Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5535
Peer-review started: April 26, 2020
First decision: August 23, 2020
Revised: September 4, 2020
Accepted: September 16, 2020
Article in press: September 16, 2020
Published online: November 26, 2020
Processing time: 212 Days and 21.5 Hours
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in China, constitutes a Public Health Emergency of International Concern. It is well known that COVID-19 patients may have increased serum lactate dehydrogenase (LDH) levels in the early stage. The clinical changes in LDH may have predictive value in disease evolution and prognosis in critically ill COVID-19 patients.
To examine serum LDH and clinical characteristics in patients with COVID-19 and their predictive value for prognosis.
This retrospective study analyzed the clinical data of forty-seven critical COVID-19 patients in the intensive care unit of the Third People's Hospital of Yichang City from January 27 to March 25, 2020 and divided them into survivors and non-survivors. The patients were diagnosed according to the World Health Organization interim guidance and critical cases met any one of the following criteria: Respiratory failure and required mechanical ventilation, the occurrence of shock, and the combined failure of other organs that required intensive care unit monitoring and treatments, according to the diagnostic criteria of critical COVID-19. Clinical data including symptoms, detection of SARS-CoV-2, chest computed tomography (CT) images, changes in serum LDH in different clinical phases, and prognosis were collected. Statistical analysis of the data was performed. Continuous variables were expressed as median (interquartile range) and compared with the Mann-Whitney U test. Categorical variables were compared with the Chi-square test. Survival data were analyzed using Kaplan-Meier survival curves and log-rank tests.
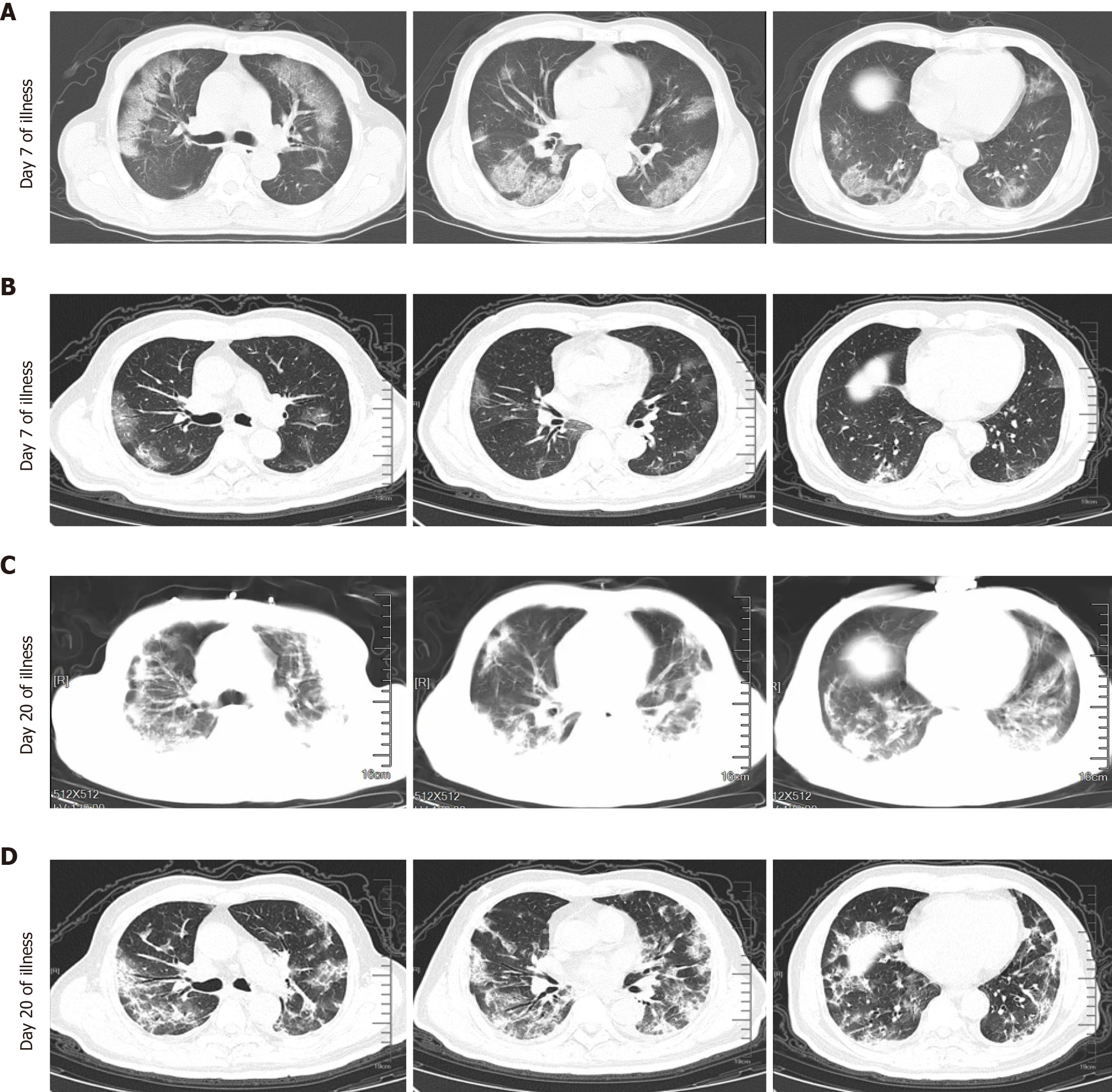
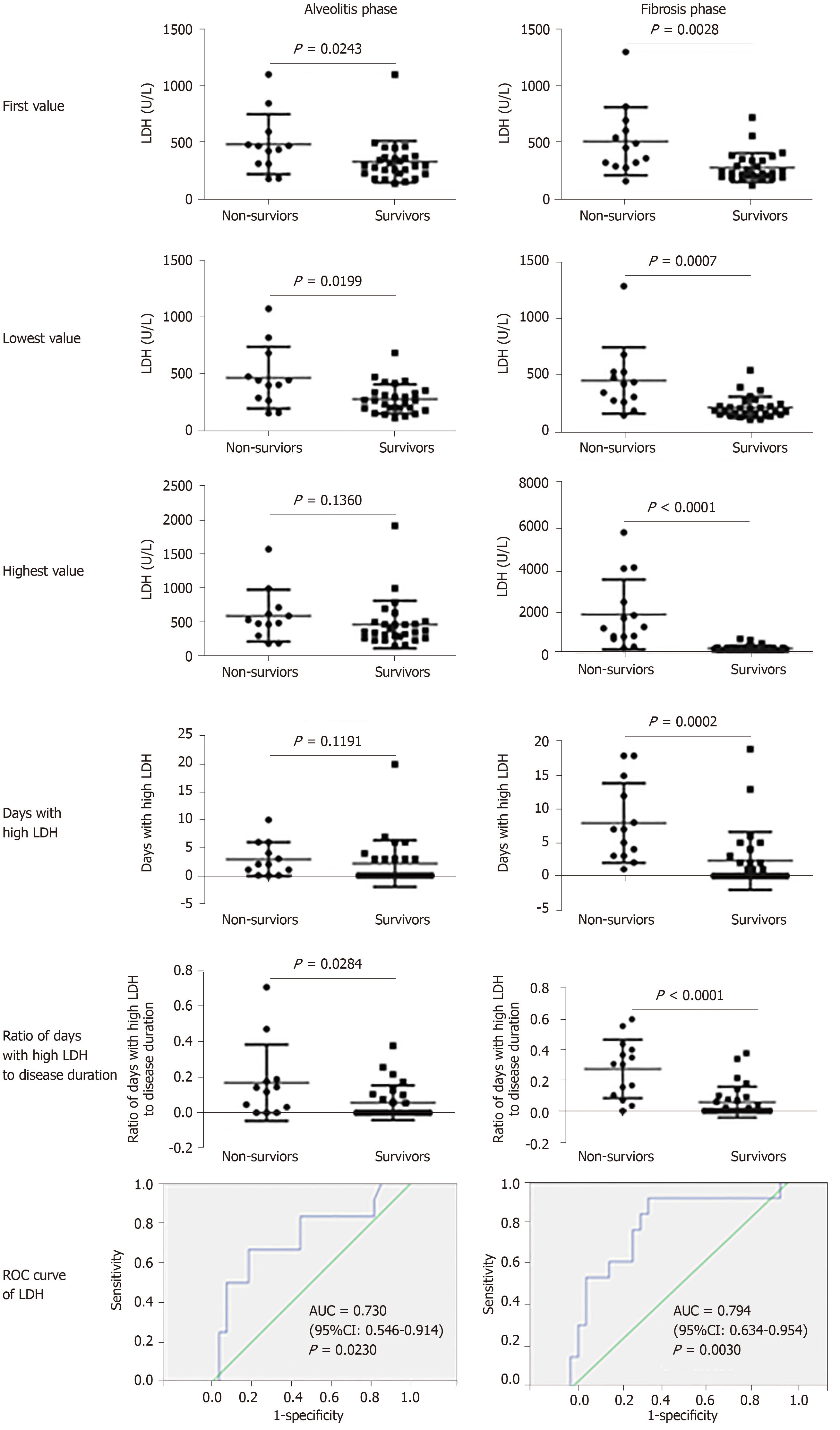
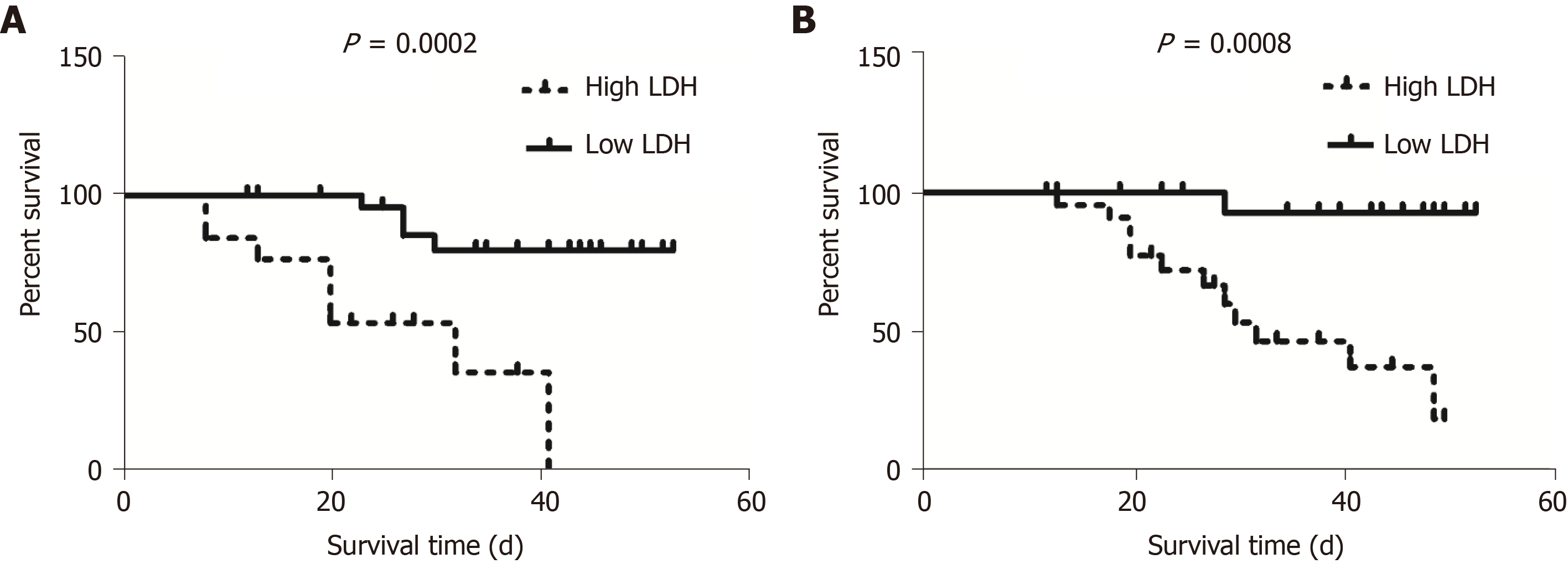
According to chest CT images, we observed the alveolitis and fibrosis stages in all critical patients in this study. Most non-survivors died in the fibrosis stage. Non-survivors had fewer days of hospitalization, shorter disease duration, shorter duration of alveolitis and fibrosis, and had dyspnea symptoms at disease onset (P = 0.05). Both first and lowest LDH values in the alveolitis stage were more pronounced in non-survivors than in survivors (449.0 U/L vs 288.0 U/L, P = 0.0243; 445.0 U/L vs 288.0 U/L, P = 0.0199, respectively), while the first, lowest and highest values of serum LDH in non-survivors were all significantly increased compared to survivors in the fibrosis phase (449.0 U/L vs 225.5 U/L, P = 0.0028; 432.0 U/L vs 191.0 U/L, P = 0.0007; 1303.0 U/L vs 263.5 U/L, P = 0.0001, respectively). The cut-off points of first LDH values in the alveolitis and fibrosis phase for distinction of non-survivors from survivors were 397.0 U/L and 263.0 U/L, respectively. In the fibrosis stage, non-survivors had more days with high LDH than survivors (7.0 d vs 0.0 d, P = 0.0002). Importantly, patients with high LDH had a significantly shorter median survival time than patients with low LDH in the alveolitis phase (22.0 d vs 36.5 d, P = 0.0002), while patients with high LDH also had a significantly shorter median survival time than patients with low LDH in the fibrosis phase (27.5 d vs 40.0 d, P = 0.0008). The proportion of non-survivors with detectable SARS-CoV-2 until death in the alveolitis stage was significantly increased compared with that in the fibrosis stage (100% vs 35.7%, P = 0.0220).
High LDH and dyspnea symptoms were positive predictors of an adverse outcome in critical COVID-19. The rapid progressive fibrosis stage was more perilous than the alveolitis stage, even if SARS-CoV-2 is undetectable.
Core Tip: We examined the serum lactate dehydrogenase (LDH) and clinical characteristics in critical coronavirus disease 2019 (COVID-19) patients and their predictive value for prognosis. We retrospectively evaluated 47 critical COVID-19 patients and divided them into non-survivors and survivors. Clinical data including symptoms, detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), chest computed tomography images, changes in serum LDH in different clinical phases, and prognosis were collected. In this study, high LDH was positively correlated with worsening overall survival. High LDH and dyspnea symptoms were positive predictors of adverse outcome in critical COVID-19 patients. The rapid progressive fibrosis stage may be more perilous than the alveolitis stage, even if SARS-CoV-2 is undetectable.
- Citation: Lv XT, Zhu YP, Cheng AG, Jin YX, Ding HB, Wang CY, Zhang SY, Chen GP, Chen QQ, Liu QC. High serum lactate dehydrogenase and dyspnea: Positive predictors of adverse outcome in critical COVID-19 patients in Yichang. World J Clin Cases 2020; 8(22): 5535-5546
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5535.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5535
The large-scale new coronavirus disease pneumonia (coronavirus disease 2019, COVID-19) broke out in Wuhan City, China and the basic reproductive number was 2.68 (95%CI: 2.47-2.86)[1,2]. Millions of cases have been reported worldwide, and the fatality rate was up to 7%[3]. The pathogen, officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was confirmed to be related to Middle East respiratory syndrome and severe acute respiratory syndrome (SARS)[3-6]. However, the prognosis of critically ill and severe patients is extremely poor. It is well known that some patients have increased lactate dehydrogenase (LDH) levels in the early stage of COVID-19[7] and predictors of poor outcome in SARS were increased age, comorbidities and high LDH in 2003[8]. Whether serum LDH can be used as a marker related to COVID-19 treatment response as well as SARS is inconclusive.
Computed tomography (CT) images and pathological findings of critical COVID-19 patients have characteristic manifestations in the alveolitis and fibrosis stages. Chest CT images are characterized by multiple ground-glass opacities (GGO) and early infiltration in the acute alveolitis stage. Furthermore, critical cases often subsequently progressed to the fibrosis stage and showed consolidation, reticular pattern, and other fibrosis patterns on chest CT[7,9-11]. In addition, autopsy results in critical COVID-19 patients who died within 14 d demonstrated typical pathological changes of acute respiratory distress syndrome such as desquamation of pneumocytes, hyaline membrane formation, interstitial monocytes and lymphocytes inflammatory infiltrates[12], while pathological findings of patients who died within 17-19 d showed lighter exudation of alveolar fluid and cellulose, less hyaline membrane, obvious changes in the proliferation of type II alveolar epithelial cells and alveolar fleshy change and interstitial fibrosis[13]. The pathological findings were in accordance with CT images in the different stages of COVID-19. Early recognition and isolation of critical COVID-19 patients in the alveolitis and fibrosis stages are crucial in controlling this outbreak.
The aim of this study was to describe the clinical characteristics, and to examine the clinical changes in LDH in the alveolitis and fibrosis stages according to the CT findings in critically ill COVID-19 patients in the intensive care unit (ICU) and their predictive value for clinical prognosis.
A total of 47 patients in the ICU of the Third People's Hospital of Yichang City (one of the highly impacted epidemic areas in China) from January 27 to March 25, 2020 were enrolled in this study. The Third People's Hospital of Yichang is a teaching hospital affiliated to Sanxia University and is also an infectious disease specialist hospital responsible for the treatment of COVID-19 patients as assigned by the government. The patients were diagnosed according to the World Health Organization interim guidance[14] and critical cases met any one of the following criteria: respiratory failure and required mechanical ventilation, the occurrence of shock, and the combined failure of other organs that required ICU monitoring and treatments, according to the diagnostic criteria of critical COVID-19[7]. The protocols for the study and informed consents were approved by the ethics committee of First Affiliated Hospital of Fujian Medical University [Approval No. (2020) 153]. All survivors were discharged following two negative nucleic acid tests of throat swab samples[7].
General information included the gender, age, days of hospitalization, disease duration, duration of viral shedding, days from illness onset to hospital admission, duration of alveolitis and fibrosis phases, the complications of chronic obstructive pulmonary disease, hypertension, coronary heart disease, cerebrovascular disease, diabetes, renal dysfunction, malignant tumors and other underlying diseases, the numbers of complications, the initial symptoms such as fever, cough, dyspnea, fatigue and so on were obtained. Chest CT images were used to make a distinction between the alveolitis and fibrosis phases.
The serum concentrations of LDH were detected using an appropriate detection Kit (No. A0701) ordered from Sichuan Chengdu New Health City Biological Co., Ltd. (China), according to the manufacturer’s instructions. The recommended reference range for the normal population is (109-245) U/L. Throat swab samples were collected from the patients and fluorescent polymerase chain reaction was used to detect the ribonucleic acid (RNA) of SARS-CoV-2 using the new coronavirus 2019-nCov nucleic acid detection kit (No. DA0930-DA0932) provided by Sun Yat-sen University Daan Gene Co., Ltd. (China). Patients with positive detection of SARS-CoV-2 RNA were identified as confirmed cases.
Statistical analysis was performed using the Statistic Package for Social Science 18.0 software package (Statistic Package for Social Science Inc., Chicago, IL, United States). Continuous variables were expressed as median (interquartile range) and compared using the Mann-Whitney U test. Categorical variables were compared using the Chi-square test. Survival data were analyzed using Kaplan-Meier survival curves and the log-rank test. For all tests, P < 0.05 was considered statistically significant.
Forty-seven COVID-19 patients were divided into non-survivors (n = 17) and survivors (n = 30). The baseline characteristics of the patients with critical COVID-19 are summarized in Table 1. Non-survivors had fewer days of hospitalization, shorter disease duration and more dyspnea symptoms than survivors (P = 0.01). There was no significant difference between the two groups in terms of age (P = 0.595), gender (P = 0.330), days from illness onset to hospital admission (P = 0.689), duration of viral shedding (P = 0.118), complications as well as the number of complications (P = 0.139).
| Clinical features | Groups | P value | |
| Non-survivors (n = 17) | Survivors (n = 30) | ||
| Age (yr) | 69.0 (63.5-78.0) | 67.0 (55.5-77.0) | 0.595 |
| Male (%) | 11 (64.7) | 15 (50.0) | 0.33 |
| Days of hospitalization (d) | 14.0 (8.0-24.5) | 33.0 (16.8-38.3) | 0.001b |
| Disease duration (d)1 | 23.0 (15.5-29.5) | 38.0 (24.5-45.3) | 0.005b |
| Duration of viral shedding (d) | 8.0 (6.0-18.0) | 11.0 (8.8-20.5) | 0.118 |
| Days from illness onset to hospital admission (d) | 5.0 (2.0-10.0) | 5.0 (3.75-7.25) | 0.689 |
| Duration of alveolitis phase (d) | 12.0 (8.0-17.5) | 17.0 (13.8-21.0) | 0.020a |
| Duration of fibrosis phase (d) | 10.0 (5.5-15.5) | 19.0 (10.0-26.0) | 0.016a |
| Complication of COPD (%) | 3 (17.6) | 3 (10.0) | 0.764 |
| Complication of hypertension (%) | 10 (58.8) | 11 (36.7) | 0.142 |
| Complication of coronary heart disease (%) | 7 (41.2) | 6 (20.0) | 0.222 |
| Complication of cerebrovascular disease (%) | 3 (17.6) | 7 (23.3) | 0.931 |
| Complication of diabetes (%) | 5 (29.4) | 7 (23.3) | 0.912 |
| Complication of renal dysfunction (%) | 2 (11.8) | 5 (16.7) | 0.978 |
| Complication of malignant tumors (%) | 2 (11.8) | 3 (10.0) | 1.000 |
| Other complications (%)2 | 7 (41.2) | 6 (20.0) | 0.222 |
| Number of complications | 2.0 (1.5-3.5) | 1.5 (0.0-3.0) | 0.139 |
| Fever (%) (temperature ≥ 37.3°C) | 13 (76.5) | 26 (86.7) | 0.624 |
| Cough (%) | 7 (41.2) | 20 (66.7) | 0.089 |
| Dyspnea (%) | 9 (52.9) | 4 (13.3) | 0.010a |
| Fatigue (%) | 5 (29.4) | 4 (13.3) | 0.337 |
| Other symptoms (%)3 | 5 (29.4) | 4 (13.3) | 0.337 |
According to chest CT images, we observed the alveolitis and fibrosis stages in all critical patients included in the study. Chest CT images of the alveolitis stage showed that patients with critical COVID-19 had bilateral lung involvement, mainly with peripheral and diffuse distribution and focal GGO. Following progression to the fibrosis stage, chest CT showed consolidation and pulmonary interstitial fibrosis changes such as the air bronchogram sign, bronchiectasis and reticular pattern. Some non-survivors showed more consolidation lesions than the survivors (Figure 1). According to the imaging findings, we found that the duration of inflammation and fibrosis was shorter in non-survivors than in survivors (P = 0.020 and 0.016).
The results (Figure 2) showed that both first and lowest LDH values in the alveolitis stage were more pronounced in non-survivors than in survivors (449.0 U/L vs 288.0 U/L, P = 0.0243; 445.0 U/L vs 288.0 U/L, P = 0.0199, respectively). No difference at the highest value of serum LDH between non-survivors and survivors in this stage was found (499.0 U/L vs 349.0 U/L, P = 0.1360). The cutoff point of the first serum LDH concentration in the alveolitis stage for differentiating non-survivors from survivors was 397.0 U/L, according to receiver operating characteristic curve analysis. The ratio of days with high LDH (more than 397.0 U/L) to disease duration in non-survivors was markedly higher compared to survivors (0.14 vs 0.00, P = 0.0284), although the days with high LDH was not statistically significant between the two groups in the alveolitis stage.
In the fibrosis phase, the first, lowest and highest values of serum LDH in non-survivors were all significantly increased compared to survivors (449.0 U/L vs 225.5 U/L, P = 0.0028; 432.0 U/L vs 191.0 U/L, P = 0.0007; 1303.0 U/L vs 263.5 U/L, P = 0.0001, respectively). The cutoff value of serum LDH for differentiating non-survivors from survivors was 263.0 U/L. Non-survivors had more days with LDH above 263.0 U/L than survivors (7.0 vs 0.0 d, P = 0.0002) in the fibrosis phase. Moreover, the ratio of days with high LDH to disease duration in non-survivors was higher than that in survivors (0.31 vs 0.00, P = 0.0001). Our results revealed that serum LDH was increased in non-survivors, and the elevated levels of serum LDH were positively related to adverse outcome in the acute alveolitis and fibrosis phases.
Kaplan-Meier analysis and the log-rank test showed that patients with high LDH (≥ 397.0 U/L) had a significantly shorter median survival time compared to those with low LDH (< 397.0 U/L) in the alveolitis phase (22.0 d vs 36.5 d, P = 0.0002) (Figure 3A). In addition, patients with high LDH (≥ 263.0 U/L) also had a significantly shorter median survival time compared to those with low LDH (< 263.0 U/L) in the fibrosis phase (27.5 d vs 40.0 d, P = 0.0008) (Figure 3B).
All non-survivors who died in the alveolitis stage had detectable SARS-CoV-2 until death, but only some patients (35.7%) who died in the fibrosis stage had detectable SARS-CoV-2. The proportion of non-survivors with detectable SARS-CoV-2 until death in the alveolitis stage was significantly increased compared to that in the fibrosis stage (P = 0.0220) (Figure 4).
Our results showed that fewer days of hospitalization, shorter course of disease, shorter duration of alveolitis and fibrosis, and more dyspnea symptoms at disease onset were positively associated with adverse outcome in critical COVID-19. In addition, the first and lowest values of LDH in the alveolitis and fibrosis phase, the highest LDH value in the fibrosis phase of non-survivors were higher than those in survivors, together with more days with high LDH (≥ 263.0 U/L) in the fibrosis phase and the higher ratio of days with high LDH levels (≥ 397.0 U/L) in the alveolitis phase and ≥ 263.0 U/L in the fibrosis phase, respectively, to disease duration. The elevated levels of first LDH in the acute alveolitis and fibrosis phases were correlated with worsening overall survival. The rapid progressive fibrosis stage may be more perilous than the alveolitis stage, even if SARS-CoV-2 is undetectable.
It was reported that older age was correlated with unfavorable outcomes in COVID-19, SARS and Middle East respiratory syndrome[15-18]. A previous study confirmed that SARS-CoV-infected aged macaques developed an exacerbated innate host response with an increased expression of genes associated with inflammation[18]. The current study showed that there was no significant difference between non-survivors and survivors in terms of the age of patients in Yichang, and the median age of survivors with critical COVID-19 was greater than COVID-19 survivors in Wuhan (67.0 years vs 52.0 years)[15]. These results supported that older age may be a predictor of death in patients with critical COVID-19. In addition, there was no significant difference between the two groups in terms of gender as well as the median number of days from illness onset to hospital admission, similar to COVID-19 patients in Wuhan[15].
The median number of days from illness onset to hospital admission (5.0 d) was similar in survivors and non-survivors, and this value in Wuhan patients was 11.0 d. The concerns related to COVID-19 in Wuhan city helped patients receive earlier recognition and treatment in Yichang. Our results indicated that shorter disease course, shorter duration of alveolitis and fibrosis were associated with death in Yichang, which suggested non-survivors had more rapid progression, irrespective of disease stage.
Based on chest CT appearance, critical patients had diffuse GGO in the alveolitis phase, and massive consolidation lesions in the fibrosis phase. Fewer GGO and consolidation lesions were found in survivors than in non-survivors. Most non-survivors in our study died in the fibrosis phase (14/17), and few died in the alveolitis phase (3/17). It was reported that the virus was continuously detectable until death in all non-survivors[15]. Our study demonstrated that the alveolitis phase was significantly associated with detectable SARS-CoV-2 in non-survivors and only 35.7% of non-survivors had detectable SARS-CoV-2 until death in the fibrosis phase. The replicated SARS-CoV injured alveolar epithelial cells, leading to alveolitis and fibrotic lesions[19]. Combined with shorter duration of the fibrosis stage in non-survivors, the rapid progressive fibrosis stage may be more perilous than the alveolitis stage, even if SARS-CoV-2 is undetectable. Accumulating evidence suggests that a subgroup of patients with severe COVID-19 might have cytokine storm syndrome[20], and it was difficult for those who died in the alveolitis stage to eradicate the virus, and duration of viral shedding in these patients may be longer.
In addition, dyspnea symptoms at disease onset were associated with fatal outcome in the COVID-19 patients in our study. Dyspnea was also an independent clinical factor for H1N1 pneumonia[21]. There are many possible causes for dyspnea. On the one hand, pathological findings in COVID-19 patients showed pulmonary edema and hyaline membrane formation[12]. Aquaporin 5 (AQP5) and AQP1, located in the endothelial cells and secretory cells of terminal bronchioles and the alveolar type I cells, play an important role in water clearance in lungs. It was reported that porcine reproductive and respiratory syndrome virus infection usually caused pulmonary inflammation and edema in the infected lungs[13]. The expression of AQPs and Na, K-adenosine triphosphatase may be downregulated, causing lung edema with apoptosis of alveolar epithelial cells in porcine reproductive and respiratory syndrome virus infection. The replicated SARS-CoV may cause alveolitis and fibrotic changes, leading to acute lung injury that may develop into life-threatening acute respiratory distress syndrome[18,19]. On the other hand, a substantial portion of SARS patients had evidence of respiratory muscle weakness leading to air trapping, whereas inspiratory muscle weakness may lead to atelectasis, as at least 40% of patients suffered from acute respiratory failure requiring supplemental oxygen[22]. Respiratory muscle weakness may be one cause of dyspnea.
LDH has been reported to be a useful inflammatory biomarker of community acquired pneumonia, Mycoplasma pneumoniae pneumonia, and complicated pneumonia[23-25]. Our findings indicated that COVID-19 was associated with high levels of LDH, and elevated serum LDH could be used as a severity and poor prognosis indicator in patients with critical COVID-19 at different stages. Moreover, the best threshold of LDH level for predicting COVID-19 in the alveolitis and fibrosis phase was 397.0 U/L and 263.0 U/L, respectively. Among non-survivors, the first and lowest values of LDH in the alveolitis and fibrosis phase, and the highest LDH value in the fibrosis phase were higher than those in survivors, together with more days with high LDH (≥ 263.0 U/L) in the fibrosis phase and a higher ratio of days with high LDH levels (≥ 397.0 U/L in the alveolitis phase and ≥ 263.0 U/L in the fibrosis phase, respectively) to disease duration. Laboratory results showed that increases in LDH (28.3%) were more common in patients with COVID-19[3]. The level of LDH in severe patients was significantly higher than those in mild patients in Wuhan[15,26].
Possible sources of elevated serum LDH levels during infection may be immunologic changes after SARS-COV-2 infection of the upper and lower respiratory tract which result in an early acute respiratory inflammatory response with consequent release of pro-inflammatory cytokines, including interleukin-1β, followed by inflammasome activation and production of active mature interleukin-1β which is a mediator of lung inflammation and fibrosis[27]. High levels of serum LDH were also reported to be associated with the severe form of H1N1 influenza, and serum LDH level above 500 U/L was significantly more common in patients with pneumonia[21,28]. Lung parenchymal cells and/or local inflammatory cells may be potential sources of elevated LDH in serum[29,30] and elevated serum values of LDH indirectly indicate lung tissue damage[31,32]. Therefore, the best LDH threshold for predicting COVID-19 in the acute alveolitis phase was higher than that in the fibrosis phase. The first, lowest and highest values of LDH in the alveolitis and fibrosis phase, together with more days with high LDH and a higher ratio of days with high LDH levels, were important for predicting the severity of critical COVID-19. As critical COVID-19 in the ICU is often combined with bacterial infection, LDH can convert pyruvate to lactate and might be the key enzyme for pneumococcal pyruvate metabolism and thus pneumococcal survival in blood[33].
High serum LDH and dyspnea symptoms in the early stages of infection may predict the severity and poor prognosis in patients with critical COVID-19. The disease in non-survivors developed rapidly in both the alveolitis stage and fibrosis stage, and the progressive fibrosis stage may be more perilous than the alveolitis stage, even if SARS-CoV-2 is undetectable.
Millions of new coronavirus disease pneumonia (coronavirus disease 2019, COVID-19) cases have been reported worldwide. Moreover, the prognosis of critically ill and severe COVID-19 patients is extremely poor.
COVID-19 patients may have increased serum lactate dehydrogenase (LDH) levels in the early stage. The clinical changes in LDH may have predictive value in disease evolution and prognosis in critically ill COVID-19 patients.
To describe the clinical characteristics and examine the clinical changes in LDH in the alveolitis and fibrosis stages according to computed tomography findings in critically ill COVID-19 patients and their predictive value for clinical prognosis.
We analyzed the clinical data of forty-seven critical COVID-19 patients in the intensive care unit of the Third People's Hospital of Yichang City and divided them into non-survivors and survivors. Clinical data including symptoms, detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), chest computed tomography images, changes in serum LDH in different clinical phases, and prognosis were collected.
Most non-survivors died in the fibrosis stage. Non-survivors had fewer days of hospitalization, shorter disease duration, shorter duration of alveolitis and fibrosis, and had dyspnea symptoms at disease onset. Both first and lowest LDH values in the alveolitis and fibrosis stage were more pronounced in non-survivors than in survivors. Importantly, patients with high LDH had a significantly shorter median survival time in the alveolitis and fibrosis phase.
High serum LDH and dyspnea symptoms in the early stages of infection were positive predictors of severity and poor prognosis in patients with critical COVID-19. The rapid progressive fibrosis stage was more perilous than the alveolitis stage, even if SARS-CoV-2 is undetectable.
The immunologic mechanism of elevated serum LDH during SARS-COV-2 infection requires further investigation.
Thanks to all the medical teams that went to Hubei province to battle the COVID-19 epidemic in China. The authors thank Yujie Lin for reviewing and editing the article.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maurea S, Phan T S-Editor: Zhang L L-Editor: Webster JR P-Editor: Xing YX
| 1. | Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health? Lancet. 2020;395:542-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 580] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 2. | Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3012] [Cited by in RCA: 2500] [Article Influence: 500.0] [Reference Citation Analysis (0)] |
| 3. | Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 886] [Cited by in RCA: 846] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 4. | Malik YS, Sircar S, Bhat S, Sharun K, Dhama K, Dadar M, Tiwari R, Chaicumpa W. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;40:68-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 5. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8473] [Cited by in RCA: 7586] [Article Influence: 1517.2] [Reference Citation Analysis (0)] |
| 6. | Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2713] [Cited by in RCA: 2763] [Article Influence: 552.6] [Reference Citation Analysis (0)] |
| 7. | National Health Commission. Guideline of Diagnosis and Treatment for COVID-19 (7th Edition). Available from: http://www.gov.cn/zhengce/zhengceku/2020-03/04/5486705/files/ae61004f930d47598711a0d4cbf874a9.pdf. |
| 8. | Auyeung TW, Lee JS, Lai WK, Choi CH, Lee HK, Lee JS, Li PC, Lok KH, Ng YY, Wong WM, Yeung YM. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51:98-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Zhou M, Zhang X, Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med. 2020;14:126-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 10. | Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381-4389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 896] [Cited by in RCA: 790] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 11. | Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295:715-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1617] [Cited by in RCA: 1756] [Article Influence: 351.2] [Reference Citation Analysis (0)] |
| 12. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5776] [Article Influence: 1155.2] [Reference Citation Analysis (2)] |
| 13. | Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 461] [Reference Citation Analysis (0)] |
| 14. | World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/330893/WHO-nCoV-Clinical-2020.3-eng.pdf?sequence=1&isAllowed=y. |
| 15. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18177] [Article Influence: 3635.4] [Reference Citation Analysis (0)] |
| 16. | Choi KW, Chau TN, Tsang O, Tso E, Chiu MC, Tong WL, Lee PO, Ng TK, Ng WF, Lee KC, Lam W, Yu WC, Lai JY, Lai ST; Princess Margaret Hospital SARS Study Group. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 277] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Hong KH, Choi JP, Hong SH, Lee J, Kwon JS, Kim SM, Park SY, Rhee JY, Kim BN, Choi HJ, Shin EC, Pai H, Park SH, Kim SH. Predictors of mortality in Middle East respiratory syndrome (MERS). Thorax. 2018;73:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Smits SL, de Lang A, van den Brand JM, Leijten LM, van IJcken WF, Eijkemans MJ, van Amerongen G, Kuiken T, Andeweg AC, Osterhaus AD, Haagmans BL. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6:e1000756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 19. | Xie L, Liu Y, Xiao Y, Tian Q, Fan B, Zhao H, Chen W. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127:2119-2124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6741] [Article Influence: 1348.2] [Reference Citation Analysis (0)] |
| 21. | Kanchana S, Kanchana S, Vijitsopa T, Thammakumpee K, Yamwong S, Sawanyawisuth K. Clinical factors predictive of pneumonia caused by pandemic 2009 H1N1 influenza virus. Am J Trop Med Hyg. 2013;88:461-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, Ko FW, Chan MC, Chan DP, Tong MW, Rainer TH, Ahuja AT, Cockram CS, Sung JJ. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 359] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 23. | Tao RJ, Luo XL, Xu W, Mao B, Dai RX, Li CW, Yu L, Gu F, Liang S, Lu HW, Chen KB, Bai JW, Ji XB, Gu SY, Sun XL, Dai FH, Jiang P, Cao WJ, Xu JF. Viral infection in community acquired pneumonia patients with fever: a prospective observational study. J Thorac Dis. 2018;10:4387-4395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Saraya T, Ohkuma K, Tsukahara Y, Watanabe T, Kurai D, Ishii H, Kimura H, Goto H, Takizawa H. Correlation between clinical features, high-resolution computed tomography findings, and a visual scoring system in patients with pneumonia due to Mycoplasma pneumoniae. Respir Investig. 2018;56:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Breuer O, Picard E, Benabu N, Erlichman I, Reiter J, Tsabari R, Shoseyov D, Kerem E, Cohen-Cymberknoh M. Predictors of Prolonged Hospitalizations in Pediatric Complicated Pneumonia. Chest. 2018;153:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, Huang X, Lv J, Luo Y, Shen L, Yang H, Huang G, Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 643] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 27. | Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 592] [Reference Citation Analysis (0)] |
| 28. | Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA; INER Working Group on Influenza. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 940] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 29. | Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 362] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Liu TY, Lee WJ, Tsai CM, Kuo KC, Lee CH, Hsieh KS, Chang CH, Su YT, Niu CK, Yu HR. Serum lactate dehydrogenase isoenzymes 4 plus 5 is a better biomarker than total lactate dehydrogenase for refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Neonatol. 2018;59:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Mura M, Andrade CF, Han B, Seth R, Zhang Y, Bai XH, Waddell TK, Hwang D, Keshavjee S, Liu M. Intestinal ischemia-reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock. 2007;28:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Ding CY, Peng L, Lin YX, Yu LH, Wang DL, Kang DZ. Elevated Lactate Dehydrogenase Level Predicts Postoperative Pneumonia in Patients with Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019;129:e821-e830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Gaspar P, Al-Bayati FA, Andrew PW, Neves AR, Yesilkaya H. Lactate dehydrogenase is the key enzyme for pneumococcal pyruvate metabolism and pneumococcal survival in blood. Infect Immun. 2014;82:5099-5109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |