Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.5866
Peer-review started: April 18, 2020
First decision: September 14, 2020
Revised: September 23, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 6, 2020
Processing time: 229 Days and 20.7 Hours
Single umbilical artery (SUA) is the most common umbilical cord malformation in prenatal diagnosis. The presence of an SUA can cause blood circulation disorder in the foetus and functional changes of the foetal heart, affecting foetal circulation. The right ventricular diastolic functions in foetuses with isolated SUA and in normal foetuses in the third trimester were evaluated using the spectral Doppler of blood flow in the foetal ductus venosus (DV).
To evaluate the right ventricular diastolic functions in foetuses with isolated SUA and in normal foetuses in the third trimester.
Colour Doppler was used to measure the spectrum of foetal DV and tricuspid orifice in 34 foetuses with isolated SUA aged 28-39 wk and in age-matched healthy controls. The DV flow velocities and velocity ratios were measured. The early passive/late active (E/A) ratio at the tricuspid orifice and tissue Doppler Tei index of the foetal right ventricular in the two groups were also measured.
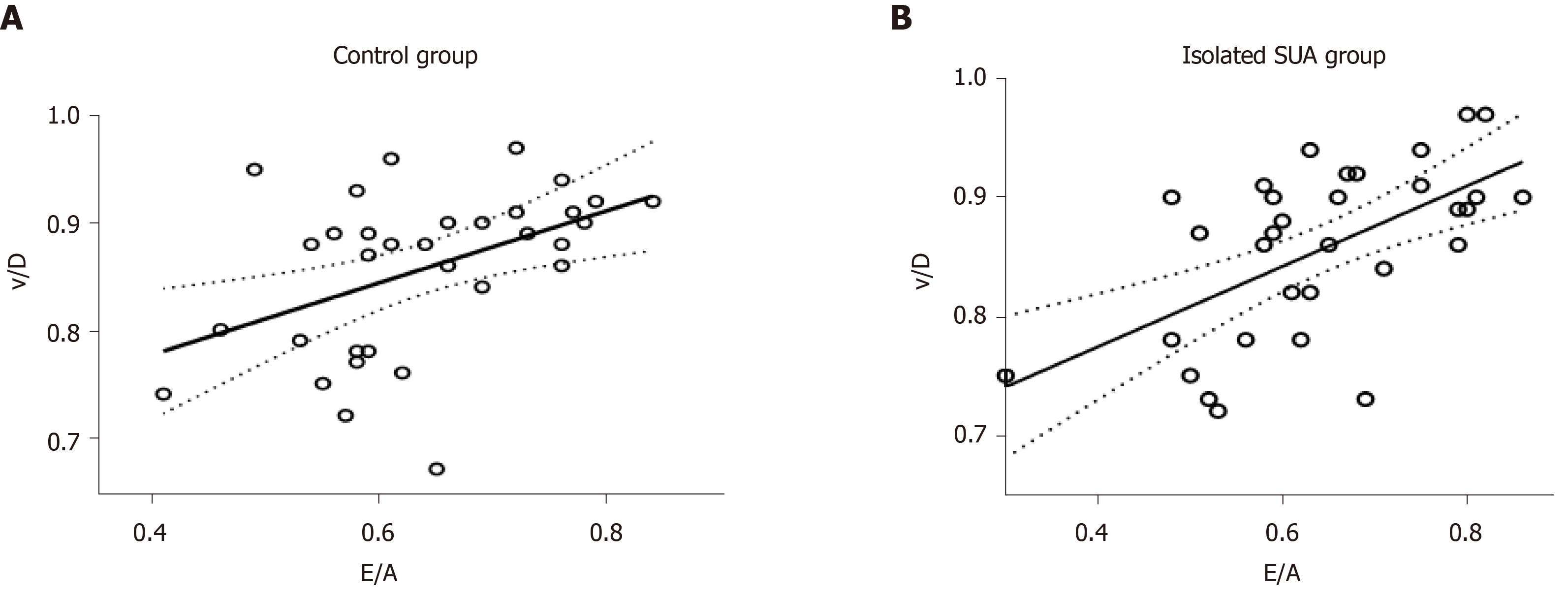
During the third trimester, the isolated SUA group showed a lower ‘a’-wave peak velocity in the DV than the control group (P < 0.05). The correlations between the velocity ratios and E/A ratio at the tricuspid orifice in the two groups were analysed, and the correlation between the ventricular late diastolic velocity/ventricular diastolic peak flow velocity and E/A ratios was the best (R2 of the isolated SUA group: 0.520; R2 of the control group: 0.358). The correlations between the velocity ratios and tissue Doppler Tei index of foetal right ventricular in the two groups were analysed, and the correlation between the pulsatility index for veins (PIV) and tissue Doppler Tei index ratios was the best (R2 of the isolated SUA group: 0.865; R2 of the control group: 0.627).
In the isolated SUA group, the atrial systolic peak velocity ‘a’ decreased, and this finding might be related to the changes in foetal cardiac functions. The ratio of ventricular late diastolic velocity to ventricular diastolic peak flow velocity was closely related to the E/A ratio at the tricuspid valve and can be used to identify changes in the right ventricular diastolic functions of isolated SUA and healthy foetuses. PIV was closely related to the tissue Doppler Tei index of the foetal right ventricular and can be used to identify the right ventricular overall functions of isolated SUA and healthy foetuses.
Core Tip: Single umbilical artery (SUA) is the most common umbilical cord malformation in prenatal diagnosis. The presence of an SUA can cause blood circulation disorder in the foetus and functional changes of the foetal heart, affecting foetal circulation. We used ductus venosus velocity firstly for the evaluation of right ventricular diastolic function in foetuses with isolated SUA and in those with normal cardiac anatomy. This study performed an objective assessment of the occurrence and development of isolated SUA in foetuses, predicted its prognosis and guided clinical work.
- Citation: Li TG, Nie F, Xu XY. Correlation between ductus venosus spectrum and right ventricular diastolic function in isolated single-umbilical-artery foetus and normal foetus in third trimester. World J Clin Cases 2020; 8(23): 5866-5875
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/5866.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.5866
Normal umbilical cords contain two umbilical arteries (UAs) and one umbilical vein (UV). A condition in which only one UA is present, single UA (SUA), is the most common malformation of the umbilical cord. SUA is a soft marker for chromosomal abnormalities, congenital structural malformations and preterm birth. Depending on the presence of other structural malformations and/or karyotype abnormalities, SUA is divided into isolated and nonisolated SUA, with the former accounting for approximately 65% of all SUA cases[1-3]. Isolated SUA results in the development of certain obstetric complications, such as foetal growth restriction and increased perinatal mortality[4-7].
The presence of an SUA can cause blood circulation disorder in the foetus and functional changes of the foetal heart, affecting foetal circulation[6,8,9]. Anatomically, the ductus venosus (DV) enters directly into the right atrium or is connected to the right atrium via the inferior vena cava (IVC). Changes in the right ventricular function of the foetus may be reflected in changes in the DV blood flow pattern, as can be observed on spectral Doppler[10-12]. Currently, pulsatility index for veins (PIV) is clinically used for evaluating changes in the DV blood flow spectrum[12]. However, the foetal cardiac cycle involves four different stages, including ventricular systole, ventricular diastole, atrial diastole and atrial systole, and DV is greatly affected by factors such as the cardiac cycle, volume and pressure[13,14]. As a result, evaluating foetal cardiac functions using PIV alone may not be objective and accurate. Evaluating the right ventricular function changes in foetuses with isolated SUA in the third trimester aids in the assessment of intrauterine conditions and perinatal outcomes[15].
In this study, foetal DV flow velocities, flow velocity ratios and the tricuspid valve inflow Doppler pattern were used to evaluate changes in the right ventricular diastolic function of foetuses with isolated SUA in the third trimester to identify sensitive indicators for evaluating the right ventricular diastolic function of foetuses with isolated SUA and thereby provide an objective basis for clinical practice.
We prospectively studied 34 foetuses with prenatally identified isolated SUA with gestational age of 28-39 wk and 34 gestational age-matched healthy foetuses from the Gansu Provincial Maternity and Child-care Hospital between July 2017 and December 2018. The study protocol was approved by the Medical Ethics Committee of Gansu Provincial Maternity and Child-care Hospital ethics committee (No. 2017-04), and the pregnant mothers provided their written informed consent. We excluded pregnant mothers with multiple gestations, pregnancies presenting associated foetal anomalies, including structural abnormalities, congenital heart disease and abnormal karyotype and pregnant mothers with conditions that may affect foetal haemodynamics, such as maternal diabetes, pre-eclampsia, preterm labour or endocrinological disorders such as thyroid disease.
The diagnosis of isolated SUA was confirmed using colour Doppler ultraso-nography by observing the absence of one UA at the level of the foetal abdominal cord insertion. In all cases, the diagnosis of isolated SUA was confirmed by postnatal pathological examination, and all newborns were determined to be anatomically normal at delivery. The newborns were diagnosed with a small-for-gestational-age (SGA) condition when their birth weight was below the 10th percentile for gestational age. Demographic data, including maternal age, weight, body mass index, parity and medical history, were collected. Gestational age was calculated based on the first day of the last menstrual period and confirmed by crown–rump length measurement at the first-trimester ultrasound scan.
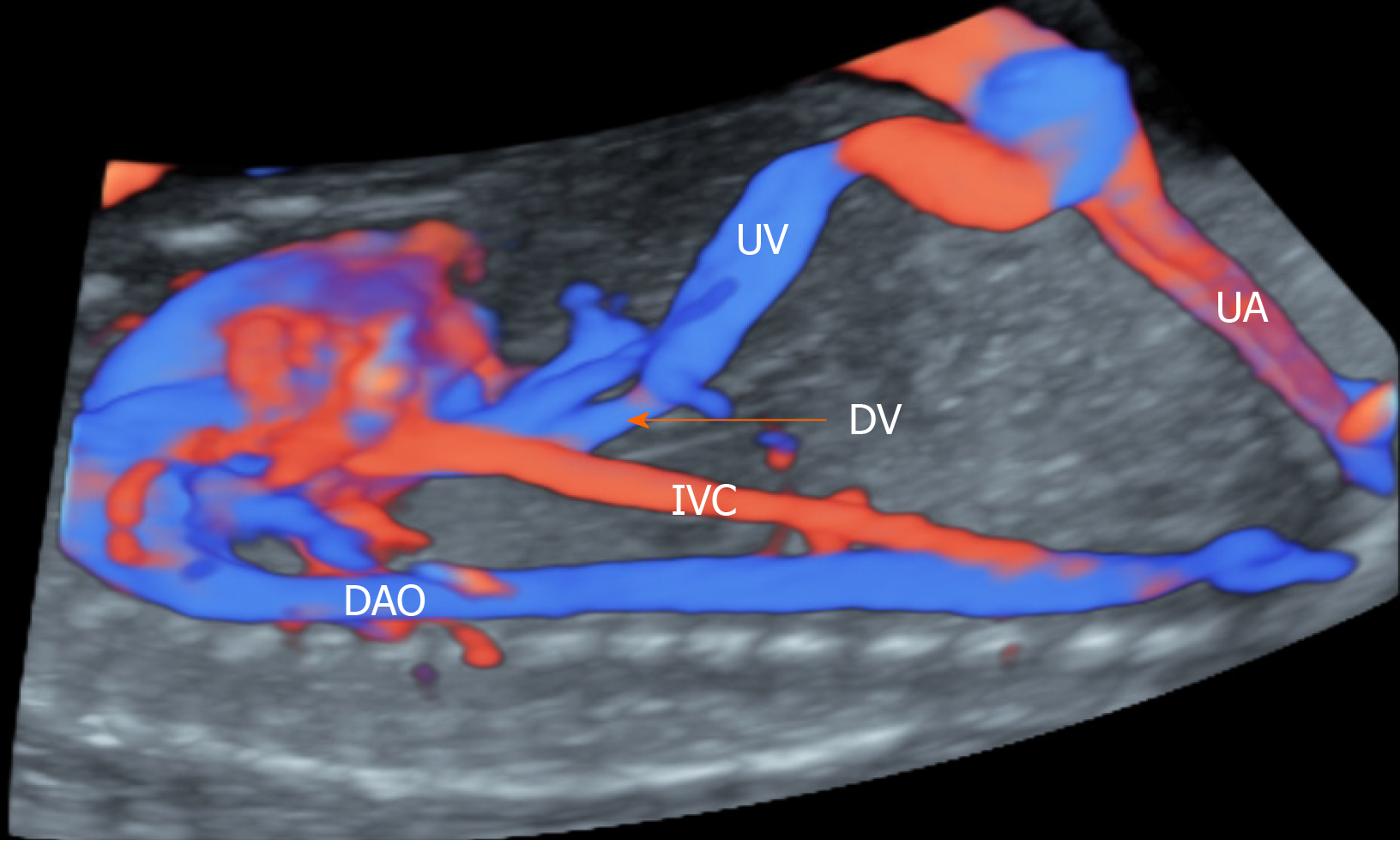
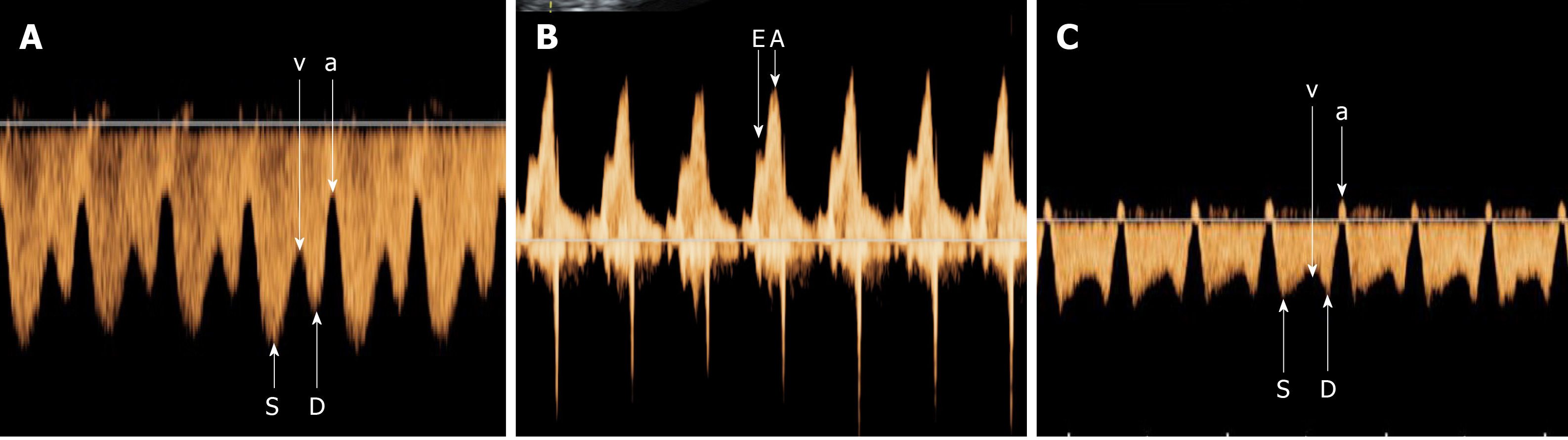
E10 (GE Healthcare, United States) and EPIQ5 (Philips, The Netherlands) ultrasound systems were used. Foetal biometric measurements were performed during each scan. The pregnant women were asked to lie in a supine position. The median sagittal section or oblique transection of the upper abdomen of the foetus was assessed to display the long axis of the UV and track it toward the foetus head. Before the UV turned toward the left branch of the portal vein, a small tubular structure was shown to be connected to the IVC. After turning on the colour Doppler function, bright blood flow signals were identified as the DV blood flow (Figure 1). To obtain the DV blood flow spectrum, we initiated colour Doppler ultrasonography with the Doppler sampling line paralleling the DV blood flow (angle < 30 °). Blood flow parameters, including the ventricular systolic peak flow velocity (S), ventricular late diastolic velocity (v), ventricular diastolic peak flow velocity (D) and the atrial systolic peak velocity (a), were measured (Figure 2A), and the velocity ratios, including S/v, S/D, S/a, v/D, v/a and D/a, were calculated based on these parameters. During measurement, the sample volume was placed inside the DV to reduce interference of the surrounding vessels. A four-chamber view of the foetus was obtained, and the sample volume was placed at the tip of the tricuspid valve to measure the early passive (E) and late active (A) peak blood flow velocities to calculate the E/A ratio (Figure 2B).
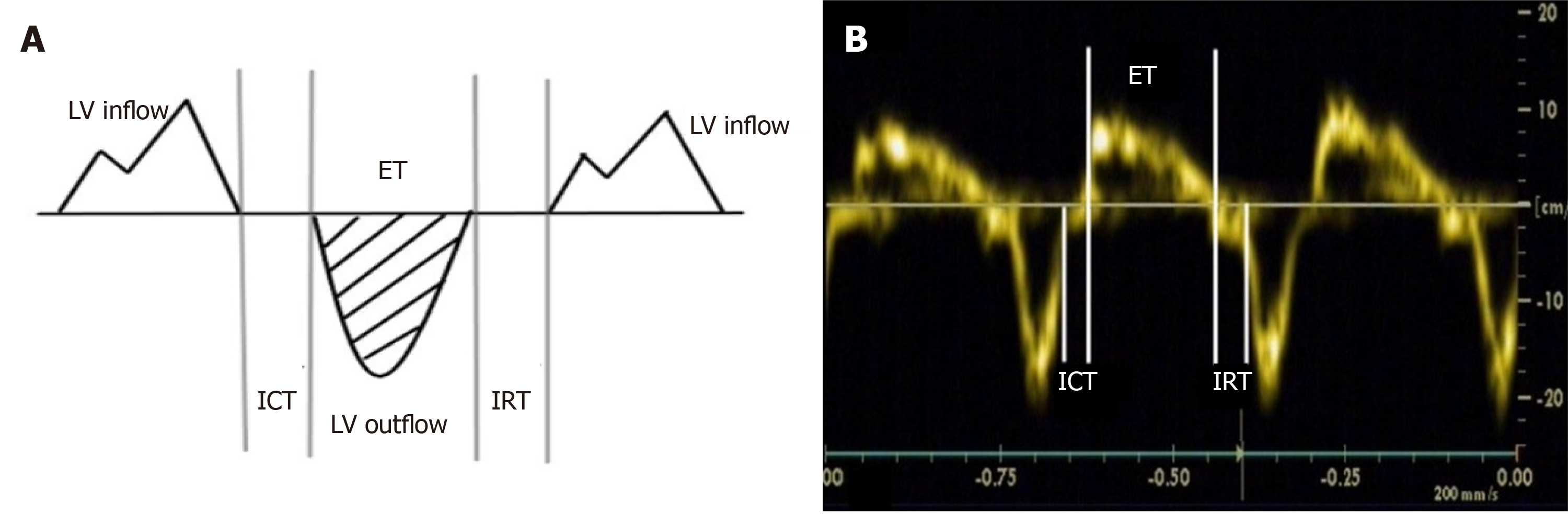
The right ventricular function was evaluated using the tissue Doppler Tei index described in the literature[16]. In the four-chamber view, the tissue Doppler sample volume was placed at the junction of the free wall of the right ventricle and posterior leaflet of the tricuspid valve, the sample line was parallel to the direction of movement (angle < 20 °), the tissue Doppler sample volume was 2 mm3 and the scanning speed was adjusted to 10-15 cm/s to obtain tissue Doppler spectrum images to measure the isovolumic contraction time (ICT), ejection time (ET) and isovolumic relaxation time (IRT). The tissue Doppler Tei index was calculated in accordance with the measurement method (as shown in Figure 3A): Tei = (ICT + IRT)/ET (Figure 3B). When the foetal position was poor, measurement was conducted again after the foetus changed position. The above parameters were measured three consecutive times, and the average values were calculated.
The data analysis was performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, United States). Continuous variables were presented as the mean ± standard deviation or median (interquartile range) as appropriate. The independent sample t test was used for comparisons between groups, and P < 0.05 was considered statistically significant. The velocity ratios of DV with the E/A ratio at the tricuspid orifice and the tissue Doppler Tei index of the foetal right ventricular were analysed using linear regression, and the regression coefficient R2 was calculated accordingly.
The colour flow Doppler of the umbilical cords in all 34 healthy foetuses showed two UAs and one UV. The colour flow Doppler of umbilical cords in the 34 isolated SUA foetuses showed one UA and one UV. In the latter group, the left branch was absent in 20 cases, and the right branch was absent in 14 cases. One healthy foetus displayed mild tricuspid regurgitation (TR), whereas in the isolated SUA, two foetuses had mild TR, and three had moderate TR. TR was considered abnormal if holosystolic with a maximum velocity of more than 2 m/s[17].
In the control group, the foetal DV spectrum ‘a’-wave was a forward wave. In the isolated SUA group, the ‘a’-waves of 32 foetuses were forward, whereas those of two foetuses, both of which were SGA, were reversed (Figure 2C). A comparison of blood flow parameters between the two groups showed that the isolated SUA group exhibited a lower DV atrial systolic peak velocity ‘a’-wave than the control group (P < 0.05), whereas the PIV, S/v, S/D, S/a, v/D, v/a, D/a and E/A ratios at the tricuspid orifice showed no significant change (P > 0.05) (Table 1).
| Isolated SUA group | Control group | P value | |
| S, cm/s | 32.8 ± 14.0 | 34.9 ± 14.3 | 0.552 |
| v, cm/s | 22.6 ± 9.4 | 24.1 ± 9.8 | 0.508 |
| D, cm/s | 26.7 ± 12.0 | 28.2 ± 11.6 | 0.600 |
| a, cm/s | 13.0 ± 7.1 | 17.5 ± 7.4 | 0.013 |
| PIV | 0.81 ± 0.33 | 0.73 ± 0.34 | 0.310 |
| S/v | 1.48 ± 0.30 | 1.47 ± 0.30 | 0.816 |
| S/D | 1.26 ± 0.20 | 1.24 ± 0.15 | 0.778 |
| S/a | 2.37 ± 0.79 | 2.13 ± 0.77 | 0.214 |
| v/D | 0.86 ± 0.71 | 0.86 ± 0.75 | 0.921 |
| v/a | 1.61 ± 0.48 | 1.44 ± 0.41 | 0.126 |
| D/a | 1.90 ± 0.60 | 1.70 ± 0.53 | 0.163 |
| E/A | 0.64 ± 0.13 | 0.64 ± 0.10 | 0.975 |
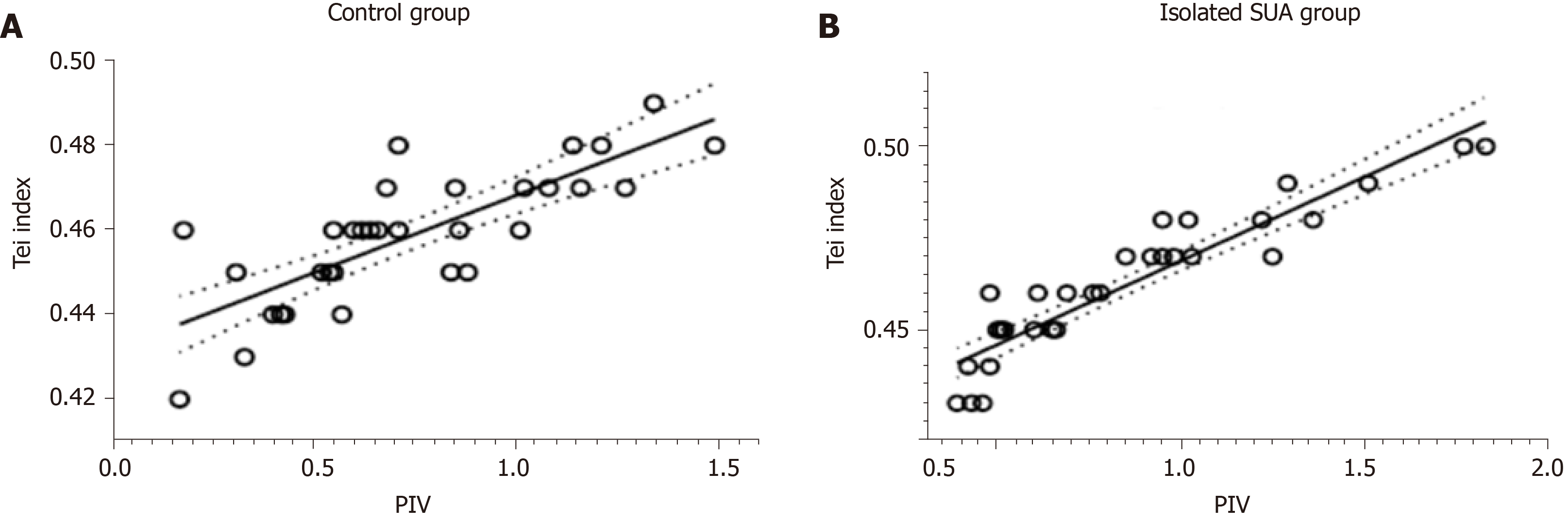
The correlations between the velocity ratios and E/A ratio at the tricuspid orifice in the two groups were analysed. The correlation between the v/D and E/A ratios was the best in both groups (R2 of 0.520 in the isolated SUA group and 0.358 in the control group) (Figure 4A and 4B). The correlations between the velocity ratios and tissue Doppler Tei index of foetal right ventricular in the two groups were analysed. The correlation between the PIV and tissue Doppler Tei index of foetal right ventricular was the best in both groups (R2 of 0.865 in the isolated SUA group and 0.627 in the control group) (Figure 5A and 5B).
During the follow-up of all foetuses until birth, the general conditions of the newborns at birth were analysed, and their bodyweight and placental mass in the two groups were compared. The differences were statistically significant (P < 0.05). In the univariate analysis, the presence of an isolated SUA was associated with a low birth weight (2940 g vs 3260 g) and a high prevalence of SGA (13.0% vs 3.9%; P < 0.01). No statistically significant difference was observed in the pregnant women’s age, gravidity and parity and gestational age (P > 0.05) (Table 2).
| Isolated SUA group | Control group | P value | |
| Maternal weight, kg | 61.9 ± 5.8 | 61.8 ± 5.5 | 0.978 |
| Body mass index, kg/m2 | 24.1 ± 3.6 | 24.6 ± 3.7 | 0.853 |
| Maternal age in yr | 27.7 ± 5.2 | 27.5 ± 4.5 | 0.382 |
| Delivery at wk | 37.9 ± 1.1 | 38.8 ± 0.9 | 0.139 |
| Birth weight, kg | 2.9 ± 0.3 | 3.3 ± 0.4 | 0.011 |
| Placental weight, kg | 461 ± 59 | 523 ± 62 | < 0.001 |
In foetuses, the diastolic function changes before the cardiac function. The foetal DV directly delivers the umbilical venous blood with high oxygen saturation through the IVC to the right atrium, the pressure of which is the major factor affecting DV blood flow[18]. When dynamic changes in foetal heart blood flow lead to changes in the right atrial pressure, changes in the DV spectral blood flow parameters occur, most evidently PIV[19,20]. This study evaluated the relationship between the DV Doppler flow velocity ratios and right ventricular function.
The foetal DV spectral waveform is closely related to the four periods of the cardiac cycle[13]. The ‘S’-wave corresponds to ventricular systole, which is produced by an increase in the venous forward blood flow velocity caused by atrial diastole during ventricular systole and followed by the ‘v’ wave during end-systolic ventricular relaxation and the ascent of the atrioventricular (AV) valves before the onset of diastole. With the opening of the AV valves, the ‘D’- and ‘a’-waves correspond to E and A diastolic filling, respectively. In particular, the highest velocity occurs at the ‘S’-wave and the lowest at the ‘a’-wave. The S/v ratio quantifies the relative forward flow into the atria as the ventricle relaxes before the AV valves open. The v/D ratio reflects early diastolic filling immediately following this event. The D/a ratio is a diastolic parameter relating to the magnitude of forward flow during passive and active diastolic filling; it is analogous to the E/A ratio but for the AV valves. Three ratios describe nonconsecutive cardiac events: The S/D ratio that quantifies ventricular systolic to E diastolic filling[13]; the S/a ratio that quantifies ventricular systolic to active diastolic filling[21]; and the v/a ratio that quantifies end-systolic relaxation and active diastolic filling. The relative decrease in the ‘a’-wave is closely associated with the increase in PIV.
Currently, PIV is used as the primary indicator for evaluating changes in the DV blood flow spectrum[22-24]. However, this study showed that in both isolated SUA and healthy foetuses, the v/D ratio significantly correlated with the E/A ratio at the tricuspid orifice. Thus, the E/A ratio can be used for the evaluation of conventional Doppler foetal cardiac diastolic function[25]. The PIV ratio was significantly correlated with the tissue Doppler Tei index of foetal right ventricular. Therefore, the tissue Doppler Tei index can be used for the evaluation of foetal cardiac overall function. These findings suggest that when evaluating cardiac function using the DV spectrum, in addition to monitoring PIV, attention should be paid to the correlation between the ‘v’ wave-related ratios and right ventricular function.
A decline in cardiac diastolic function is often observed as an increase in the DV spectrum PIV, a decrease in ‘a’-wave velocity and disappearance or reversal of blood flow[22,23]. This condition may occur because subjects in previous studies were foetuses with significant changes in cardiac functions, including gestational hypertension, intrauterine growth restriction and twin-to-twin transfusion syndrome[24-30]. In this study, all the foetal DV spectrum ‘a’-waves in the control group were forward and can be observed throughout the entire cardiac cycle, potentially because of the high resistance of the foetal DV venous system and the weak atrial systolic force. Consequently, the DV pressure in the entire cardiac cycle was consistently greater than the atrial pressure. Thus, the blood was unable to flow in the reverse direction into the DV. In the isolated SUA group, however, the DV spectrum ‘a’-waves of two cases, both of which were SGA foetuses, were backward. Reversal of the ‘a’-wave might indicate a decrease in ventricular compliance, which is caused by atrial systolic venous blood reflux resulting in increased atrial pressure[28]. Therefore, close attention should be paid to whether the DV reversed ‘a’-wave occurs. In such a case, maternal and foetal examinations should be enhanced and the pregnancy be terminated if necessary.
This study showed that the ‘a’-wave flow velocity was lower in foetuses with isolated SUA than in the controls. This condition might have occurred because foetuses with isolated SUA are prone to having a low bodyweight at birth resulting in decreased circulating blood volume and DV blood flow volume. Moreover, none of the DV blood flow parameters in the foetuses with isolated SUA changed significantly compared with the healthy controls. This finding might be explained as follows: although the foetuses with isolated SUA exhibited dynamic changes in their blood flow in the absence of one UA, such changes were inadequate to cause an insufficient blood supply, which led to increased right heart load and right atrial pressure and blocked DV reflux.
In this study, one of the healthy controls displayed a mild TR, whereas five of the isolated SUA foetuses had mild or moderate TR. Thus, the isolated SUA group had a slightly higher TR rate than the control group. Therefore, once isolated SUA is diagnosed, TR should be monitored to facilitate the preliminary determination of right ventricular function in the foetus.
First, given that atrial pressure is a major factor affecting the changes in DV blood flow and both respiration and motion of the foetus can affect the DV spectrum, atrial pressure should preferably be measured with the foetus in the resting state. Second, mapping of the foetal DV spectrum can be easily affected by adjacent blood vessels, particularly the vena cava. Given that vena cava displays continuous reversed blood flow under normal circumstances of atrial systole, it can be easily misjudged as the DV spectrum. Finally, acquisition of the tissue Doppler Tei index of the foetal right ventricular is difficult and affected by the foetal position, and thus, the measurement may be inaccurate.
Foetuses with isolated SUA are prone to having a low bodyweight at birth. Changes in the cardiac functions of foetuses with isolated SUA can be evaluated by the DV spectrum velocities and velocity ratios. When monitoring the DV spectrum, v/D and PIV may be used to identify changes in the right ventricular function of isolated SUA foetuses early.
Single umbilical artery (SUA) is the most common umbilical cord malformation in prenatal diagnosis. The presence of an SUA can cause blood circulation disorder in the foetus and functional changes of the foetal heart, affecting foetal circulation.
We used ductus venosus (DV) velocity for the evaluation of right ventricular diastolic function in foetuses with isolated SUA and in those with normal cardiac anatomy.
The right ventricular diastolic functions in foetuses with isolated SUA and normal foetuses in the third trimester were evaluated using the spectral Doppler of blood flow in the foetal DV.
Colour Doppler was used to measure the spectrum of foetal DV and tricuspid orifice in SUA foetuses and in age-matched healthy controls. The DV flow velocities and velocity ratios were measured. The early passive/late active ratio at the tricuspid orifice and tissue Doppler Tei index of the foetal right ventricular in the two groups were measured.
During the third trimester, the isolated SUA group showed a lower ‘a’-wave peak velocity in the DV than the control group. The correlation between the ventricular late diastolic velocity/ventricular diastolic peak flow velocity and early passive/late active ratios was the best. The correlation between the pulsatility index for veins and tissue Doppler Tei index ratios was the best.
The spectral Doppler of blood flow in the foetal DV can be used to identify the right ventricular diastolic functions of isolated SUA and healthy foetuses.
Through the detection of spectral Doppler of blood flow in the foetal DV between the isolated SUA group and the control group, we can accurately evaluate the foetal ventricular diastolic function of isolated SUA to provide more accurate and objective diagnosis and treatment basis for the clinic.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Daniilidis A S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX
| 1. | Voskamp BJ, Fleurke-Rozema H, Oude-Rengerink K, Snijders RJ, Bilardo CM, Mol BW, Pajkrt E. Relationship of isolated single umbilical artery to fetal growth, aneuploidy and perinatal mortality: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Bombrys AE, Neiger R, Hawkins S, Sonek J, Croom C, McKenna D, Ventolini G, Habli M, How H, Sibai B. Pregnancy outcome in isolated single umbilical artery. Am J Perinatol. 2008;25:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Granese R, Coco C, Jeanty P. The value of single umbilical artery in the prediction of fetal aneuploidy: findings in 12,672 pregnant women. Ultrasound Q. 2007;23:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Araujo Júnior E, Palma-Dias R, Martins WP, Reidy K, da Silva Costa F. Congenital heart disease and adverse perinatal outcome in fetuses with confirmed isolated single functioning umbilical artery. J Obstet Gynaecol. 2015;35:85-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Bugatto F, Quintero-Prado R, Melero-Jiménez V, Fajardo-Expósito MA, Hervías-Vivancos B, Bartha JL. Ultrasound predictors of birth weight in euploid fetuses with isolated single umbilical artery. Ultrasound Obstet Gynecol. 2010;36:724-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | De Catte L, Burrini D, Mares C, Waterschoot T. Single umbilical artery: analysis of Doppler flow indices and arterial diameters in normal and small-for-gestational age fetuses. Ultrasound Obstet Gynecol. 1996;8:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Martínez-Payo C, Gaitero A, Tamarit I, García-Espantaleón M, Iglesias Goy E. Perinatal results following the prenatal ultrasound diagnosis of single umbilical artery. Acta Obstet Gynecol Scand. 2005;84:1068-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Raio L, Ghezzi F, Di Naro E, Cromi A, Buttarelli M, Sonnenschein M, Dürig P. Ductus venosus blood flow velocity characteristics of fetuses with single umbilical artery. Ultrasound Obstet Gynecol. 2003;22:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Murphy-Kaulbeck L, Dodds L, Joseph KS, Van den Hof M. Single umbilical artery risk factors and pregnancy outcomes. Obstet Gynecol. 2010;116:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Crispi F, Gratacós E. Fetal cardiac function: technical considerations and potential research and clinical applications. Fetal Diagn Ther. 2012;32:47-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 11. | Baschat AA, Turan OM, Turan S. Ductus venosus blood-flow patterns: more than meets the eye? Ultrasound Obstet Gynecol. 2012;39:598-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Baschat AA. Examination of the fetal cardiovascular system. Semin Fetal Neonatal Med. 2011;16:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Turan OM, Turan S, Sanapo L, Willruth A, Berg C, Gembruch U, Harman CR, Baschat AA. Reference ranges for ductus venosus velocity ratios in pregnancies with normal outcomes. J Ultrasound Med. 2014;33:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Smrcek JM, Krapp M, Axt-Fliedner R, Kohl T, Geipel A, Diedrich K, Gembruch U, Berg C. Atypical ductus venosus blood flow pattern in fetuses with severe tricuspid valve regurgitation. Ultrasound Obstet Gynecol. 2005;26:180-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Li TG, Nie F, Li ZD, Wang YF, Li Q. Evaluation of right ventricular function in fetuses with isolated single umbilical artery using spatiotemporal image correlation M-mode. Cardiovasc Ultrasound. 2019;17:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Bokiniec R, Własienko P, Borszewska-Kornacka MK, Madajczak D, Szymkiewicz-Dangel J. Myocardial performance index (Tei index) in term and preterm neonates during the neonatal period. Kardiol Pol. 2016;74:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Respondek ML, Kammermeier M, Ludomirsky A, Weil SR, Huhta JC. The prevalence and clinical significance of fetal tricuspid valve regurgitation with normal heart anatomy. Am J Obstet Gynecol. 1994;171:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Hassan WA, Brockelsby J, Alberry M, Fanelli T, Wladimiroff J, Lees CC. Cardiac function in early onset small for gestational age and growth restricted fetuses. Eur J Obstet Gynecol Reprod Biol. 2013;171:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Crispi F, Hernandez-Andrade E, Pelsers MM, Plasencia W, Benavides-Serralde JA, Eixarch E, Le Noble F, Ahmed A, Glatz JF, Nicolaides KH, Gratacos E. Cardiac dysfunction and cell damage across clinical stages of severity in growth-restricted fetuses. Am J Obstet Gynecol 2008; 199: 254.e1-254. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Rychik J, Tian Z, Bebbington M, Xu F, McCann M, Mann S, Wilson RD, Johnson MP. The twin-twin transfusion syndrome: spectrum of cardiovascular abnormality and development of a cardiovascular score to assess severity of disease. Am J Obstet Gynecol 2007; 197: 392.e1-392. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Kanzaki T, Chiba Y. Evaluation of the preload condition of the fetus by inferior vena caval blood flow pattern. Fetal Diagn Ther. 1990;5:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Seravalli V, Miller JL, Block-Abraham D, Baschat AA. Ductus venosus Doppler in the assessment of fetal cardiovascular health: an updated practical approach. Acta Obstet Gynecol Scand. 2016;95:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Morris RK, Selman TJ, Verma M, Robson SC, Kleijnen J, Khan KS. Systematic review and meta-analysis of the test accuracy of ductus venosus Doppler to predict compromise of fetal/neonatal wellbeing in high risk pregnancies with placental insufficiency. Eur J Obstet Gynecol Reprod Biol. 2010;152:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Turan OM, Turan S, Berg C, Gembruch U, Nicolaides KH, Harman CR, Baschat AA. Duration of persistent abnormal ductus venosus flow and its impact on perinatal outcome in fetal growth restriction. Ultrasound Obstet Gynecol. 2011;38:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Hernandez-Andrade E, Benavides-Serralde JA, Cruz-Martinez R, Welsh A, Mancilla-Ramirez J. Evaluation of conventional Doppler fetal cardiac function parameters: E/A ratios, outflow tracts, and myocardial performance index. Fetal Diagn Ther. 2012;32:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Stirnemann JJ, Mougeot M, Proulx F, Nasr B, Essaoui M, Fouron JC, Ville Y. Profiling fetal cardiac function in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2010;35:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Hofstaetter C, Gudmundsson S. Venous Doppler in the evaluation of fetal hydrops. Obstet Gynecol Int. 2010;2010:430157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Nakagawa K, Tachibana D, Nobeyama H, Fukui M, Sumi T, Koyama M, Ishiko O, Hecher K. Reference ranges for time-related analysis of ductus venosus flow velocity waveforms in singleton pregnancies. Prenat Diagn. 2012;32:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Tachibana D, Glosemeyer P, Diehl W, Nakagawa K, Wada N, Kurihara Y, Fukui M, Koyama M, Hecher K. Time-interval analysis of ductus venosus flow velocity waveforms in twin-to-twin transfusion syndrome treated with laser surgery. Ultrasound Obstet Gynecol. 2015;45:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Wada N, Tachibana D, Kurihara Y, Nakagawa K, Nakano A, Terada H, Tanaka K, Fukui M, Koyama M, Hecher K. Alterations in time intervals of ductus venosus and atrioventricular flow velocity waveforms in growth-restricted fetuses. Ultrasound Obstet Gynecol. 2015;46:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |