Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.5894
Peer-review started: June 16, 2020
First decision: July 25, 2020
Revised: August 11, 2020
Accepted: October 19, 2020
Article in press: October 19, 2020
Published online: December 6, 2020
Processing time: 170 Days and 22.7 Hours
Intracranial infection is a common clinical disease. Computed tomography (CT) and magnetic resonance imaging (MRI) have certain sensitivity and have good diagnostic efficacy.
To study the application value of MRI and CT in the diagnosis of intracranial infection after craniocerebral surgery.
We selected 82 patients who underwent craniocerebral surgery (including 40 patients with intracranial infection and 42 patients without infection) during the period from April 2016 to June 2019 in our hospital. All 82 patients received CT and MRI examinations, and their clinical data were reviewed. A retrospective analysis was performed, and the coincidence rate of positive diagnosis and the overall diagnosis coincidence rate of different pathogenic infection types were measured with the two examination methods. The diagnostic sensitivity and specificity as well as the positive and negative predictive values of the two examination methods were compared.
For all types of pathogenic infections (Staphylococcus aureus, Staphylococcus hemolyticus, Staphylococcus epidermidis, and others), MRI scans had higher positive diagnostic coincidence rates than CT scans; the overall diagnostic coincidence rate, sensitivity, specificity, positive predictive value, and negative predictive values were significantly higher with MRI examinations than with CT examinations, and the differences were statistically significant (P < 0.05).
MRI examination can accurately diagnose intracranial infection after clinical craniocerebral surgery. Compared with CT, MRI had higher diagnostic efficiency. The diagnostic sensitivity and specificity, the diagnostic coincidence rate, and the positive and negative predictive values were significantly higher with MRI than with conventional CT, which can be actively promoted.
Core Tip: In this article, the diagnostic value of magnetic resonance imaging and computed tomography for intracranial infection after craniocerebral surgery was assessed.
- Citation: Gu L, Yang XL, Yin HK, Lu ZH, Geng CJ. Application value analysis of magnetic resonance imaging and computed tomography in the diagnosis of intracranial infection after craniocerebral surgery. World J Clin Cases 2020; 8(23): 5894-5901
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/5894.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.5894
Intracranial infection is a common clinical disease and a common complication after craniocerebral surgery[1]. The typical manifestation of patients with intracranial infection is increased intracranial pressure, and various symptoms and signs including disturbance of consciousness, and nerve damage also occur[2-4]. Clinical research suggests that craniocerebral infection will cause irreversible damage to patients’ brain tissue. Failure to provide effective and timely treatment may cause memory loss, seizures, etc., and in severe cases will lead to hemiplegia and even death[5].
Clinical craniocerebral surgery is mainly performed for the treatment of intracranial tumors, intracranial injuries and other diseases. Such patients are prone to intracranial infection after surgical treatment, and relevant examinations should be performed in a timely manner for early effective treatment[6,7]. Commonly used clinical diagnostic tools for intracranial infection include bacteriological examinations, cerebrospinal fluid examinations[8,9], and imaging laboratory examinations, including CT, MRI and other imaging techniques. In recent years, there have been advances in clinical head CT and MRI examinations of the temporal lobe to visualize focal hemorrhagic brain tissue. The softening foci have certain sensitivity and have good diagnostic efficacy[10].
Some scholars believe that diagnosis should include more conventional CT examinations, MRI examinations of additional infected lesions with brain tissue sensitivity, a higher performance of the overall diagnosis, and clinical priority for inspection after brain surgery in patients with intracranial infection[11]. The present study was conducted to verify the diagnostic value of MRI and CT for intracranial infection after craniocerebral surgery.
Data from a total of 40 patients with infection after craniocerebral surgery and 42 patients without infection after craniocerebral surgery admitted to our hospital from August 2017 to October 2019 were collected. The clinical data of these 82 patients were retrospectively analyzed. The inclusion criteria were as follows: indications for surgery related to brain surgery; all patients were given anti-infective treatment in accordance with standard procedures; clinical data were complete; after being informed of the nature of this study, patients were voluntarily included; and the ethics committee of our hospital was informed and approved the study. The exclusion criteria were as follows: Poor compliance and incomplete imaging data; and the multi-item analysis was not in full compliance with the inclusion criteria. Of the 82 patients, 43 were male, and 39 were female; age ranged from 26-56 years, with an average age of 41.52 ± 3.64 years. Forty patients with intracranial infections were diagnosed from cerebrospinal fluid via lumbar puncture, and 16 cases of Staphylococcus aureus infection, 8 cases of Staphylococcus hemolyticus infection, 9 cases of Staphylococcus epidermidis injection, and 7 cases of other types of infection were confirmed.
Patients underwent a secondary MRI and CT examination 7 d after admission and 7 d before discharge. CT examination was performed with a Siemens raw 16-slice spiral CT system, with an axial scanning routine, a controlled layer thickness of 10 mm, and a remaining layer pitch of 10 mm. MRI examination was performed with a Siemens MAGNETOM Skyra3.0T superconducting MRI, and the data parameters used have been reported previously: Rectangular array parameter of 256 × 384, auxiliary sagittal and coronal T1WI, FLAIR (TE 72 ms, TR 6000 ms), TSE T2WI (TR 4600 ms, TE 103 ms), and DWI (TR 6202 ms, TE 92 ms), seT1WI (TR 500 ms, TE 12 ms), and the brain was scanned 2 to 3 times at 1-mm intervals. After completing the routine examinations, all patients underwent enhanced scans, and after obtaining the image data, these were handed over to the senior attending doctor of our hospital for diagnosis.
Taking lumbar puncture for cerebrospinal fluid examination as the gold standard, statistics of the positive diagnosis conformity and overall diagnosis conformity were retrieved for various types of infections (Staphylococcus aureus, Staphylococcus hemolyticus, Staphylococcus epidermidis, and others); the diagnostic coincidence rate = (total number of cases-missed/misdiagnosis number of cases)/total number of cases × 100%.
The sensitivity, specificity, and positive and negative predictive values of the two examination methods were calculated as follows: Sensitivity = true positive/(true positive + false negative) × 100%, specificity = true negative/(true negative + false positive) × 100%, positive predictive value = true positive/(true positive + false positive) × 100%, and negative predictive value = true negative/(true negative + false negative) × 100%.
The data analysis was completed with the assistance of SPSS 22.0 software. Mean ± SD was used to represent the measurement data, which were in accordance with a normal distribution, and the homogeneity of variance was subjected to t tests. The rate (%) indicates the count data, and the χ2 test was performed. The difference was statistically significant when P < 0.05.
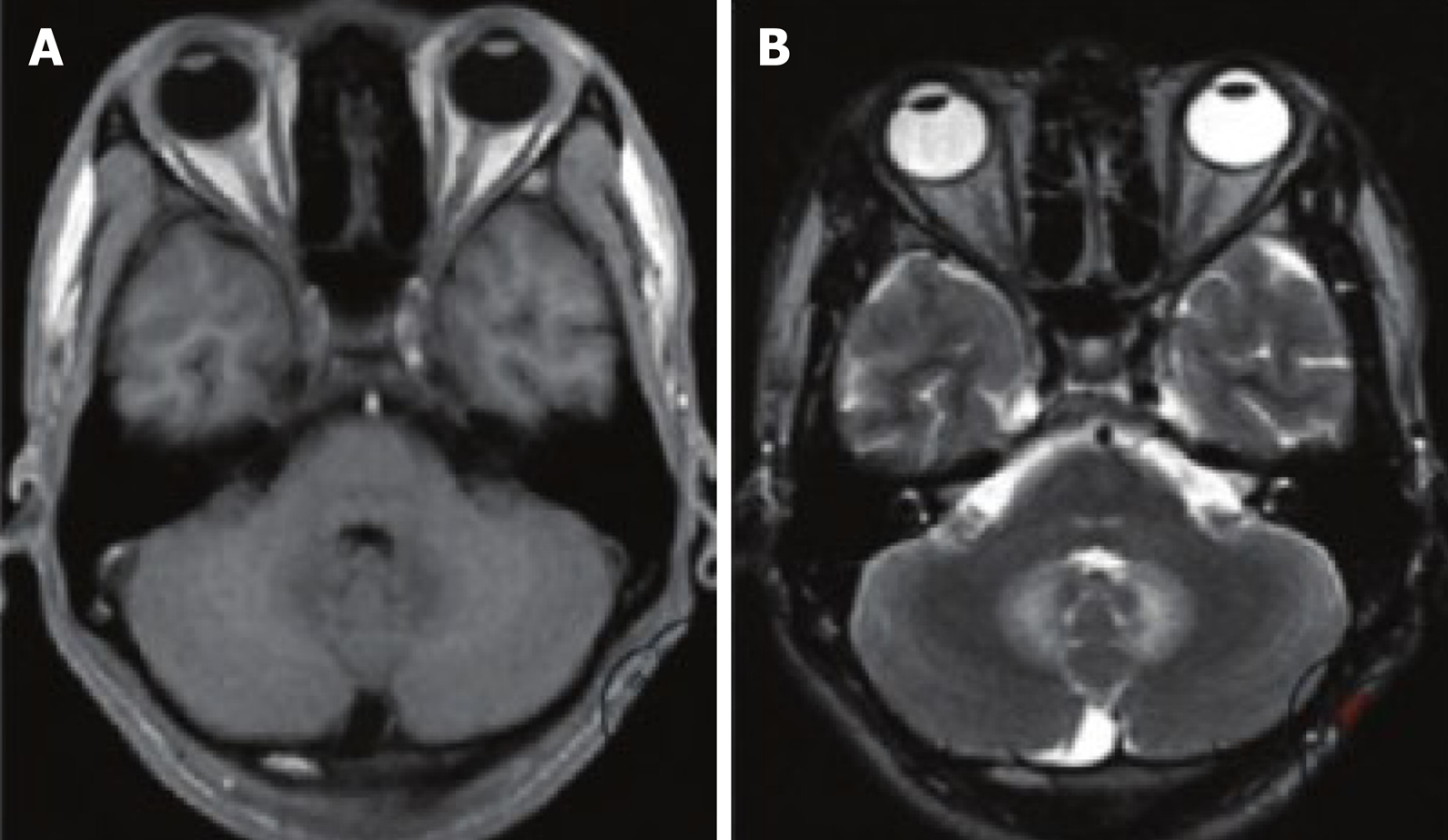
According to CT examination, 26 patients had abnormal CT images, mainly manifesting as diffuse cerebral edema and plaque-like low-density foci. Among them, 18 patients showed high-density enhancement of their lesions and surrounding areas after enhanced scanning. MRI examinations in 39 patients showed abnormal images, mainly due to multiple irregular lengths on Tl and long T2 signal lesions; 21 demonstrated enhanced disease after reflected scanning of patients’ tumors and surrounding signal enhancement (Figure 1).
The statistics showed that the numbers of false-positive and true-positive results in 39 patients with abnormal images on MRI examination were 2 and 37, respectively, and the numbers of false-negative and true-negative results in 43 patients without abnormalities were 3 and 3, respectively. Among the 26 patients with abnormal CT images, 19 had true-positive results, and 7 had false-positive results; and among the 56 patients without abnormalities, 35 had true-negative results, and 21 had false-negative results (Table 1).
| Examination method | True positive (n) | False positive (n) | True negative (n) | False negative (n) |
| CT | 19 | 7 | 35 | 21 |
| MRI | 37 | 2 | 40 | 3 |
The diagnostic sensitivity, specificity, and negative and positive predictive values of the MRI examinations were significantly higher than those of the CT examinations, and the difference was statistically significant (P < 0.05, Table 2)
| Examination method | Sensitivity(%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| CT | 47.50 (19/40) | 83.33 (35/42) | 73.08 (19/26) | 62.50 (39/56) |
| MRI | 92.50 (37/40) | 95.24 (40/42) | 94.87 (37/39) | 93.02 (40/43) |
| χ2 | 48.214 | 7.414 | 17.642 | 26.931 |
| P value | 0.001 | 0.006 | 0.001 | 0.001 |
The data in Table 3 show that the coincidence rate of MRI and CT in infections [Staphylococcus aureus (47.50% vs 25%), Staphylococcus hemolyticus (20% vs 10%), Sta-phylococcus epidermidis (22.5% vs 10%), and others (12.50% vs 2.5%)] was significantly different.
| Examination methods | Number of gold standard positive cases | Staphylococcus aureus infection | Staphylococcus epidermidis infection | Staphylococcus hemolyticus infection | Other |
| CT | 40 | 10 (25.00) | 4 (10.00) | 4 (10.00) | 1 (2.50) |
| MRI | 40 | 19 (47.50) | 8 (20.00) | 9 (22.50) | 5 (12.50) |
| χ2 | - | 10.953 | 3.922 | 5.741 | 7.207 |
| P value | - | 0.001 | 0.048 | 0.017 | 0.007 |
The overall diagnostic coincidence rate of the MRI examinations was significantly higher than that of the CT examinations (93.90% vs 65.85%, P < 0.001, Table 4).
| Examination method | Total number of cases | Missed diagnosis/ misdiagnosis | Diagnostic coincidence rate |
| CT | 82 | 28 (34.15) | 54 (65.85) |
| MRI | 82 | 5 (6.10) | 77 (93.90) |
| χ2 | - | 24.473 | |
| P value | - | 0.001 | |
The brain is an important organ for human physiological regulation, cognition, thinking and other activities. When brain disturbance occurs due to infection, trauma, ischemia or other factors, it may cause serious damage to patients' health and cognitive function[7]. At present, craniocerebral surgery includes open surgery, minimally invasive surgery, etc., with a variety of operative methods, all of which are somewhat invasive and traumatic. Under normal circumstances, brain tissue is protected by the blood-brain barrier and is not easily infected. External pathogens can easily invade brain tissue and cause infectious diseases such as brain abscess and meningitis, which seriously threaten the safety of patients’ lives[12-14].
Current common intracranial infection pathogens include E. coli, Staphylococcus, Proteus, Salmonella, Haemophilus influenzae, etc. Infection caused by the invasion of such pathogens is one of the most common complications of craniocerebral surgery and craniocerebral trauma[15,16]. When intracranial infection occurs, the common clinical manifestations of patients include meningeal irritation, varying degrees of consciousness, and increased intracranial pressure. Relevant analysis suggests that the common causes of intracranial infections are residual foreign bodies, such as hair and skull fragments, as well as shrapnel and cap fragments, when craniocerebral trauma occurs. After such intracranial infections, patients are prone to critical illnesses such as acute cerebral edema and intracranial hematoma. The risk of death is high, and timely corresponding treatment is necessary[17]. It is generally believed that the direct route of intracranial infection includes infection, focal infection, cerebrospinal fluid path of infection, and blood infection from 4 types of infections. Regardless of the route of infection, patients will have irreversible neurological damage, and early diagnosis and early treatment to protect patients’ lives and health are essential[18].
The determination of craniocerebral infection in patients undergoing clinical craniocerebral surgery is generally performed by cerebrospinal fluid examination following lumbar puncture. Although this examination method can effectively determine whether there is intracranial infection in patients and can identify the infected pathogen, it is somewhat traumatic, and the operation is complicated by poor repeatability. With the development of modern imaging technology, an increasing number of clinical diseases can be diagnosed by imaging examinations, contrast puncture examinations, and imaging examinations, which are noninvasive, easy to operate, highly repeatable, more acceptable to patients and have a higher application value[19]. CT and MRI are currently the most frequently used imaging techniques for the examination of brain diseases; these two methods can effectively check the position of lesions in patients, as well as the range, to help physicians intuitively grasp the circumferential surrounding lesion or the absence of diffusion, infiltration, etc. The development of craniocerebral surgery has a guiding role[6].
CT scanning uses X, γ and other rays at a certain thickness of the human body, using corresponding electronic components to receive the rays that have penetrated the layer, digital conversion after processing, and then subjected to a series of image processing to form the subject. Examination methods are part of the image information[20]. MRI consists of the use of a static magnetic field applying ra-diofrequency pulses to the body, causing the body to produce proton magnetic resonance; the proton pulse stops relaxation and generates the MR signal, using the processing electronic MR signals to obtain image information of the portion of the subject. Brain tissue is an important soft tissue organ of the human body, and its main components include protein and fat. Theoretically, MRI examination has higher resolution than CT examination.
Compared with CT examination, MRI examination has a higher sensitivity in terms of brain edema, inflammatory infiltrates, and the presence of a lesion when there is brain edema. Tl-weighted images may be displayed in patients with cerebral gray matter and white matter junctions of irregular shape, and the boundaries have blurred clarity. The low-signal effect of T2-weighted imaging shows a wider range of lesions than that of T1-weighted imaging. When patients have typical meningeal enhancement and thickening, MRI may indicate purulent encephalitis.
Sensitivity, specificity, positive predictive value, and negative predictive value are all effective indicators for testing the diagnostic efficacy of a test method, of which sensitivity mainly reflects the efficacy of the evaluated test method in diagnosing patients, and specificity reflects the elimination of noninfected patients. The efficacy and positive and negative predictive values reflect the proportion of detecting true positive/negative patients and positive/negative patients in the test method, respectively. In this observation, the four diagnostic efficacy evaluation indices of MRI examination were significantly higher than those of CT examination, and MRI examination had a higher positive diagnosis rate of different infection types and overall diagnosis coincidence rate than CT examination (P < 0.05), suggesting that in the diagnosis of intracranial infection, MRI has greater diagnostic performance than CT, both in terms of removing the accuracy of diagnosis of infection and the lack of infection as shown on MRI. In addition to the differences in diagnostic performance, the MRI technology does not involve radiation, and the application safety is high. However, MRI is a new imaging technology, and its examination cost is generally higher than that of CT; thus, its clinical promotion is still hindered. As a result, the positive rate of diagnosis of various types of pathogenic infections by MRI examination was significantly higher than that by CT examination, and for early and late MRI abnormalities, the positive rate of diagnosis was significantly higher with MRI than with CT. The results of this study are similar to those of our previous study, further confirming that MRI has a higher diagnostic value than CT in the diagnosis of intracranial infection.
In summary, in patients with intracranial infection after brain surgery, MRI has a higher diagnostic efficiency than CT, conducive to the preference for carrying out timely detection and early treatment for infection.
Intracranial infection is a common clinical disease and a common complication after craniocerebral surgery. In recent years, there have been advances in clinical head computed tomography (CT) and magnetic resonance imaging (MRI) examinations of the temporal lobe to visualize focal hemorrhagic brain tissue.
To study the application value of MRI and CT in the diagnosis of intracranial infection after craniocerebral surgery and improve the diagnosis rate of intracranial infection.
This study determined the diagnostic value of MRI and CT for intracranial infection after craniocerebral surgery.
We selected 82 patients who underwent craniocerebral surgery. All 82 patients received CT and MRI examinations, and a retrospective analysis was performed. The coincidence rate of positive diagnosis and the overall diagnosis coincidence rate of different pathogenic infection types were measured with the two examination methods.
MRI scans had higher positive diagnostic coincidence rates than CT scans; the overall diagnostic coincidence rate, sensitivity, specificity, positive predictive value, and negative predictive value were significantly higher with MRI examinations than with CT examinations, and the differences were statistically significant (P < 0.05).
MRI can diagnose intracranial infections after clinical craniocerebral surgery more accurately than CT.
To further compare the difference between CT and MRI in the diagnosis of brain diseases.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hashash JG, Kato S, Kim ES S-Editor: Gong ZM L-Editor: Webster JR P-Editor: Zhang YL
| 1. | Jiang L, Guo L, Li R, Wang S. Targeted surveillance and infection-related risk factors of nosocomial infection in patients after neurosurgical operation. Pak J Pharm Sci. 2017;30:1053-1056. [PubMed] |
| 2. | Yu Y, Li HJ. Diagnostic and prognostic value of procalcitonin for early intracranial infection after craniotomy. Braz J Med Biol Res. 2017;50:e6021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Ruan L, Wu D, Li X, Huang Q, Lin L, Lin J, Chen L, Xu P, Jin J, Yang N, Li X. Analysis of microbial community composition and diversity in postoperative intracranial infection using high‑throughput sequencing. Mol Med Rep. 2017;16:3938-3946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Mashiko R, Taguchi S, Tobita T, Shibata Y. Intracranial infection caused by minor skin contusion associated with previous craniotomy. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Bahubali VKH, Vijayan P, Bhandari V, Siddaiah N, Srinivas D. Methicillin-resistant Staphylococcus aureus intracranial abscess: An analytical series and review on molecular, surgical and medical aspects. Indian J Med Microbiol. 2018;36:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Shi ZH, Xu M, Wang YZ, Luo XY, Chen GQ, Wang X, Wang T, Tang MZ, Zhou JX. Post-craniotomy intracranial infection in patients with brain tumors: a retrospective analysis of 5723 consecutive patients. Br J Neurosurg. 2017;31:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Guerin JB, Vork DL, Eguiguren L, Marston AP, Driscoll CLW, Carlson ML, Henry NK, Lane JI. Labyrinthine Sequestrum: A Case Report and Review of the Literature. Otol Neurotol. 2018;39:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Wang H. Higher Procalcitonin Level in Cerebrospinal Fluid than in Serum Is a Feasible Indicator for Diagnosis of Intracranial Infection. Surg Infect (Larchmt). 2020;21:704-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Hrishi AP, Sethuraman M. Cerebrospinal Fluid (CSF) Analysis and Interpretation in Neurocritical Care for Acute Neurological Conditions. Indian J Crit Care Med. 2019;23:S115-S119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Fahradyan A, Ohanisian L, Tsuha M, Park MJ, Hammoudeh JA. An Unusual Complication of Bone Wax Utilization. J Craniofac Surg. 2018;29:976-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Suu-Ire R, Begeman L, Banyard AC, Breed AC, Drosten C, Eggerbauer E, Freuling CM, Gibson L, Goharriz H, Horton DL, Jennings D, Kuzmin IV, Marston D, Ntiamoa-Baidu Y, Riesle Sbarbaro S, Selden D, Wise EL, Kuiken T, Fooks AR, Müller T, Wood JLN, Cunningham AA. Pathogenesis of bat rabies in a natural reservoir: Comparative susceptibility of the straw-colored fruit bat (Eidolon helvum) to three strains of Lagos bat virus. PLoS Negl Trop Dis. 2018;12:e0006311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Lin C, Zhao X, Sun H. Analysis on the risk factors of intracranial infection secondary to traumatic brain injury. Chin J Traumatol. 2015;18:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Yu H, Liu G. [Analysis of factors of intracranial infection after transnasal endoscopic crannialbase approach]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28:1544-1546. [PubMed] |
| 14. | Mo S, Wei L, Chen H, Li R, Li S, Luo G. A chinese case of prevotella intermedia and streptococcus constellatus intracranial mixed infection. Metab Brain Dis. 2018;33:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Hussein K, Rabino G, Feder O, Eghbaryeh H, Zayyad H, Sviri G, Benenson R, Paul M. Risk factors for meningitis in neurosurgical patients with cerebrospinal fluid drains: prospective observational cohort study. Acta Neurochir (Wien). 2019;161:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Akhaddar A, Hall W, Boucetta M. Subgaleal and brain abscesses due to Salmonella enteritidis following craniotomy for giant cell glioblastoma multiforme: A case report and literature review. Surg Neurol Int. 2019;10:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Peng Y, Liu X, Pan S, Xie Z, Wang H. Anti-N-methyl-D-aspartate receptor encephalitis associated with intracranial Angiostrongylus cantonensis infection: a case report. Neurol Sci. 2017;38:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Offiah CE, Naseer A. Spectrum of imaging appearances of intracranial cryptococcal infection in HIV/AIDS patients in the anti-retroviral therapy era. Clin Radiol. 2016;71:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Ramgopal S, Obeid R, Zuccoli G, Cleves-Bayon C, Nowalk A. Lyme disease-related intracranial hypertension in children: clinical and imaging findings. J Neurol. 2016;263:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Sica GT, Norton KI. Intracranial human immunodeficiency virus infection in an infant: sonographic findings. Pediatr Radiol. 1990;21:64-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |