tomography (CT)-based 3D reconstruction technique. Next, the stereoscopic 3D imaging data is input to a 3D printer. Using exclusive mesh materials and specific computational programs, the 3D printing device gradually stacks the materials layer-by-layer to complete the construction of a “3D mesh”. Currently, the application of CT imaging in hernia repair surgery is mostly focused on preoperative diagnosis and classification[11], determination of mesh size[12], and assessment of postoperative infection of the inguinal region for treatment plan formulation. Reports on the use of CT imaging for the precise assessment of the MPO and sites of hernia and its application to the production of 3D biologic meshes are absent. The purpose of the present study was to conduct statistical analysis of the MPO data obtained from preoperative CT-based 3D reconstruction and real-world intraoperative measurements so as to identify the differences between them and determine whether the multislice spiral CT-based 3D reconstruction technique can be an ideal method to obtain precise data for the production of 3Dprinted biologic meshes.
A total of 60 patients who underwent laparoscopic tensionfree repair for inguinal hernia at the Department of General Surgery of the First Hospital of Shanxi Medical University from September 2019 to December 2019 were retrospectively analyzed. This study included 30 males and 30 females, with a mean age of 40 ± 5.6 years. All patients were confirmed to have no history of diseases that affected the anatomy of the inguinal region, such as disorders of collagen metabolism; history of inguinal hernia; history of lower abdominal surgery; and history of prostate, seminal vesicle, or uterus resection. The patients underwent preoperative thin-slice CT scanning of the inguinal region. Data of the MPO collected through CT-based 3D reconstruction and real-world intraoperative measurements were compared.
The following measurement points were set according to the definition of MPO: A: The pubic tubercle; B: Intersection of the horizontal line extending from the summit of the inferior edge of the internal oblique and transversus abdominis and the outer edge of the rectus abdominis; C: Intersection of the horizontal line extending from the summit of the inferior edge of the internal oblique and transversus abdominis and transversus abdominis and the inguinal ligament; D: Intersection of the iliopsoas muscle and inguinal ligament; and E: The intersection of the iliopsoas muscle and the superior pubic ramus. The distance between points AB, AC, BC, DE, and AE in the patients was measured using the state-of-the-art dual-source dual-energy CT scanner FORCE (Siemens,Berlin, Germany), purchased by our hospital. These line segments were not in the same plane and did not intersect with each other. The region enclosed by their projections was free of muscle and bone tissues, i.e., the stereoscopic 3D structure of the MPO. All intraoperative measurements were obtained during laparoscopic totally extraperitoneal (TEP) inguinal hernia repair: Under general anesthesia, a 10-mm infraumbilical incision was made and extended in layers to the posterior rectus sheath. A pneumoperitoneum was established after a trocar was inserted. A preliminary preperitoneal space was established by advancing the laparoscope with sweeping motions. Next, two trocars were placed at the superior 1/3 and inferior 1/3 of the midline, as instrument portals. The spaces of Retzius and Bogros were fully exposed, and the superior edge of the dissected space was 2 cm beyond the conjoint tendon. Spermatic cord peritoneal stripping must be performed in male patients, while the round ligament of the uterus in female patients could be dissected if necessary. Finally, the distance between points AB, AC, BC, DE, and AE in the operative field was measured using a sterile soft ruler and recorded.
Comparison of the measurement points marked in the CT-based 3D reconstruction images and during the surgery is shown in Figure 1.
The SPSS 20.0 statistical software was used to perform paired sample t-test analysis on the distance between the measurement points of the MPO predicted by the multislice spiral CT-based 3D reconstruction data and the real-world intraoperative measurement data. P > 0.05 indicated that there was no statistical difference between the two sets of data. The distance between the measurement points of the MPO predicted using the multislice spiral CT-based 3D reconstruction technique and that predicted using the real-world intraoperative measurement data was considered to be similar. Comparison of preoperative CT measurement data and intraoperative measurement data is shown in Table 1 and Figure 2.
| Connections between markers | mean ± SD | t value | P value |
| AB, preoperative | 7.576 ± 0.212 | 0.014 | 0.990 |
| AB, intraoperative | 7.573 ± 0.266 | ||
| AC, preoperative | 7.627 ± 0.212 | 0.147 | 0.891 |
| AC, intraoperative | 7.627 ± 0.212 | ||
| BC, preoperative | 7.677 ± 0.229 | 0.752 | 0.494 |
| BC, intraoperative | 7.567 ± 0.786 | ||
| DE, preoperative | 7.589 ± 0.204 | 0.585 | 0.590 |
| DE, intraoperative | 7.512 ± 0.21 | ||
| AE, preoperative | 7.617 ± 0.231 | 0.214 | 0.841 |
| AE, intraoperative | 7.582 ± 0.189 |
The MPO data for all 60 patients were collected by preoperative CT-based 3D reconstruction as well as intraoperative measurements. All surgeries were completed successfully. The preoperative and intraoperative data showed that the distance between points AB, AC, BC, DE, and AE was 7.576 ± 0.212 cm vs 7.573 ± 0.266 cm, 7.627 ± 0.212 cm vs 7.627 ± 0.212 cm, 7.677 ± 0.229 cm vs 7.567 ± 0.786 cm, 7.589 ± 0.204 cm vs 7.512 ± 0.21 cm, and 7.617 ± 0.231 cm vs 7.582 ± 0.189 cm, respectively. The differences were not statistically significant (P > 0.05). These data fully supported that the distance between the measurement points of the MPO predicted using the multislice spiral CT-based 3D reconstruction technique and that predicted using real-world intraoperative measurement data was similar.
Three-dimensional printing technology has been playing an increasingly important role in numerous fields. In the medical field, 3D-printing technology has also been investigated in many disciplines, with some progresses achieved. Specifically in hernia repair surgery, the use of 3D-printed biologic meshes has drawn increasing attention due to their advantages, such as low recurrence rate, infection rate, and frequency of adverse reactions and rapid integration with the body. The first step of 3D-printed biologic mesh production is based on CT-based 3D reconstruction, i.e., generation of stereoscopic images of the MPO and defect sites for data analysis. First, all patients underwent CT of the MPO and defect sites to obtain the tomographic images of the relevant sites. These images were saved and transferred to the computer. Analyses on the scans of the relevant sites were conducted by an experienced physician with the aid of a software. Then, 3D images were constructed by the computer. After filling, 3D models were generated, and the anatomic measurement points were set on the model as mentioned above. The distance between the points was measured. The data obtained from the aforementioned model were compared with the data from the corresponding measurement points obtained from intraoperative measurements. The totally extraperitioneal repair was used for all the operations. The midline trocars layout was set intraoperatively, and trocars could be moved up moderately according to actual needs. It has been confirmed by studies that the final operation effect is not affected by differences in patients’ height, age and BMI[13]. The results showed that the two sets of data were not significantly different. Further reduction in their differences may make the CT-based 3D reconstruction technique an ideal measurement method that can rapidly provide precise data to support the production of 3D-printed biologic meshes.
In summary, the increasing number of hernia operations has led to an increasing demand for meshes. In addition, the gradual improvement of living standards also raises the bar for the requirements on mesh materials and postoperative outcomes. However, existing meshes often lead to a series of complications, such as pain, infection, hematoma, edema, recurrence, and abdominal adhesions. The development of these conditions is mostly associated with inappropriate mesh sizes, postoperative mesh displacement, and inflammation. In addition, 3D-printed biologic meshes are individualized and their production is entirely based on the patient’s MPO and defects. They exhibit precise distribution in 3D space and can fit precisely in the surgical site, which greatly reduces mesh displacement and the risk of recurrence and other postoperative complications. Three-dimensional meshes can also be easily implanted during surgery, greatly reducing operative difficulty and significantly increasing their popularity. Patients’ quality of life can also be significantly improved, allowing them to better serve the community. Biologic meshes produced via 3D printing may benefit the majority of patients in the near future, which will in turn benefit the society.
Hernia is a common condition requiring abdominal surgery. The current standard treatment for hernia is tension-free repair using meshes. Globally, more than 200 new types of meshes are licensed each year. However, their clinical applications are associated with a series of complications, such as recurrence and infection, leading to a bottleneck in the development of hernia repair surgery techniques. Statistics show that the recurrence rate after hernia repair surgery ranges from 10% to 24%, and postoperative mesh infection rate ranges from 0.5% to 9.0%.
The existing drawback has led to the advent of 3D-printed meshes. Since their emergence, 3D-printed meshes have significantly reduced postoperative complications in patients. They have also shortened operating time and minimized the loss of mesh materials. However, it is difficult to obtain accurate data of the pectineal foramen before surgery. This study aims to find a simple, effective, non-invasive and accurate method to provide data support for the production of 3D-printed mesh.
The purpose of the present study was to conduct statistical analysis of the myopectineal orifice (MPO) data obtained from preoperative computer tomography (CT)-based 3D reconstruction and real-world intraoperative measurements so as to identify the differences between them and determine whether the CT-based 3D reconstruction technique can be an ideal method to obtain precise data for the production of 3D-printed biologic meshes.
This was a retrospective analysis of 60 patients who underwent laparoscopic tension-free repair for inguinal hernia in the Department of General Surgery of the First Hospital of Shanxi Medical University from September 2019 to December 2019. This study included 30 males and 30 females, with a mean age of 40 ± 5.6 years. Data on the MPO were obtained from preoperative CT-based 3D reconstruction as well as from real-world intraoperative measurements for all patients. All preoperative and intraoperative data were analyzed using the t test. Differences with P < 0.05 were considered significant in comparative analysis.
The distance between points AB, AC, BC, DE, and AE based on preoperative and intraoperative data was 7.576 ± 0.212 cm vs 7.573 ± 0.266 cm, 7.627 ± 0.212 cm vs 7.627 ± 0.212 cm, 7.677 ± 0.229 cm vs 7.567 ± 0.786 cm, 7.589 ± 0.204 cm vs 7.512 ± 0.21 cm, and 7.617 ± 0.231 cm vs 7.582 ± 0.189 cm, respectively. All differences were not statistically significant (P > 0.05).
The use of multislice spiral CT-based 3D reconstruction technique before hernia repair surgery allows accurate measurement of data and relationships of different anatomic sites in the MPO region. This technique can provide precise data for the production of 3D-printed biologic meshes.
Specifically in hernia repair surgery, the use of 3D-printed biologic meshes has drawn increasing attention due to their demonstrated advantages, such as low recurrence rate, infection rate, and frequency of adverse reactions and rapid integration with the body. The first step of 3D-printed biologic mesh production is based on CT-based 3D reconstruction, i.e., generation of stereoscopic images of the MPO and defect sites for data analysis, which allows the CT-based 3D reconstruction technique to become an ideal measurement method that can rapidly provide precise data to support the production of 3D-printed biologic meshes.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kassir R S-Editor: Huang P L-Editor: MedE-Ma JY P-Editor: Ma YJ
| 1. | Wang P, Huang Y, Gao G, Zhang F, Ye J. Updated interpretation to the International Endohernia Society guidelines for laparoscopic treatment for ventral and incisional abdominal wall hernias. Zhonghua Shan He Fubi Waike Zazhi (Electronic Edition). 2019;13:492-496. |
| 2. | Saxena AK. Surgical perspectives regarding application of biomaterials for the management of large congenital diaphragmatic hernia defects. Pediatr Surg Int. 2018;34:475-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Sun X, Zhang X, Wang J. Surgical outcomes and quality of life post-synthetic mesh-augmented repair for pelvic organ prolapse in the Chinese population. J Obstet Gynaecol Res. 2014;40:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Sun W, Zan P, Ma X, Hua Y, Shen J, Cai Z. Surgical resection and reconstructive techniques using autologous femoral head bone-grafting in treating partial acetabular defects arising from primary pelvic malignant tumors. BMC Cancer. 2019;19:969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Alam AY. The challenge of dealing with animal derived ingredients in medical/surgical products. J Pak Med Assoc. 2017;67:1646-1647. [PubMed] |
| 6. | Pizarro-Berdichevsky J, Borazjani A, Pattillo A, Arellano M, Li J, Goldman HB. Natural history of pelvic organ prolapse in symptomatic patients actively seeking treatment. Int Urogynecol J. 2018;29:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Dumanian GA, Tulaimat A, Dumanian ZP. Experimental study of the characteristics of a novel mesh suture. Br J Surg. 2015;102:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Tanabe K, Mori S, Kita Y, Wada M, Kenji B, Itaru O, Takaaki A, Satoshi I, Kosei M, Natsugoe S. A rare case report of bilateral recurrent inguinal hernia due to persistent Müllerian duct syndrome treated by transabdominal preperitoneal repair. Medicine (Baltimore). 2020;99:e19079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Bökkerink WJV, Koning GG, Malagic D, van Hout L, van Laarhoven CJHM, Vriens PWHE. Long-term results from a randomized comparison of open transinguinal preperitoneal hernia repair and the Lichtenstein method (TULIP trial). Br J Surg. 2019;106:856-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Cybułka B. Inguinal pain syndrome. The influence of intraoperative local administration of 0.5% bupivacaine on postoperative pain control following Lichtenstein hernioplasty. A prospective case-control study. Pol Przegl Chir. 2017;89:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Zhu Z, Dou W, Yue J, Wang J, Wu Q, Chen J, Liang C. Features of multi-slice spiral CT examination of indirect and direct and femoral inguinal hernia in adults. Zhonghua Xiaohua Waike Zazhi. 2018;17:1127-1133. [DOI] [Full Text] |
| 12. | Yang S, Qin W, Dai Y. Application of multi-slice spiral CT 3D reconstruction technique in the selection of mesh sizes for hernia. Zhonghua Shan He Fubi Waike Zazhi (Electronic Edition). 2018;12:142-143. [DOI] [Full Text] |
| 13. | Meyer A, Blanc P, Kassir R, Atger J. Laparoscopic hernia: umbilical-pubis length versus technical difficulty. JSLS 2014; 18: e2014. 00078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |