Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5751
Peer-review started: August 10, 2020
First decision: September 13, 2020
Revised: September 23, 2020
Accepted: September 26, 2020
Article in press: September 26, 2020
Published online: November 26, 2020
Processing time: 107 Days and 6.2 Hours
Sebaceous lymphadenoma is a benign tumor that occurs rarely in the salivary glands, most commonly in the parotid glands or periparotid lymph nodes, and even more rarely undergoes malignant transformation into a sebaceous lymphadenocarcinoma.
We report an 82-year-old woman who presented with a painless mass in the right parotid region. We performed extended surgical resection of the parotid gland mass. Intraoperative pathology revealed a sebaceous lymphadenocarcinoma with metastasis into the periparotid cervical lymph nodes, so we also performed neck dissection and lymph node resection. Postoperative pathology confirmed the diagnosis. The literature review revealed that this was the seventh reported case of sebaceous lymphadenocarcinoma and the second reported case of cervical lymph node metastasis and infiltration of the skin of the parotid gland.
Treatment of sebaceous lymphadenocarcinoma depends on the typing and clinical staging of the cancer. Extensive resection is the first choice, and adjuvant radiotherapy should be given to patients with high-grade tumors or those at an advanced clinical stage.
Core Tip: We present a case of sebaceous lymphadenocarcinoma in the parotid gland of an elderly woman, with metastases to the periparotid lymph nodes. Because these tumors are so rare, we conducted a literature review of sebaceous lymphadenocarcinoma, which revealed that our case is only the seventh reported case of sebaceous lymphadenocarcinoma and the second with cervical lymph node metastasis and infiltration of the skin of the parotid gland. We believe that because these tumors are rare, our case report will add valuable information to the growing reports of their occurrence.
- Citation: Hao FY, Wang YL, Li SM, Xue LF. Sebaceous lymphadenocarcinoma of the parotid gland: A case report. World J Clin Cases 2020; 8(22): 5751-5757
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5751.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5751
Sebaceous glands, which are a component of the pilosebaceous unit, are abundant in the head and neck. They are uncommon in the salivary glands, occurring mostly in the parotid glands (10%-42%)[1]. Sebaceous gland tumors in the parotid glands and periparotid cervical lymph nodes are even more rare[1]. Salivary gland and sebaceous gland tumors are currently classified into sebaceous adenomas, sebaceous lymphadenomas, sebaceous adenocarcinomas, and sebaceous lymphadenocarcinomas. Sebaceous lymphadenocarcinoma is a malignant type of sebaceous lymphadenoma, which has a very rare incidence and is the rarest sebaceous gland tumor in the salivary glands. Before the case presented here, there were only six reports of sebaceous lymphadenocarcinoma[2,3]. This report discusses a case of sebaceous lymphadenocarcinoma of the parotid gland, with a collateral cervical lymph node metastasis.
An 82-year-old woman presented with a 4-mo history of a painless mass in the right parotid gland.
The painless mass in the right parotid gland had rapidly grown over the previous 4 mo. The symptoms were not relieved after anti-inflammatory treatment for 7 d.
The patient had a 10-year history of hypertension.
The patient’s medical history and family history were unremarkable.
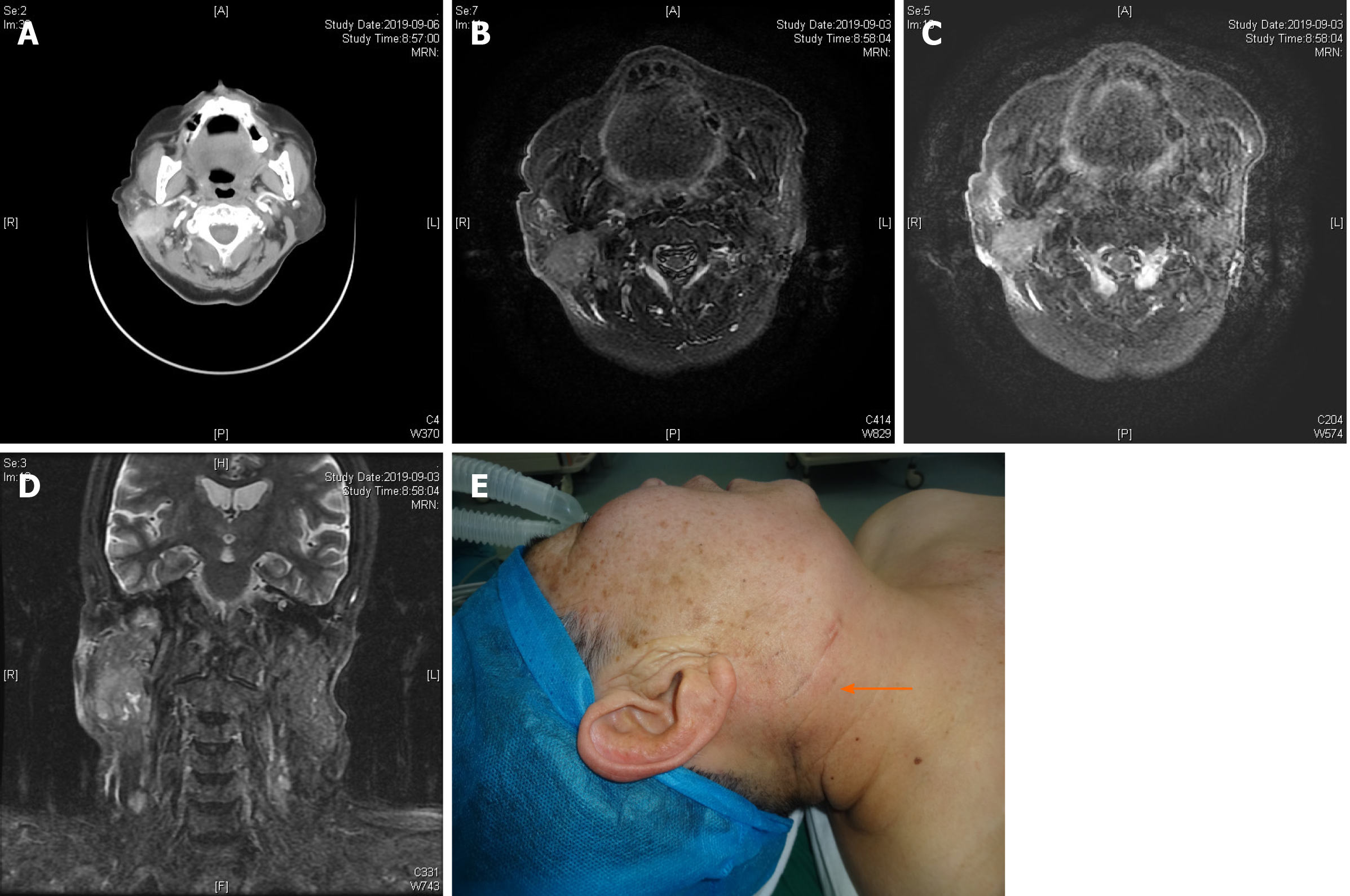
Physical examination showed a 3 cm diameter mass in the right parotid region, with localized reddish skin, clear boundaries, medium texture, mild tenderness, moderate mobility, no fluctuation, and no facial paralysis. No obvious bulge was found on the parapharyngeal wall, and secretions from the parotid duct were clear.
Laboratory tests showed the following results. Urine analysis showed urine occult blood (BLD) (+ -); liver and kidney functions were normal; preoperative routine electrocardiography (ECG) and dynamic ECG suggested sinus bradycardia; and other auxiliary examination results were unremarkable.
Enhanced computed tomography (CT) of the neck and magnetic resonance imaging (MRI) of the parotid gland (Figure 1) demonstrated an approximately 28 mm × 21 mm irregular mass shadow at the lower pole of the right parotid gland, with unclear boundaries, obvious inhomogeneous enhancement, and thickened and enhanced adjacent skin. A malignant tumor in the right parotid gland and involvement of the adjacent skin were suspected. Multiple lymph node shadows in the right parotid gland and the right II, V regions were consistent with possible lymph node metastases.
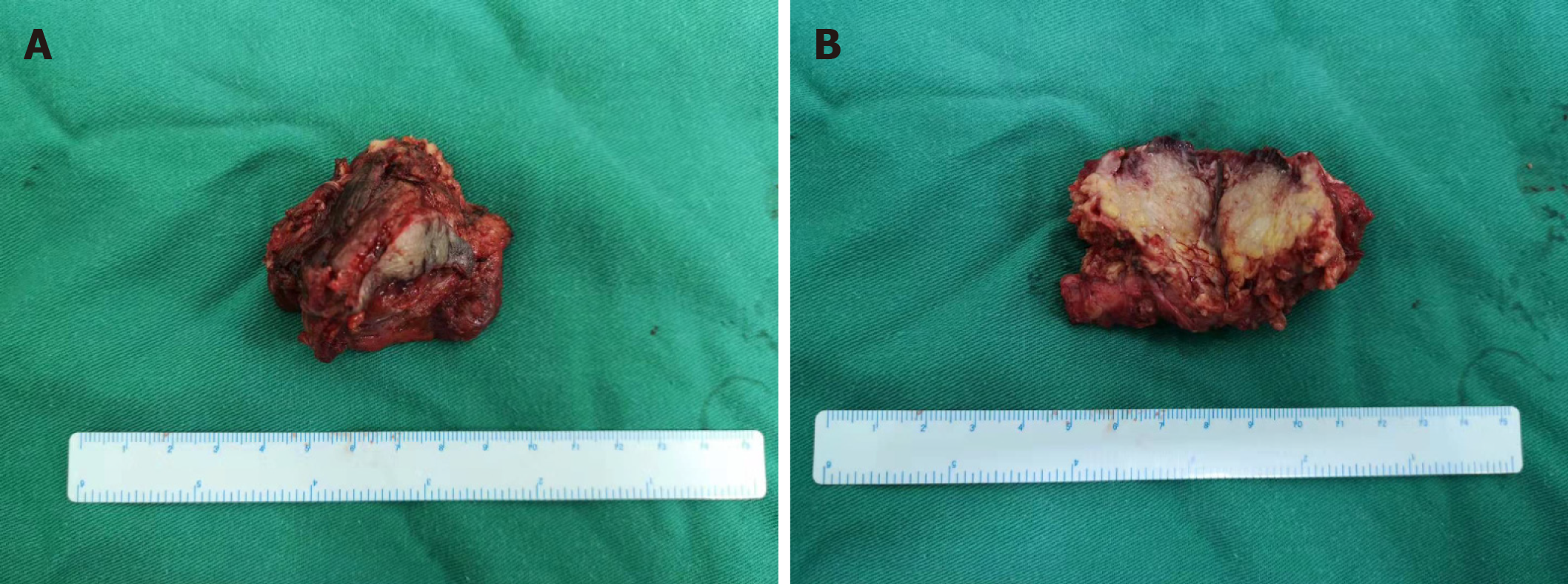
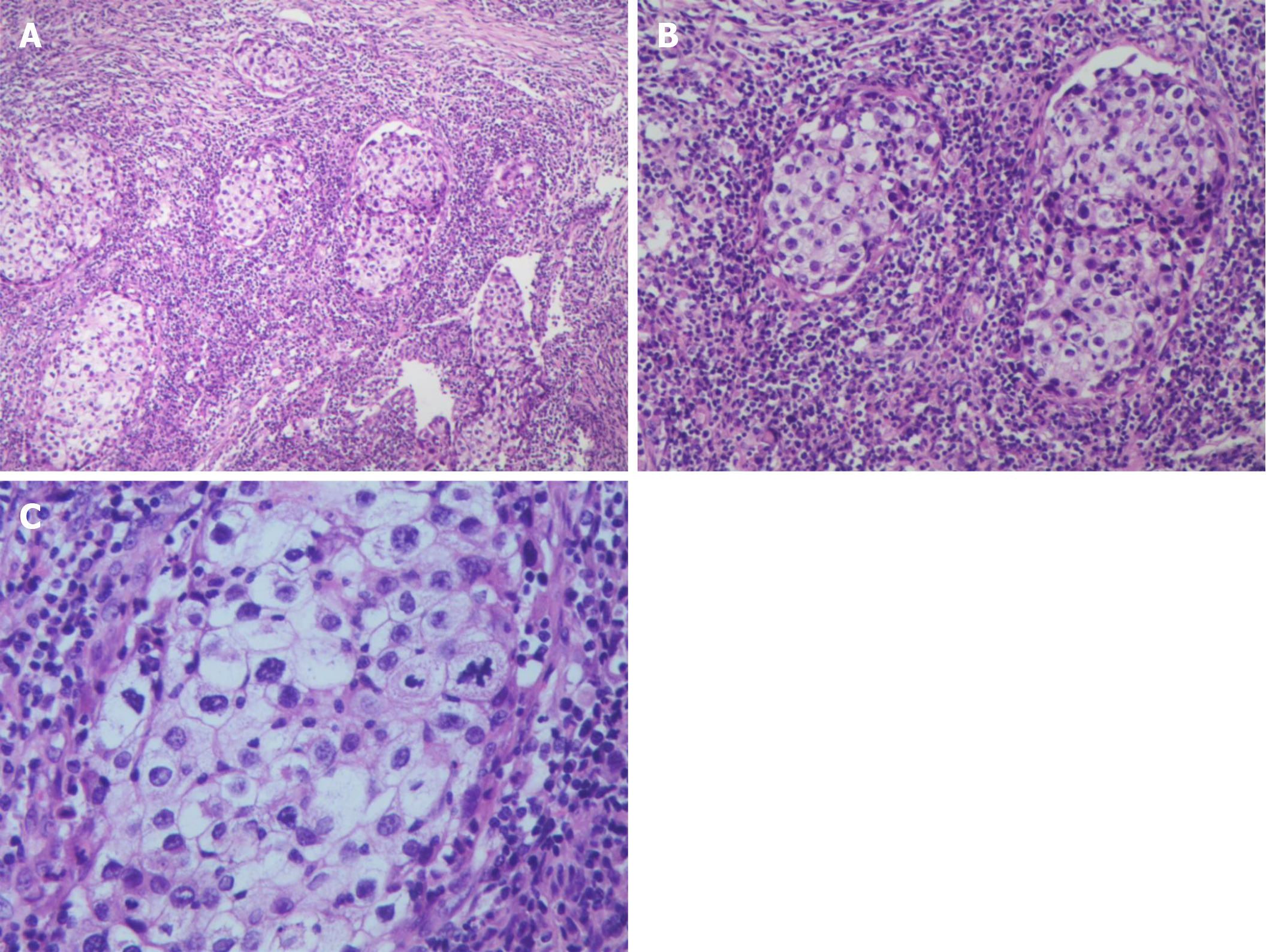
A generally greyish-red, 5 cm × 4 cm × 4 cm specimen of parotid tissue, which had a 3.5 cm × 1 cm segment of skin tissue attached and an underlying 3 cm × 2 cm × 1.5 cm, grayish-yellow nodule (Figure 2), was sent for postoperative pathological examination. Microscopic examination showed nest-like tumor cell growth that infiltrated the surrounding striated muscle tissue. Tumor cell cytoplasm was bright and transparent, and some cells were eosinophilic. A large number of interstitial lymphocytes were found, and lymphoid follicles were formed in some regions. Immunohistochemistry results demonstrated that the tumor cells were p40+, CK7-, CK5/6+, p63+, CD117-, MUC5AC-, CEA+ (a small number of cells), EMA+, CK19+, Dog-1-, SMA-, S-100-, AR-, HER2-0, ER-, PR-, GCDFP-15-, and calponin-. In situ hybridization indicated that the tumor cells were EBER- and HER2-. The combined results of hematoxylin and eosin (H&E) morphology, immunohistochemistry, and EBER in situ hybridization were highly consistent with sebaceous lymphadenocarcinoma. The remaining cervical tissue was cleared, and metastases were found in regions 2 (1/6) and 5 (1/4) of the detected lymph nodes (Figure 3).
Based on radiographic results and histopathology, we finally confirmed the diagnosis of a sebaceous lymphadenocarcinoma with lymph node metastasis.
We implanted a temporary pacemaker under local anesthesia. Under general anesthesia, we resected the right parotid gland tumor, performed superficial lobectomy of the parotid gland, and dissected the facial nerve. After rapid intraoperative pathology showed poorly differentiated carcinoma of the parotid gland, we dissected the periparotid cervical lymph nodes. No apparent surgical complications occurred after surgery, and the patient was discharged 9 d after surgery.
The family members disagreed with our recommendation for chemotherapy or local radiotherapy because of the advanced age of the patient. No recurrence or metastasis occurred at 10-mo follow-up. We are still following the patient at the time of writing the report.
High-grade transformation of sebaceous glands has been found in 11%-28% of normal parotid glands and 6% of submandibular glands[1]. Differentiated sebaceous glands rarely appear in the salivary glands and are found most commonly in the parotid glands, with an incidence of 10%-42%. Sebaceous glands in the submandibular gland are even rarer (5%-6%), and have never been reported in the minor salivary glands. Cortical glands in the oral cavity, called Fordyce granules, have been found in 80% of individuals, most commonly in the buccal mucosa and the vermilion of the upper lip[2-4]. However, sebaceous gland tumors occur only rarely.
The 2005 World Health Organization (WHO) histopathological classification of tumors divides sebaceous gland tumors arising from the salivary glands into four types: Sebaceous adenoma, sebaceous lymphadenoma, sebaceous adenocarcinoma, and sebaceous lymphadenocarcinoma. The WHO 2017 classification of salivary gland tumors categorizes sebaceous lymphadenocarcinoma as the malignant transformation of sebaceous lymphadenoma. It is the rarest malignant sebaceous tumor in the salivary glands. At the time of writing this report, fewer than 10 cases have been reported (Table 1)[3-9].
| Ref. | Age, yr/sex | History | Location | Histology | Treatment | Follow-up |
| Linhartová et al[4] (1974) | 68/female | 2.5 yr | Parotid gland | Sebaceous carcinoma arising from lymphadenoma | Excision and postoperative radiotherapy | NER at 6 yr |
| Gnepp et al[5] (1984) | 70/male | 70 yr | Parotid gland | Poorly differentiated carcinoma arising from lymphadenoma | Excision and superficial parotidectomy | Died of other causes at 18 mo |
| Gnepp et al[6] (1983) | 70/male | 1 mo | Periparotid lymph nodes | Salivary duct carcinoma with focal epithelial– myoepithelial carcinoma arising from lymphadenoma | Excision and superficial parotidectomy | NER at 14 mo |
| Croitoru1 et al[7] (2003) | 55/male | 3 yr | Parotid gland | Sebaceous carcinoma arising from lymphadenoma | Excision and postoperative radiotherapy | NER at 4 mo |
| Ahn et al[8] (2006) | 36/male | > 10 yr | Parotid gland | Sebaceous carcinoma arising from lymphadenoma | Superficial parotidectomy and postoperative radiotherapy | NER at 24 mo |
| Claudius et al[3] (2011) | 87/male | 2–6 mo | Parotid gland | Sebaceous carcinoma arising from lymphadenoma with lymphangiosis carcinomatosa and cervical lymph node metastases | Excision, neck dissection and postoperative adjuvant radiotherapy | Died of respiratory failure at 8 mo |
The origins of sebaceous lymphadenomas and their malignant transformations have been controversial. Gnepp and Brannon reported that these tumors are probably caused by ectopic salivary gland tissue in the lymph nodes, and that their occurrence is similar to that of Warthin tumors[2,5]. Other theories have shown that there are residual or inclusion bodies of sebaceous glands in the lymph nodes around the parotid gland.
In a case where the ages of the patients were between 30 and 70 years, the tumors were located in the parotid gland or periparotid lymph nodes. The disease courses ranged from 1 mo to 20 years. In some cases, the skin of the parotid region was involved, which appeared as confluent purplish-red papules[3].
The tumors have been variously reported as brown, yellowish-brown to gray, grayish-white, and white–brown in color, and the largest was 6 cm in diameter. The tumors have partial capsules, present with local infiltrative growth, and include both benign and malignant components of a sebaceous lymphadenoma[10]. The benign components include cortices, sebaceous lymphadenomas, epithelial infiltration of different sizes in the lymphoid tissue, formation of lymphoid follicles, and different degrees of invasive pleomorphic cancer cells. The malignant components comprise squamous cell carcinomas, sebaceous adenocarcinomas, and lamellar undifferentiated carcinomas, accompanied by regional ductal differentiation. Adenoid cystic carcinomas and epithelial myoepithelial lesions have also been found, with obvious heteromorphism and nuclear division. Several cases of nerve invasion have been reported. As many as 24 cell divisions have been seen for every × 10 high-power magnification. The tumors present with tissue and cell aggregation and multinucleated foreign body giant cells[7]. No atypical cells have been found in the sebaceous lymphadenomas.
Sebaceous lymphadenocarcinomas should be differentiated from sebaceous adenocarcinomas, mucoepidermoid carcinomas, epithelial myoepithelial carcinomas, squamous cell carcinomas accompanied by clear cells, and metastatic sebaceous adenocarcinomas.
The origins and immunohistochemical characteristics of sebaceous adenocarcinomas and sebaceous lymphadenocarcinomas are different. Based on Ahn et al[8] research, sebaceous lymphadenocarcinoma tumor tissue is SMA-positive. Another difference is that sebaceous lymphadenocarcinomas arise from the malignant transformation of sebaceous lymphadenomas, which is the primary form.
Cortical lymphadenocarcinomas may be confused with mucoepidermoid carcinomas histologically. Mucoepidermoid carcinomas have clear cytoplasm as well as intracellular mucin in some cells. In the presence of foreign bodies, the foreign body reaction also helps to differentiate cortical lymphadenocarcinomas from mucoepidermoid carcinomas. Mucoepidermoid carcinomas contain intracellular mucin in some transparent cells, but mucin-positive cells are not found in transparent sebaceous cells[2,9].
Glandular epithelial cells are common in myoepithelium-predominant epithelial–myoepithelial carcinomas, appearing in tubular arrangements. Immunohistochemistry has shown CK-positive cells, and clear myoepithelial cell SMA, actin, or myosin. Scanning electron microscopy has been used to visualize the myofilaments.
In squamous cell carcinomas combined with Periodic Acid-Schiff-positive transparent cells with glycogen aggregation, adjacent epithelial hyperplasia has occurred. Basaloid squamous carcinoma can be characterized by the presence of highly atypical basaloid cells accompanied by clear squamous cell differentiation, occasionally acne-like necrosis, and no foam-like cytoplasm[2,9].
Metastatic sebaceous adenocarcinomas of the parotid glands are rare and can metastasize to the parotid lymph nodes. A reported case of a sebaceous adenocarcinoma of the eyelid metastasizing to the parotid lymph nodes underscores the importance of considering patient medical histories when screening for the presence of sebaceous lymphadenocarcinomas[11].
The treatment of these tumors depends on the typing and clinical staging of the cancer. Extensive surgical resection is the first choice, and adjuvant radiotherapy should be given to patients with high-grade tumors or those determined to be at an advanced clinical stage. There is currently no long-term, follow-up survival data for the few published cases. One report did not include follow-up information and two others reported one patient death each after 8 mo and 1.5 years postoperatively; both from causes unrelated to their cancer. Another four patients survived without recurrence during the reported 4-mo to 6-year follow-up. In our case, the family members disagreed with our recommendation for chemotherapy or local radiotherapy because of the advanced age of the patient. No recurrence or metastasis occurred at 10-mo follow-up. We are still following the patient at the time of writing this report.
The treatment of sebaceous lymphadenocarcinoma depends on the typing and clinical staging of the cancer. Extensive surgical resection is the first choice, and adjuvant radiotherapy should be given to patients with high-grade tumors or those determined to be at an advanced clinical stage. For such rare tumor sites, it is important for oral and maxillofacial surgeons to review the clinical presentation, histology, and management of sebaceous lymphadenocarcinoma.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dereci O S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | Gnepp DR, Sporck FT. Benign lymphoepithelial parotid cyst with sebaceous differentiation--cystic sebaceous lymphadenoma. Am J Clin Pathol. 1980;74:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Gnepp DR. My journey into the world of salivary gland sebaceous neoplasms. Head Neck Pathol. 2012;6:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Claudius K, Ginzkey C, Gattenlöhner S, Müller J, Demmer P, Bröcker EB. A red cheek as first clinical sign of a sebaceous lymphadenocarcinoma of the parotid gland with lymphangiosis carcinomatosa and lymph node metastases. Am J Dermatopathol. 2011;33:e50-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Linhartová A. Sebaceous glands in salivary gland tissue. Arch Pathol. 1974;98:320-324. [PubMed] |
| 5. | Gnepp DR, Brannon R. Sebaceous neoplasms of salivary gland origin. Report of 21 cases. Cancer. 1984;53:2155-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Gnepp DR. Sebaceous neoplasms of salivary gland origin: a review. Pathol Annu. 1983;18 Pt 1:71-102. [PubMed] |
| 7. | Croitoru CM, Mooney JE, Luna MA. Sebaceous lymphadenocarcinoma of salivary glands. Ann Diagn Pathol. 2003;7:236-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Ahn SH, Park SY. Sebaceous lymphadenocarcinoma of parotid gland. Eur Arch Otorhinolaryngol. 2006;263:940-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Seethala RR, Stenman G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol. 2017;11:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 272] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 10. | Seethala RR, Thompson LD, Gnepp DR, Barnes EL, Skalova A, Montone K, Kane S, Lewis JS Jr, Solomon LW, Simpson RH, Khan A, Prasad ML. Lymphadenoma of the salivary gland: clinicopathological and immunohistochemical analysis of 33 tumors. Mod Pathol. 2012;25:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Lally SE, Rao R, Shields JA, Shields CL. Comparison of posterior lamellar resection vs lumpectomy for initial management of localized tarsal conjunctival sebaceous carcinoma in 54 cases. Indian J Ophthalmol. 2018;66:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |