Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5250
Peer-review started: September 1, 2020
First decision: September 13, 2020
Revised: September 16, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: November 6, 2020
Processing time: 66 Days and 6.1 Hours
Since the beginning of the pandemic, coronavirus disease-2019 (COVID-19) in children has shown milder cases and a better prognosis than adults. Although the respiratory tract is the primary target for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), cardiovascular involvement is emerging as one of the most significant and life-threatening complications of SARS-CoV-2 infection in adults.
To summarize the current knowledge about the potential cardiovascular involvement in pediatric COVID-19 in order to give a perspective on how to take care of them during the current pandemic emergency.
Multiple searches in MEDLINE, PubMed were performed using the search terms “COVID-19” or “SARS-CoV-2" were used in combination with “myocardial injury” or "arrhythmia" or “cardiovascular involvement” or "heart disease" or "congenital heart disease" or “pulmonary hypertension” or "long QT" or “cardiomyopathies” or “channelopathies” or "Multisystem inflammatory system" or "PMIS" or “MIS-C” or ”Pediatric multisystem inflammatory syndrome" or "myocarditis" or "thromboembolism to identify articles published in English language from January 1st, 2020 until July 31st, 2020. The websites of World Health Organization, Centers for Disease control and Prevention, and the Johns Hopkins Coronavirus Resource Center were reviewed to provide up to date numbers and infection control recommendations. Reference lists from the articles were reviewed to identify additional pertinent articles. Retrieved manuscripts concerning the subject were reviewed by the authors, and the data were extracted using a standardized collection tool. Data were subsequently analyzed with descriptive statistics. For Pediatric multisystemic inflammatory syndrome temporally associated with COVID-19 (PMIS), multiple meta-analyses were conducted to summarize the pooled mean proportion of different cardiovascular variables in this population in pseudo-cohorts of observed patients.
A total of 193 articles were included. Most publications used in this review were single case reports, small case series, and observational small-sized studies or literature reviews. The meta-analysis of 16 studies with size > 10 patients and with complete data about cardiovascular involvement in children with PMIS showed that PMIS affects mostly previously healthy school-aged children and adolescents presenting with Kawasaki disease-like features and multiple organ failure with a focus on the heart, accounting for most cases of pediatric COVID-19 mortality. They frequently presented cardiogenic shock (53%), ECG alterations (27%), myocardial dysfunction (52%), and coronary artery dilation (15%). Most cases required PICU admission (75%) and inotropic support (57%), with the rare need for extracorporeal membrane oxygenation (4%). Almost all of these children wholly recovered in a few days, although rare deaths have been reported (2%). Out of PMIS cases we identified 10 articles reporting sporadic cases of myocarditis, pulmonary hypertension and cardiac arrythmias in previously healthy children. We also found another 10 studies reporting patients with pre-existing heart diseases. Most cases consisted in children with severe COVID-19 infection with full recovery after intensive care support, but cases of death were also identified. The management of different cardiac conditions are provided based on current guidelines and expert panel recommendations.
There is still scarce data about the role of cardiovascular involvement in COVID-19 in children. Based on our review, children (previously healthy or with pre-existing heart disease) with acute COVID-19 requiring hospital admission should undergo a cardiac workup and close cardiovascular monitoring to identify and treat timely life-threatening cardiac complications.
Core Tip: Cardiovascular involvement has emerged as a remarkable risk-factor for poor outcomes of primary respiratory diseases such as coronavirus disease-2019 (COVID-19). Nevertheless, the body of evidence of cardiac complications in pediatric COVID-19 is still scarce to extract definitive conclusions about the adequate management for these patients. This review establishes a perspective on how COVID-19 impacts on the heart of both previously healthy children and those with pre-existing heart diseases, and how to take care of them during the current pandemic emergency.
- Citation: Rodriguez-Gonzalez M, Castellano-Martinez A, Cascales-Poyatos HM, Perez-Reviriego AA. Cardiovascular impact of COVID-19 with a focus on children: A systematic review. World J Clin Cases 2020; 8(21): 5250-5283
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5250.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5250
The coronavirus disease-2019 (COVID-19) was first discovered in a cluster of patients with severe respiratory symptoms in Hubei Province, China, in December 2019[1,2]. By early January 2020, analysis of bronchoalveolar lavage (BAL) fluid from infected patients revealed that COVID-19 is caused by the novel coronavirus strain, named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[3], a single-stranded RNA virus belonging to the beta genus coronavirus in the coronaviridae family. COVID-19 rapidly swept China and spread worldwide, becoming a global pandemic causing significant mortality and morbidity[4]. The current count of confirmed cases and deaths from COVID-19 worldwide can be found at the website of the Johns Hopkins Coronavirus Resource Center. At the time of this review (July 31, 2020), COVID-19 has caused more than 18 million cases and an estimate of more than 700000 associated deaths[5].
Since the beginning of the pandemic, COVID-19 in children has shown milder cases and a better prognosis than adults[6]. Children have worldwide so far accounted for approximately 2%-5% of diagnosed COVID-19 cases[7-14]. A review of 72314 patients by the Chinese Center for Disease Control and Prevention showed that only 1% of COVID-19 cases were in children younger than 10 years old[15]. Epidemiological studies have consistently demonstrated across the world that children are at a lower risk of developing severe symptoms or critical illness compared with adults. Dong et al[16] reported, in the most extensive pediatric study in China, that only 5.2% had severe disease, while 0.6% had a critical illness, with a low case-fatality rate of less than 0.1%. In North America, data from the Centers for Disease Control and Prevention (CDC) as of March 2020 found that 2% to 3% of pediatric patients with positive SARS-CoV-2 testing required hospitalization, with hospitalization rates for children of 0.3/100000 in patients aged 0 to 4 years and 0.1/100000 in patients aged 5 to 17 years. At the time of this publication, there had been relatively few reported cases of pediatric deaths attributed to COVID-19. In the United States the CDC reported a total of 121/391814 (0.03%) deaths associated with SARS-CoV-2 infection among persons aged < 21 years from February to July 2020. This supposed 121/190000 (0.06%) of all reported death associated with COVID-19 in the same period. The median age of death in this age group was 16 years, with 30% of deaths occurring in children younger than 10 years old[17-21]. DeBiasi et al[22] reported that 9/177 (5%) cases of confirmed COVID-19 required pediatric intensive care unit (PICU) admission (no mortality) in Washington, DC Metropolitan region. Chao et al[23] observed in New York City that 13/67 (28%) of pediatric COVID-19 cases required PICU admission with a mortality rate of 1.5%. Shekerdemian et al[24] reported that only 48 pediatric cases required PICU admission between March 14 and April 3, 2020, in 46 North American PICUs, with a mortality rate of 4%. Götzinger et al[25] in a multicenter (82 hospitals) cohort study, including 582 individuals with PCR-confirmed SARS-CoV-2 infection across 25 European countries, found that 363 (62%) individuals were admitted to hospital and 48 (8%) individuals required PICU admission. Only four children died (case-fatality rate 0.69%) suggesting that the case-fatality rate of COVID-19 in children is substantially lower than in older adult patients. The mechanism by which children seem less susceptible to severe infection caused by SARS-CoV-2 has yet to be elucidated (Table 1)[26-31].
| Factor involved | Explanation |
| ACE2 receptor | The ACE2 receptor is necessary for the entry of the COVID-19 into the cells. It is postulated that in children the development, function, or activity of this protein could be lower. Also, ACE2 receptors are up-regulated in those with chronic obstructive pulmonary disease or hypertension, which may partially explain more serious disease in those with comorbid conditions. |
| Reduced exposures | Children may have fewer opportunities than adults to be exposed to the virus or to those with COVID-19. Children have fewer outdoor activities and make fewer international trips, making infection less likely. Compared to adults, children have had less lifetime exposure to toxins such as cigarette smoke and air pollution, factors which may affect the health of an individual’s epithelium. |
| Comorbidities | Compared to adults, children have a low rate of comorbidities and most children with COVID-19 infection are young and previously healthy. |
| Other viruses | Children are susceptible to a wide variety of viral illnesses. The presence of other viruses may limit COVID-19 infection by competitive mechanism. In addition, cross-reactive antibodies generated by exposure to other viruses may cause a partial protective response. |
| Inflammatory response | Children have fewer pro-inflammatory cytokines secretion than adults. This may mean that adults experience a more pronounced inflammatory response than children with a similar exposure to SARS-CoV-2. |
| Immune response | Children have a more active innate immune response than adults. The innate immune system, which acts earlier than the adaptive immune response, is more active in children, and may prevent more serious illness. Natural involution of the thymus over time leads to a decline in circulating naïve T cells. Due to this normal process, immune systems in adults are less able to be adaptive than those of children. |
Clinical manifestations of COVID-19 in children overlap with many other pediatric viral infections. Most commonly, children presented with a mild flu-like state that can progress to potentially lethal acute respiratory distress syndrome, fulminant pneumonia, and multi-organ failure[10,32-35]. Although the respiratory tract is the primary target for SARS-CoV-2, the virus may interact with the cardiovascular system producing myocardial injury through different mechanisms (Table 2)[36-40], increasing morbidity in both, previously healthy patients and those with underlying cardiovascular conditions. Thus, cardiovascular involvement is emerging as one of the most significant and life-threatening complications of SARS-CoV-2 infection in adults[41,42].
| Mechanism | Explanation |
| COVID-19–related myocarditis | SARS-CoV-2 may directly cause myocardial damage by entering cardiomyocytes using the ACE2 receptor, a human cell receptor with a strong binding affinity to the virus spike protein of SARS-CoV-2 (highly expressed in the heart). The virus is also capable of active CD8+ T lymphocytes migrate to the cardiomyocytes and cause myocardial inflammation through cell-mediated cytotoxicity. There is evidence demonstrating that SARS-CoV-2 infects the myocardial tissue. |
| Deregulated immune response & cytokine storm | SARS-CoV-2 infection may lead to deregulated immune response with higher neutrophil-lymphocyte-ratio, lower levels of both T helper and T suppressor cells, and higher expression of pro-inflammatory cytokines (IFN-γ, TNF, IL-1, IL-6 and IL-18), which are released into the circulation. This cytokine storm syndrome has a role in cardiovascular system injury, causing multi-system inflammation and multi-organ failure with direct cardiotoxicity and rapid onset of severe cardiac dysfunction, hemodynamically instability and vascular leakage with peripheral and pulmonary edema. |
| Oxygen supply and demand imbalance | Myocardial injury may result from the imbalance between oxygen supply and demand due to a severe acute respiratory distress syndrome and systemic hypotension with myocardial hypo-perfusion in association with increased cardio metabolic demand in the myocardial tissue. This can result in myocytes hypoxia and necrosis. |
| Thromboembolic events | The systemic inflammation secondary to the cytokine storm also causes endothelial dysfunction and increases the procoagulant activity of the blood, which can further contribute to the formation of multi-organ micro thrombi and also occlusive thrombi over a ruptured coronary plaque. |
| Cardiotoxicity of drugs used against SARS-CoV-2 | Off-label drugs available for COVID-19 treatment can produce myocardial dysfunction, severe systemic hypotension, QT prolongation with ventricular arrhythmia and AV block |
| Deleterious effects of inotropes and mechanical ventilation | Mechanical ventilation in critically ill children is another possible cause of cardiovascular adverse effects, such as a decrease in cardiac output due to decreased venous return to the right heart, right ventricular dysfunction, and impaired left ventricular elastics. Increased right ventricular after-load due to the pulmonary infection can be worsened by mechanical ventilation with high PEEP, leading to right ventricular failure and subsequent myocardial injury. Inotropes can provoke an increased cardio metabolic demand during an hypoxemic condition, |
| Pre-existing Heart diseases | Patients with pre-existing heart diseases have increased morbidity and mortality related to viral infection It is reasonable to assume that patients with underlying heart diseases with low cardiopulmonary reserve are susceptible to cardiac injury, and once such patients are infected with COVID-19, myocardial ischemia or infarction, and left ventricular systolic dysfunction or ventricular arrhythmia are more likely to occur, ultimately leading to a sudden deterioration. |
Given the smaller numbers of pediatric cases of COVID-19 regarding adults, there is still scarce data about the role of cardiovascular involvement in COVID-19 in children. Of note, up to 34% of children with COVID admitted to the PICU in Spain presented with signs of heart dysfunction[43]. Besides, there is a suggestion that, as with other viral illnesses such as respiratory syncytial virus or influenza, children with underlying cardiac conditions are also at greater risk of cardiac complications or develop a more severe SARS-CoV-2 infection[21]. Furthermore, from April 2020, increasing cases of previously healthy children showing a hyper-inflammatory state and features similar to Kawasaki-Shock disease are being reported in Europe and America[44-49]. Remarkably, initial reports suggest many of these patients have myocardial dysfunction and coronary artery involvement with high requirements of PICU admission, hemodynamic, and respiratory support. All the above brings out that cardiovascular involvement could be a significant risk factor for severe COVID-19 in children. Thus, all health care providers must be aware of the potential impact of COVID-19 on children.
In this article, we aimed to summarize the current knowledge about the potential cardiovascular involvement in pediatric COVID-19 to give a perspective on how to take care of them during the current pandemic emergency.
A literature search was conducted by all the authors using PubMed and MEDLINE. Also, the websites of the health organizations including World Health Organization and CDC and the website of the Johns Hopkins Coronavirus Resource Center were reviewed to provide up to date numbers and infection control recommendations. Multiple searches were performed during the writing of this article, as the COVID-19 pandemic is still evolving. Search terms “COVID-19” or “SARS-CoV-2" were used in combination with “myocardial injury” or "arrhythmia" or “cardiovascular involvement” or "heart disease" or "congenital heart disease" or “pulmonary hypertension” or "long QT" or “cardiomyopathies” or “channelopathies” or "Multisystem inflammatory system" or "PMIS" or “MIS-C” or ”Pediatric multisystem inflammatory syndrome" or "myocarditis" or "thromboembolism to identify publications from January 1st, 2020 until July 31st, 2020. Reference lists of the articles identified by this search strategy were reviewed to capture additional studies. No randomized trials neither interventional studies were available at the time this article was written; hence, observational studies, long-term prospective cohort studies, case-control, cross-sectional studies, case series, or case reports were also included in this review. Few additional articles before our search time period were included if they were referenced in existing articles and included pertinent essential data for this present article. Only articles published in the English language were included in this review. After the initial search, the authors separately screened all abstracts based on the eligibility criteria. Any abstracts or articles for which there was disagreement or uncertainty were reviewed again and discussed until consensus was reached. We finally included 193 articles. The included studies were categorized by whether the study involved previously healthy patients or patients with pre-existing cardiac conditions. As the vast majority of cases reported of pediatric cardiovascular involvement are patients with pediatric multisystemic inflammatory syndrome temporally (PMIS) associated with COVID-19, multiple meta-analyses were conducted to summarize the pooled mean proportion of different cardiovascular variables in this population. All the statistical analyses were performed using the STATA 14.0 (StataCorp. College Station, TX, United States).
Most publications used in this review were single case reports, small case series, and observational small-sized studies or literature reviews. The most relevant articles were 16 studies with size > 10 patients and with complete data about cardiovascular involvement in children with PMIS, 10 articles reporting sporadic cases of myocarditis, pulmonary hypertension and cardiac arrythmias in previously healthy children, and another 10 studies reporting patients with pre-existing heart diseases. Most cases consisted in children with severe COVID-19 infection with full recovery after intensive care support, but cases of death were also identified. The management of the different cardiac conditions was extracted from the correspondent clinical guidelines or expert panel recommendations.
Pediatric multisystemic inflammatory syndrome temporally associated with COVID-19: The incidence of severe COVID-19 in children is lower than in adults; however, Pediatric multisystemic inflammatory syndrome temporally associated with COVID-19 (PMIS) has been recognized worldwide over the past few months, changing the paradigm that children are not severely affected by SARS-CoV-2. Since April 2020, various alerts were issued in Italy, United Kingdom, and Spain about a local increase in the number of these cases[44-46]. After these reports, the first cases in North America were reported in May 2020[47,48]. Recently, cases of PMIS are currently also diagnosed in Latinamerica[49]. In May 2020, case definitions for PMIS have been produced by the U.S. Centers for Disease Control and Prevention and the U.K.’s Royal College of Paediatrics and Child Health, as well as by the World Health Organization (Table 3)[50-52]. Following these case definitions, 570 cases of PMIS have been notified to the CDC from March to July 2020[53]. Taking into account that on July 29th there were 4.5 million cases of COVID-19 and that children account for 2%-5% of these cases, the estimated incidence PMIS accounts for approximately 0.2%-0.6% of pediatric SARS-CoV-2 infections. The case definition is nonspecific, and confirmatory laboratory testing does not exist. Therefore, it might be challenging to distinguish PMIS from other conditions with overlapping clinical manifestations such as severe acute COVID-19 and Kawasaki disease (KD), making challenging to know the exact incidence of the disease.
| Royal College of Pediatrics and Child Health (United Kingdom) | World Health Organization | Centers for Disease Control and Prevention (United States) |
| A child presenting with persistent fever, inflammation (neutrophilia, elevated CRP, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, kidney, gastrointestinal, or neurological disorder) with additional clinical features, including children fulfilling full or partial criteria for Kawasaki disease 2 | Children and adolescents 0-19 yr of age with fever > 3 d AND 2 of the following: (1) Rash or bilateral nonpurulent conjunctivitis or mucocutaneous inflammation signs (oral, hands, or feet); (2) Hypotension or shock; (3) Features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including ECHO findings or elevated troponin/NT-proBNP); (4) Evidence of coagulopathy (by PT, APTT, elevated D-dimers); (5) Acute gastrointestinal problems (diarrhea, vomiting, or abdominal pain). | An individual aged < 21 yr presenting with fever, laboratory evidence of inflammation, and evidence of clinically severe illness requiring hospitalization, with multisystem (> 2) organ involvement (cardiac, kidney, respiratory, hematologic, gastrointestinal, dermatologic, or neurological). 1Fever > 38.0 °C for ≥ 24 h or report of subjective fever lasting ≥ 24 h. |
| And exclusion of any other microbial cause, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, infections associated with myocarditis such as enterovirus (waiting for results of these investigations should not delay seeking expert advice) | And elevated markers of inflammation such as ESR, CRP, or procalcitonin. | And no alternative plausible diagnoses |
| And SARS-CoV-2 PCR test results may be positive or negative | And no other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal or streptococcal shock syndromes | And positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or COVID-19 exposure within the 4 wk prior to the onset of symptoms |
| And evidence of COVID-19 (RT-PCR, antigen test, or serology positive), or likely contact with patients with COVID-19 | Additionally, 1some individuals may fulfil full or partial criteria for Kawasaki disease but should be reported if they meet the case definition for MIS-C. |
As the COVID-19 pandemic has evolved, case reports have appeared describing school-aged children and adolescents presenting with persistent high fever and systemic hyper-inflammation, reflected in a constellation of symptoms involving multiple organ systems[54-89]. They frequently manifested abdominal pain and gastrointestinal symptoms, KD-like features, myocardial dysfunction, coronary artery dilation, and cardiogenic shock. Most cases required PICU admission and inotropic support, with the rare need for extracorporeal membrane oxygenation (ECMO). Almost all of these children wholly recovered in a few days, although rare deaths have been reported. These patients tested positive for SARS-CoV-2 infection either by nasopharyngeal reverse transcriptase–polymerase chain reaction (RT-PCR) assay or by antibody testing. Epidemiological studies have demonstrated that higher regional incidences of PMIS are associated with the larger COVID-19 outbreaks in the countries mentioned above, with most PMIS diagnoses occurring approximately four weeks after COVID-19 diagnoses peaks[80,83]. This temporal and spatial association suggests a causal link between SARS-CoV-2 and PMIS.
If PMIS is indeed related to infection with SARS-CoV-2, the pathophysiological mechanism of disease is unclear. PMIS is presumed to reflect a post-infectious cytokine-mediated hyper-inflammatory process, triggered by COVID-19 infection[89-95]. Of note, there are striking similarities between the overall clinical picture of children affected by PMIS and the late phase of adult COVID-19 infection, characterized by cytokine storm, hyper-inflammation, and multi-organ damage[96-98]. Thus, they had laboratory findings associated with the cytokine storm described in adults, including high serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin, ferritin, Dimer-D, interleukin (IL)-6, Troponin and pro B-type natriuretic peptide (proBNP) levels. These findings suggest similar pathogenesis and a spectrum of illness from children to adults, leading to the hypothesis that PMIS is due to a post-infectious inflammatory state that occurs several weeks after a primary infection with SARS-CoV-2. Accordingly, most of the patients with PIMS were positive for IgG antibodies to SARS-CoV-2 (which typically appears 2-4 wk after primary infection) when the hyper-inflammatory symptoms appeared. Proposed mechanisms include direct triggering of auto-inflammatory response and deregulation of immune responses after COVID-19 infection, which could result in other environmental insults triggering a hyper-inflammatory pathology in predisposed patients[89-98]. One hypothesis for the marked cytokine storm experienced by children with PMIS derives from the ability of coronaviruses to block type I and type III interferon responses, which can lead to an uncontrolled viral replication in those with initially high SARS-CoV-2 viral load[99]. Myocarditis has been demonstrated by late gadolinium enhancement on cardiac magnetic resonance imaging in various children with PMIS[77]. Recently, the presence of viral particles of SARS-CoV-2 in different cell types of cardiac tissue in the autopsy of a PMIS case has been demonstrated[72]. This finding points out that SARS-CoV-2 could also produce a direct myocyte injury similar to other viral myocarditis as the mechanism of myocardial injury and heart failure during the PMIS course. Both the virus-induced damage and the local inflammatory response to cell injury could lead to necrosis of cardiomyocytes. The finding of viral particles in neutrophils within the myocardium supports the idea of local virus-induced inflammation. Of note, infection of endothelial cells in the endocardium could result in the hematogenous spread of SARS-CoV-2 to other organs and tissues, facilitating the typical multisystem failure.
Currently, available data so far suggests that PMIS shares a common pathophysiological pathway and overlapping symptoms with that described in KD[100-106]. However, clinical, laboratory, and epidemiological characteristics of PMIS appear to be different from those of KD, raising the question if they are the same entities. As the outcomes are distinct and PMIS seems to be more aggressive, it is critical to make the subtle distinction between classical KD and PMIS. The absence of cases in Asia, the predisposition of Afro-Caribbean people, the older age of the patients, the presence of gastrointestinal symptoms in almost patients, the presence of lymphopenia/Leucopenia, the abnormal coagulation indexes, the higher levels of ferritin, D-dimer, inflammatory and cardiac markers, the higher rates of cardiogenic shock, myocardial dysfunction, PICU admission, intravenous immunoglobulin (IVIG) resistance, the requirement of advanced respiratory or circulatory support and mortality could be the main differences (Table 4)[100-106].
| Characteristic | Kawasaki disease | PMIS |
| Age | 6 mo-5 yr (most cases under 2-yr-old) | School-aged children (mean age 9-yr-old) |
| Sex | Male predominance | Male = Female |
| Race | Asiatic | African/Caribbean |
| Region | Most cases at Asia | Most cases at Europe and America. No asiatic cases |
| Seasonality | Spring-Autum | Regional incidences associated with the larger regional COVID-19 outbreaks |
| Related with acute infection | Yes | 2-4 wk after primary infection (can occur also during acute phase) |
| Incomplete KD criteria | Up to 30% | < 25% |
| Gastrointestinal symptoms | Uncommon | Almost 100% |
| KD shock syndrome | 2%-7% | 50%-60% |
| Increased inflammatory biomarkers (CRP, Procalcitonin, Ferritin) | ++ | ++++ |
| Lymphocyte count | Lymphopenia rare | Lymphopenia in up to 80% |
| Platelet count | Thrombocytosis | Thrombocytopenia |
| Coagulation indexes | Normal values | Increased indexes; Very increased Dimer-D levels |
| Increased cardiac biomarkers | Natriuretic peptides (> 50%) ++; cTn (< 20%-30%) +/- | Natriuretic peptides (87%) ++++; cTn (73%) ++++ |
| Myocardial dysfunction | < 1% | Up to 52% |
| Coronary arteries anomalies | 25% without adequate treatment | 15% |
| IVIG resistance | 10%-20% | 50%-60% |
| Biologic therapy | Very rare | 15 % |
| Long-term Cardiac sequel | < 5% with adequate treatment | 5.5% |
| PICU admission | 4%-5% | 75% |
| Mechanical Ventilation | Very rare | 22 % |
| ECMO support | Extremely rare | 4%-5% |
| Exitus or Sequelae | < 1% | 2% |
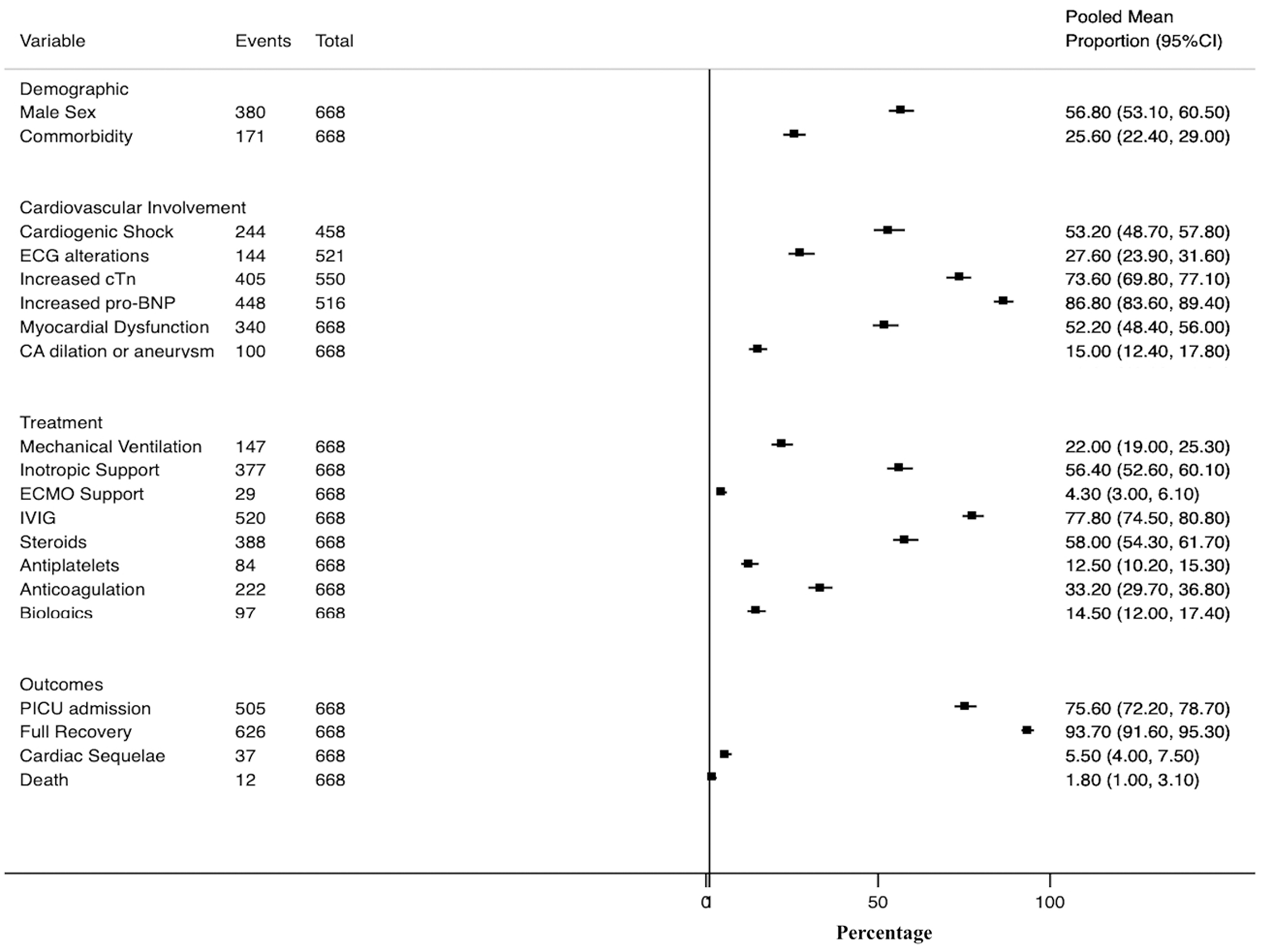
For this review we selected only those studies that included at least 10 patients with PMIS and with complete data about cardiovascular involvement (cardiogenic shock, cardiac biomarkers, ECG, echocardiography), treatment (inotropes, mechanical ventilation, ECMO support, anti-inflammatory and immunomodulatory) and outcomes[45-48,54,56,60-62,65,66,68,70,76,78,86]. We excluded those articles with suspected overlapping of patients due to multiple communications of the same patients in different papers. In our review, we found a total of 688 PMIS cases (56.8% male sex; mean age of 9 years) reported by 16 different authors from April to July 2020 across Europe and North America (Table 5, Figure 1). Of note, the vast majority of patients were previously healthy (74.4%), and the most common associated comorbidities observed were asthma and obesity, with a minimal prevalence of previous cardiac diseases. Since the first cases were reported, it was noticed that PMIS could be a severe acute condition, especially focusing on the cardiovascular system. Thus, the cardiac troponin and natriuretic peptides [N-terminal (NT)-proBNP or proBNP] were increased in most cases of PMIS (73.6% and 86.8% respectively). ECG alterations were not infrequent (27.6%), overall in the form of unspecific ST segment and T wave alterations, prolonged QT interval, and very few ventricular arrhythmia or AV blocks. However, there have also been reported cases of sustained arrhythmias leading to hemodynamic collapse and the need for ECMO support. Most important, as myocardial dysfunction and heart failure have been observed in up to 52%-53% of cases, PMIS must be bear in mind as a cause of new-onset heart failure in children during the pandemic. Also, and similar to KD, PMIS can lead to the development of coronary artery alterations (15%). Although infrequent, most of the cases of PMIS require hospitalization and intense clinical management because of the severity of the disease. Two recent multicenter studies in Spain reported that up to 31/252 (12%) of hospitalized children with COVID-19 and that 27/50 (54%) of cases that required PICU admission were diagnosed as PMIS[43,86].
| Author | Feldstein | Dufort | Miller | Capone | Kaushik | Cheung | Riollano-Cruz | Verdoni | Whittaker | Ramcharan | Hameed | Toubiana | Belhadjer | Grimaud | Pouletty | Moraleda |
| Demographic | ||||||||||||||||
| Country | United States | United States | United States | United States | United States | United States | United States | Italy | United Kingdom | United Kingdom | United Kingdom | France | France & Sw | France | France | Spain |
| Size | 186 | 99 | 44 | 33 | 33 | 17 | 15 | 10 | 58 | 15 | 35 | 21 | 35 | 20 | 16 | 31 |
| Age | 8.3 | 8.4 | 7.3 | 8.6 | 10 | 8 | 12 | 7.5 | 9 | 8.8 | 11 | 8 | 10 | 10 | 10 | 7.6 |
| Male Sex | 115 (62%) | 53 (53%) | 20 (45%) | 20 (60%) | 20 (60%) | 8 (47%) | 11 (73%) | 7 (70%) | 25 (43%) | 11 (73%) | 27 (77%) | 9 (43%) | 18 (51%) | 10 (50%) | 8 (50%) | 18 (58%) |
| Commorbidity | 59 (31%) | 35 (35%) | 16 (36%) | 4 (9%) | 16 (48%) | 3 (17%) | 5 (33%) | 0 (0%) | 7 (12%) | 0 (0%) | 0 (0%) | 0 (0%) | 10 (28.5%) | 0 (0%) | 6 (37.5%) | 10 (32%) |
| Cardiovascular involvement | ||||||||||||||||
| Shock | NR | 32 (32%) | NR | 16 (48%) | 21 (63%) | 13 (76%) | 13 (87%) | 5 (50%) | 27 (46%) | 10 (66%) | 21 (60%) | 12 (57%) | 28 (80%) | 20 (100%) | 11 (68%) | 15 (48%) |
| ECG alterations | 22 (12%) | 59 (59%) | 22 (50%) | NR | NR | 16 (94%) | 2 (13%) | NR | 4 (7%) | 9 (60%) | NR | 2 (10%) | 1 (3%) | NR | NR | 7 (23%) |
| Increased cTn | 77/153 (50%) | 63/89 (71%) | NR | 33 (100%) | 33 (100%) | 14 (82%) | 13 (87%) | 5/9 (55%) | 34/50 (68%) | 15 (100%) | 35 (100%) | 17 (81%) | 35 (100%) | 20 (100%) | 11/11 (100%) | NR |
| Increased pro-BNP | 94/128 (74%) | 74/82 (90%) | NR | 33 (100%) | 33 (100%) | 15 (88%) | 13 (87%) | 10 (100%) | 24/29 (83%) | 15 (100%) | 35 (100%) | 14/18 (78%) | 35 (100%) | 15/15 (100%) | 16 (100%) | 22 (71%) |
| Myocardial dysfunction | 70 (38%) | 51 (52%) | 22 (50%) | 19 (58%) | 22 (63%) | 6 (35%) | 7 (57%) | 5 (50%) | 18 (31%) | 12 (80%) | 15 (43%) | 16 (76%) | 35 (100%) | 20 (100%) | 7 (43%) | 15 (48%) |
| Coronary artery involvement | 15 (8%) | 9 (9%) | 0 (0%) | 16 (48%) | 0 (0%) | 7 (41%) | 3 (20%) | 2 (80%) | 8 (14%) | 14 (93%) | 6 (20%) | 8 (38%) | 6 (17%) | 0 (0%) | 3 (18%) | 3 (10%) |
| Treatment | ||||||||||||||||
| Mechanical ventilation | 37 (20%) | 10 (10%) | 1 (2%) | 6 (18%) | 5 (15%) | 0 (0%) | 3 (20%) | 0 (0%) | 25 (43%) | 4 26%) | 7 (20%) | 11 (52%) | 22 (62%) | 8 (40%) | 2 (12%) | 6 (19%) |
| Inotropic support | 90 (48%) | 61 (62%) | 22 (50%) | 25 (75%) | 17 (51%) | 10 (59%) | 8 (53%) | 2 (20%) | 29 (50%) | 10 (67%) | 20 (57%) | 15 (71%) | 28 (80%) | 19 (95%) | 6 (38%) | 15 (48%) |
| ECMO support | 8 (4%) | 4 (4%) | 0 (0%) | 0 (0%) | 1 (3%) | 0 (0%) | 1 (6%) | 0 (0%) | 3 (5%) | 0 (0%) | 2 (6%) | 0 (0%) | 10 (28%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IVIG | 144 (77%) | 69 (70%) | 36 (81%) | 33 (100%) | 18 (54%) | 13 (76%) | 12 (80%) | 8 (80%) | 41 (70%) | 10 (66%) | 35 (100%) | 21 (100%) | 25 (71%) | 20 (100%) | 15 (93%) | 20 (65%) |
| Steroids | 91 (49%) | 61 (62%) | 42 (95%) | 23 (70%) | 17 (51%) | 15 (92%) | 3 (20%) | 10 (100%) | 37 (64%) | 5 (33%) | 35 (100%) | 10 (48%) | 12 (35%) | 2 (10%) | 4 (25%) | 21 (68%) |
| Antiplatelet | 0 (0%) | 0 (0%) | 0 (0%) | 29 (87%) | 0 (0%) | 4 (24%) | 2 (13%) | 2 (20%) | 0 (0%) | 11 (73%) | 0 (0%) | 21 (100%) | 0 (0%) | 0 (0%) | 15 (93%) | 0 (0%) |
| Anticoagulation | 87 (47%) | 0 (0%) | 40 (90%) | 14 (42%) | 32 (97%) | 11 (64%) | 15 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 23 (65%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Biologics | 38 (21%) | 0 (0%) | 8 (18%) | 7 (21%) | 12 (36%) | 0 (0%) | 14 (93%) | 0 (0%) | 11 (19%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (9%) | 2 (10%) | 2 (12%) | 0 (0%) |
| Outcomes | ||||||||||||||||
| PICU admission | 148 (80%) | 79 (80%) | 22 (50%) | 26 (79%) | 33 (100%) | 15 (88%) | 14 (93%) | 5 (50%) | 29 (50%) | 10 (67%) | 25 (69%) | 17 (81%) | 35 (100%) | 20 (100%) | 7 (44%) | 20 (65%) |
| Full recovery | 182 (98%) | 97 (98%) | 44 (97%) | 24 (73%) | 29 (88%) | 16 (94%) | 13 (88%) | 10 (100%) | 56 (98%) | 12 (80%) | 33 (97%) | 21 (100%) | 25 (71%) | 20 (100%) | 14 (88%) | 30 (97%) |
| Cardiac sequelae | 0 (0%) | 0 (0%) | 0 (0%) | 9 (27%) | 2 (6%) | 1 (6%) | 1 (6%) | 0 (0%) | 0 (0%) | 3 (20%) | 0 (0%) | 0 (0%) | 19 (29%) | 0 (0%) | 2 (12%) | 0 (0%) |
| Death | 4 (2%) | 2 (2%) | 1 (2%) | 0 (0%) | 1 (3%) | 0 (0%) | 1 (6%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3%) |
In our review, we found that a PICU admission rate of 75.6%, with up to 56.4% of cases presenting with shock requiring inotropic support, and 4.3% requiring ECMO support. Conversely, only 22% of patients required mechanical ventilation, suggesting that the primary pulmonary involvement could not be a significant issue in PMIS cases. Despite prolonged PICU admissions (mean PICU stay 4-7 d), the reported clinical outcomes for pediatric patients with PMIS appear favorable. Almost all patients (93.7%) presented a rapid full recovery within the first week of the disease. Most patients recovered when the severe inflammatory state associated with the SARS-CoV-2 infection is resolved. For this purpose, a high proportion of patients diagnosed as PMIS have been treated initially with IVIG (77.8%) and steroids (58%) in a similar way to KD[100-106]. However, to resolve this severe inflammatory state associated is challenging, as these patients exhibit an increased resistance rate to these anti-inflammatory therapies regarding those rates reported for KD. Hence, biologic therapy blocking IL-1 (anakinra) and IL-6 (tocilizumab) receptors have been used in up to 14.5% of PMIS cases. COVID-19 prothrombotic effects are concerning in adults[107-110], increased use of anticoagulation (33.2%; mainly low weighted enoxaparin) and antiplatelet agents (12.5%; primarily aspirin) have been observed in these patients. Children with PMIS are at risk of thrombotic complications from multiple causes, including hypercoagulable state, possible endothelial injury, stasis from immobilization, ventricular dysfunction, and coronary artery aneurysms[111]. Therefore, antiplatelet and anticoagulation are recommended[112,113]. The low-rate of aspirin use could be explained by its prescription, especially in patients with KD-like clinical presentations (20%-25%), or in those with evidence of coronary involvement (15%). Cardiac sequelae in the form of mild myocardial dysfunction or coronary artery alterations were present in up to 5.5% of cases. Furthermore, although the case-fatality rate is known to be minimal in pediatric COVID-19, PMIS account for 15/121 (12%) of SARS-CoV-2 associated deaths among persons aged < 21 years in the United States from February 12th 2020 to July 31st 2020. Thus, we found a mortality rate of 1.8% in this review, which is higher than the 0.1%-0.6% mortality rate for pediatric COVID-19 reported before the emergence of PMIS.
As the prognosis is excellent after treatment, the early diagnosis of PMIS and prompt initiation of anti-inflammatory therapy is crucial for a successful, rapid, and full recovery and preventing end-organ damage and mortality. For this purpose, a high index of clinical suspicion is needed. As cardiovascular involvement is present in any form in almost patients with PMIS, the screening of cardiac alterations through cardiac biomarkers, ECG, or echocardiography could be useful for the early identification of PMIS cases. Data from this review indicate that PMIS cases have similar manifestations and outcomes from different studies across the world. Therefore, there are arguments to consider PMIS a new syndrome with a strong link with SARS-CoV-2 infection. As fundamental aspects of PIMS remain unknown, future studies will improve prospects for the prevention and treatment of this severe pediatric condition. Until them, a close multidisciplinary collaboration among various disciplines including pediatrics, intensive care, rheumatology, cardiology, and immunology is warranted for the adequate management of these patients.
Cardiovascular involvement in previously healthy children out of PMIS: There is also evidence of cardiac manifestation in previously healthy children out of the setting of PMIS in children. Since the beginning of the pandemic, a few cases have been reported with cardiovascular involvement similar to adults, in the form of myocarditis, pericarditis, heart failure, cardiogenic shock, cardiac arrhythmia, and pulmonary hypertension (PH) (Table 6)[114-124]. Although infrequent, these cases are usually reported as severe or critically ill patients, pointing out the relevance of the cardiac involvement also in previously healthy children with COVID-19.
| Ref. | Mechanism | Description |
| Dong et al[16] | Myocardial injury | Nationwide case series of 2135 pediatric patients with COVID-19 reported to the Chinese Center for Disease Control and Prevention. Cardiovascular involvement was found in 13 patients with myocardial injury or heart failure. No deaths were reported. |
| Cui et al[115] | Myocardial injury | Description of a 55-d-old otherwise healthy female case with COVID-19 in China. Abnormal myocardial enzyme values on admission and increased troponin I indicated myocardial injury. The patient evolved favourably. |
| Del Barba et al[116] | Myocardial injury | A 38-d-old male tested positive for SARS-CoV-2 and developed mild cardiovascular inflammation. An increase in troponin T was observed and a cardiac magnetic resonance was also performed which showed a minimal amount of pericardial effusion. The patient evolved favourably. |
| Gnecchi et al[117] | Myocardial injury | A previously healthy 16-yr-old boy presented at the emergency department with fever and chest pain. The ECG showed inferolateral ST-segment elevation and the echocardiogram showed hypokinesia of the inferior and anterolateral segments of the left ventricle, with preserved function (EF 52%). Troponin I was very increased. Cardiac MRI supported the diagnosis of myocarditis. On day 3 of illness a nasopharyngeal swab test confirmed SARS-CoV-2 infection. The patient presented a full recovery on day 12 of illness. |
| Craver et al[122] | Myocardial injury | The authors reported a previously healthy 17-yr-old male that presented with sudden cardiac death. The autopsy showed diffuse myocarditis with mixed inflammatory infiltrate with a predominance of eosinophil as the cause of the death. |
| Sun et al[123] | Myocardial injury | In a small series of 8 critically ill patients infected by SARS-CoV-2, the authors reported the case of a 13-mo-old male who developed heart failure within a multiple organ failure with full recovery after support therapy (plasmapheresis and oxygen). |
| Su et al[124] | Myocardial injury | Clinical data from nine previously healthy children and their 14 families were collected, including general status, clinical, laboratory test, and imaging characteristics. In this study, they found six children with high CK-MB, which means that SARS-CoV-2 could cause heart injury. All children presented a full recovery. |
| Kesici et al[119] | Myocardial injury | A 2-yr-old, otherwise healthy boy with a history of respiratory distress secondary to COVID-19 developed cardiogenic shock the second day of hospitalization. The patient presented elevated cardiac Troponin and severe left ventricular failure on echocardiography. The patient required ECMO support and presented cardiac arrest. The autopsy confirmed a dilated cardiomyopathy secondary to viral myocarditis with SARS-CoV-2 RT-PCR positivity in the cardiac tissue as the cause of the death. |
| Giacomet et al[118] | Myocardial injury | A 2-mo-old boy presented with fever, vomiting and diarrhoea within a confirmed SARS-CoV-2 infection. The cardiac work-up revealed increased Troponin I and NT-proBNP levels and mild left ventricular dysfunction on echocardiogram. IL-6 was elevated. After therapy with IVIG the patient presented a rapid full recovery. The clinical diagnosis was myocarditis. |
| Rodriguez-Gonzalez et al[114] | Pulmonary hypertension | A 6-mo-old male with history of small bowel disease presented with a pneumonia, cardiogenic shock and severe hypoxemia. Cardiac biomarkers and IL-6 were increased, and echocardiography showed severe pulmonary hypertension and severe right ventricular failure. Pulmonary thromboembolism was ruled-out through angio-CT scan. The patient received inotropic and respiratory support and improved rapidly with full recovery after the initiation of Tocilizumab. SARS-CoV-2 infection was confirmed by serology. |
| Samuel et al[120] | Arrhythmia | Thirty-six pediatric patients with active PCR positive SARS-CoV-2 infection were included in the study. No patients presented pre-exiting cardiac condition. Of them 6 cases developed significant arrhythmias (non-sustained ventricular tachycardia in 5 and sustained atrial tachycardia in 1). All were self-resolving episodes, and 3 of them were started on prophylactic anti-arrhythmic therapy. Four of them presented abnormal echocardiograms with mild dilation/dysfunction of the left ventricle that recovered at discharge. |
| Xia et al[121] | Arrhythmia | The authors reported the clinical, laboratory, and chest CT features of 20 pediatric inpatients with COVID-19 infection confirmed by pharyngeal swab COVID-19 nucleic acid test. The authors observed self-limited ECG alterations in four of these patients during admission (Sinus tachycardia, Atrial arrhythmia, First-degree atrioventricular block, atrial and ventricular premature beats). The patients did not require any treatment and presented a full recovery. |
Myocardial dysfunction and heart failure: Myocarditis is an inflammatory disease of the myocardium, diagnosed by established histological, immunological, and immune-histochemical criteria, caused mostly by infectious, immune-mediated or toxic agents[125]. Remarkably, SARS-CoV-2 may represent another viral etiology of myocarditis. Acute myocardial injury by SARS-CoV-2 may be due to the coincidence of various mechanisms including a direct viral myocardial injury, a secondary inflammatory response in the form of cytokine storm, the severe hypoxemia due to pneumonia, and the side-effects of therapy against SARS-CoV-2 infection (Table 1 and 2)[126-129].
To date, sporadic cases of myocarditis have been reported in pediatric COVID-19 patients as the first clinical presentation of the disease, alone or in the clinical context of pneumonia. Dong et al[16] found myocardial injury and heart failure occurring in up to 0.6% (13/2135) of pediatric patients with COVID-19 reported to the Chinese Center for Control and Prevention. In the following months, more sporadic cases have been reported around the world[115-124,130,131]. The myocardial injury affected all age ranges, from neonates to adolescents, and a not low number of these children presented in an unexpected form of severe SARS-CoV-2 infection with increased morbidity (PICU admission, inotropic support, mechanical ventilation…) and mortality, including cases of sudden cardiac death. Of note, the presence of viral particles of SARS-CoV-2 in the myocardium of children with sudden cardiac death reinforces the direct viral damage as one of the main mechanisms of myocardial injury in healthy children[72]. Based on the few published data, SARS-CoV-2 may represent a potential novel etiology of fulminant myocarditis, which it should be suspected in COVID-19 patients with acute-onset chest pain, ST-segment changes, cardiac arrhythmias and hemodynamic unstability[131,132]. Also, left ventricular dilatation, left ventricular hypo-contractility (on echocardiography), and a significant increase in cardiac troponin and proBNP levels could also be present. Myocarditis seems to be a rare event in pediatric COVID-19, but when occurred, appears early in patients’ clinical history and could lead to sudden cardiac death. However, as few studies described myocardial injuries in these COVID-19 patients, the impact on clinical prognosis needs to be clarified in this population.
Pulmonary hypertension secondary to the primary pulmonary infection or PTE: Right heart failure with increased pulmonary vascular resistance and PH should also be considered as a possible cardiac manifestation in previously healthy children, especially in the context of moderate-severe pneumonia with acute respiratory distress syndrome and hypoxemia[132]. PH is a well-known complication of pulmonary viral infections leading to adverse outcomes when present, also in the COVID-19 scenario[114,133-136]. Interestingly, nonetheless, concomitant pneumonia, PH has not been reported as one of the main characteristic of the disease in children. Thus, we only found one case of severe PH and right ventricular failure secondary to a severe pulmonary infection in our literature review[114]. In this case, the increased levels of cardiac troponin and proBNP prompted an echocardiogram realization that diagnosed severe PH. Previous cases reported of severe pulmonary hypertension in adults were secondary to pulmonary thromboembolism (PTE), but no cases of massive PTE have been demonstrated in children[137-139]. Therefore, it has been hypothesized that the pulmonary endothelial cell damage leading to the endothelium, the activation of coagulation pathways, and deregulated inflammatory cell infiltration with cytokine storm could be the primary physiopathological mechanism of the severe PH observed in these patients[88-91,140]. Small segmental pulmonary emboli (PE) have been observed in some pediatric patients affected by PMIS supporting this theory[141-143], but the clinical significance of these findings is still unclear. Finally, pulmonary artery vasoconstriction due to severe hypoxemia and respiratory acidosis may act as a coadjuvant factor for the development of PH[144,145].
Cardiac arrhythmia in healthy children: There are little data about the occurrence of arrhythmias in the context of pediatric COVID-19. Data from 2 small-sized studies showed that hospitalized pediatric COVID-19 cases could present a rate of cardiac arrhythmia as high as 16%-20%[120,121]. Initial data from Chinese observations suggest that contrary to adult patients, whose myocardial involvement is sometimes correlated with the appearance of life-threatening arrhythmias, children with COVID-19 share less harmful rhythm troubles, such as supraventricular tachycardia, premature atrial and ventricular complexes, first-degree atrioventricular blocks, and incomplete right bundle branch block[16,36,121]. Recently, Samuel et al[120] observed non-sustained ventricular tachycardia in 5/6 patients that developed cardiac arrhythmia in a case series of 36 children. Although all were self-resolving episodes, up to 50% of cases required the initiation of antiarrhythmic drugs. Based on these few data, arrhythmias represent a not rare clinical presentation of COVID-19, which could complicate the clinical course of disease during hospitalization and worsen the prognosis of infected patients. For this reason, careful electrocardiographic monitoring should be performed in COVID19 patients to early detect paroxysmal arrhythmia that does not match the disease status and might be a red flag of worsening disease.
Ventricular arrhythmias seem to be directly correlated to the COVID-19 induced myocardial injury. Accordingly, a higher incidence of ventricular arrhythmia was reported in adult patients with elevated troponin-T levels[37-42]. Notably, 4 of 5 of the cases of ventricular arrhythmia reported by Samuel et al[120], presented abnormal echocardiograms with mild dilation/dysfunction of the left ventricle. Furthermore, ventricular fibrillation has been documented in some cases of fulminant myocarditis and sudden cardiac death in children[117-119,122]. Therefore, the direct myocardial injury seems to be a determinant factor for arrhythmia also in children. Hypoxemia and electrolyte unbalance are not rare in the acute phase of severe COVID-19 and can also trigger cardiac arrhythmias[146]. The potential role of pharmacological treatments such as hydroxychloroquine (HCQ) and azithromycin in enhancing the susceptibility to QT-related life-threatening ventricular arrhythmias, particularly Torsades de pointes (TdP), is increasingly recognized in adults[147-149]. Nevertheless, pediatric observations have shown that these drugs are safe in previously healthy children[150].
The characteristic hyper-inflammatory systemic state of COVID-19 has been proposed as a potentially crucial pro-arrhythmic factor. This fact is supported by the occurrence of arrhythmic events in PMIS cases mentioned above. Strong evidence from basic and clinical studies points to pro-inflammatory cytokines, mainly IL-6, as a novel and essential risk factor for long QT-syndrome and TdP[151-153]. Systemic inflammation might additionally predispose to long QT-syndrome/Td as a result of indirect mechanisms, such as the induction of cardiac sympathetic system hyper-activation and the inhibition of cytochrome p450, particularly CYP3A4, leading to an increased bioavailability of several medications, including QT-prolonging drugs.
The coexistence of myocardial injury and cardiac arrhythmias with COVID-19 makes it challenging the diagnosis and management of this entity. The early recognition of cardiac symptoms and their timely treatment may be of pivotal importance to improve the prognosis of pediatric patients, overall in those with severe disease.
Congenital heart diseases: Congenital heart diseases (CHD) affects approximately up to 8/1000 (0.8%) newborns and remains the leading cause of infant mortality due to congenital malformations[154]. Currently, there are no reliable data with regards to the burden of infected children with CHD and the COVID-19 related morbidity and mortality in this setting. Few studies are focusing on children with CHD and COVID-19, mainly limited to sporadic case reports or small case series (Table 7)[155-162]. These children seem to be a vulnerable population to a potential clinical deterioration in the presence of bilateral pneumonia or acute respiratory distress syndrome caused by SARS-CoV-2, especially those with non-corrected complex cardiac defects and decreased cardiopulmonary functional reserve. Furthermore, even patients with corrected CHD can present with relevant residual lesions such as residual valvular or shunt lesions, ventricular dysfunction, heart failure, arrhythmias, pulmonary vascular disease, or cyanosis. Apart from the hemodynamic burden, some of these children might have associated comorbidities such as lung disease, liver impairment, renal failure, neurological sequelae, and impaired immunity associated with possible concomitant syndromes (Down syndrome, DiGeorge syndrome, heterotaxy syndromes with asplenia). Due to the documented myocardial involvement of SARS-CoV-2 infection in both adults and children, and the increased mortality observed in adult patients with pre-existing cardiovascular disease and pediatric CHD with other viral infections (Influenza and respiratory syncytial virus)[163-166], there is a possibility that SARS-CoV-2 infection may produce new-onset of cardiac complications or a worsening of the basal status in this vulnerable population. It is essential to bear in mind that, as in other viral respiratory infections, it can be challenging to differentiate pneumonia from cardiac complications in these children due to an overlapping presentation. For example, children with non-cyanotic CHD with increased pulmonary blood flow have higher than standard resting respiratory rates even in a basal state, and some signs of respiratory distress can be secondary to heart failure. Children with cyanotic CHD have low baseline saturation (< 92%) and cyanosis due to their cardiac pathology. Due to these reasons, delayed diagnosis and treatment of critical cardiac complications could also increase morbidity and mortality.
| Authors | Description |
| Grafmann et al[158] | A 16-yr-old girl with history of treated congenital mitral valve disease with pulmonary hypertension, atrial arrhythmias and mitral valve stenosis, who is admitted for signs of respiratory infection positive for SARS-CoV-2 which produced no signs of myocardial involvement with a full recovery. |
| Zheng et al[161] | A 8-mo and 1-yr-old boys with pre-existing congenital heart disease, presented critical COVID-19 with increased cardiac enzymes, requiring mechanical ventilation and venous-venous hemodiafiltration. These two patients presented the more aggressive SARS-CoV-2 infection among the 25 patients reported in this case series. They presented a full-recovery. |
| Krishnan et al[157] | A 3-yr-old boy with Down syndrome, repaired atrioventricular septal defect (AVSD), and pulmonary hypertension was presented a critical case of COVID-19 confirmed by PCR test. He received methylprednisone, azithromycin, and hydroxychloroquine, and continuous positive airway pressure. The patients presented prolongation of the QTc interval on electrocardiogram with posterior full recovery. |
| Salik et al[159] | A 15-d-old baby girl was diagnosed with Tetralogy of Fallot prenatally. The infant's mother was diagnosed with COVID- 19 postpartum. On day 7 of life, the infant experienced desaturation to SpO2 60–65%, tachypnea, worsening cyanosis. A COVID nasopharyngeal swab was positive; and the infant exhibited frequent spells requiring supplemental oxygen. Due to sustained hypoxemia with SpO2 55%-65%, so it was decided that Blalock- Taussig shunt placement with good clinical evolution. |
| Russell et al[160] | A 3-yr-old female patient with history of heart transplant in 2017 for congenital dilated cardiomyopathy. In the first week of March she developed a mild clinical picture consisting of rhinorrhoea and a productive cough and nasal congestion that did not require hospital admission. Several weeks later, in a review by protocol, COVID 1 PCR was performed with positive results. The patient evolved favourably. |
| Linnane et al[156] | A 10-yr-old boy with a background of double inlet left ventricle, pulmonary atresia, atrial septal defect, and a right aortic arch. He proceeded to have a bidirectional Glenn procedure and completed a total cavopulmonary connection via an extra cardiac fenestrated Fontan surgery at 3 yr and 10 mo. He was admitted for signs of respiratory infection positive for SARS-CoV-2. The patient required admission to intensive care, with gradual improvement and good evolution. |
| Sabatino et al[176] | An Italian, observational, multi-center survey of patients with congenital heart disease affected by COVID-19 was conducted and included two pediatric-aged patients. The first patient is one year old with a history of transposition of great arteries, pulmonary atresia and ventricular septal defect. The second patient had pulmonary atresia and ventricular septal defect and the third patient had a transposition of great arteries. No increase in mortality was observed in this group, with full recovery of all patients. |
| Xia et al[128] | The authors reported the clinical, laboratory, and chest CT features of 20 pediatric inpatients with COVID-19 infection confirmed by pharyngeal swab COVID-19 nucleic acid test. Two patients presented a pre-existing cardiac condition and survived previous surgery for atrial septal defect. The patients did not require any intensive treatment and presented a full recovery. |
| Simpson et al[155] | They presented seven children with congenital heart disease and COVID-19. Three patients had atrioventricular canal defect and trisomy 21, one had double inlet left ventricle with Fontan palliation by cardiac transplant 8 years ago, one had hypertrophic cardiomyopathy, one history of anomalous left coronary artery from the pulmonary artery surgically repaired at 2-mo-of-age. Four of the seven developed cardiac arrhythmias or new electrocardiogram abnormalities. All seven developed acute decompensation, with one death in an 18-yr-old with hypertrophic cardiomyopathy. |
| Climent et al[162] | A 5 mo-old infant with personal history of Hurler syndrome and severe dilated cardiomyopathy with myocardial dysfunction presented a worsening of his cardiac status during SARS-CoV-2 infection, leading to cardiac arrest and death after 72 h of admission. |
Children with underlying medical conditions represent 25% of the total pediatric COVID-19 cases and 80% of those hospitalized. Hoang et al[167], in an early systematic review including 7780 pediatric COVID-19 cases form 26 different countries, found that a pre-existing cardiovascular condition was present in up to 14% of patients. In the United States the CDC established on 6 April 2020 that chronic lung disease (including asthma) is the most prevalent preexisting condition (50%), followed by cardiovascular disease (31%; including obesity) and immunosuppression (12.5%). Regarding the mortality rate associated with pre-existing conditions, the CDC reported 121 deaths among persons younger than 21 years old in the United States from February to July 2020. Of them, only 25% were previously healthy people. The most frequently reported medical underlying conditions were chronic lung disease (28% including asthma), obesity (27%), neurologic conditions (22%) and cardiovascular conditions (18%). However, these reports did not analyze the association with outcomes. To date, only one multicenter observational study that only included 2 pediatric cases has focused on the clinical characteristics and outcomes in patients with CHD infected by SARS-CoV-2. The authors found that of the 76 patients included, cardiovascular complications were mainly found in the CHD-COVID-19 group, but they did not observe the worst outcome in this population[167]. An early systematic review by Sanna et al[36], including only the Chinese experience, concluded that the presence of CHD is a high-risk factor for severe COVID 19 in children. Götzinger et al[25], in a multicenter cohort study that involved 25 European countries and included 582 children, found that 25% of pediatric COVID-19 cases presented pre-existing medical conditions, which resulted in an independent risk factor for PICU admission (OR, 5.06, 95%CI: 1.72–14.87; P = 0.0035) in multivariate analysis. Of note, a total of 25 (4%) children had a previously known CHD as comorbidity, and they present a higher risk for PICU admission (OR, 2.9, 95%CI: 1.0-8.4; P = 0.029) in the univariate analysis. DeBiasi et al[22] reported a cohort of 177 children with COVID-19 in the United States. Of them, 3% presented a pre-existing cardiac condition, and these patients were more common in hospitalized as a non-hospitalized group (9% vs 1%; P = 0.004). However, when comparing the SARS-CoV-2 infected non-critically ill and critically ill hospitalized patients, there were no significant differences in the presence of underlying cardiac conditions (22% vs 6%; P = 0.180).
From these data, we can state that although plausible, there is not yet enough evidence currently to support the association of CHD and severe COVID-19 in children. It is necessary to bear in mind that these data could instead be a reflection of the overprotective management that is usually given to these patients, with preventive hospitalizations for monitoring even though they do not require treatment, nor do they have severe clinical affectations. Hence, using recommended clinical criteria for hospital admission in children with CHD might lead to many of these being hospitalized, who could otherwise have been managed at home[12]. Remarkably, the admission of these high-risk patients must be overweighted with the risk for SARS-CoV-2 nosocomial infection, and the presence of CHD should not be used in isolation for hospitalization. CHD constitutes a very heterogeneous group of patients and not all the CHD have the same risk for adverse outcomes during viral infections. Therefore, the identification of vulnerable CHD cases is crucial to improve the efficiency of the management of this population, avoiding unnecessary hospitalizations and also the late recognition of life-threatening complications in this population. In the absence of strong pediatric evidence, risk stratification, and further recommendations are currently performed based on the adult CHD anatomic and physiological classification (Table 8)[168]. In summary, any child with a CHD requiring medication for heart failure or arrhythmia may experience a worsening of their clinical status because of the hemodynamic impact of the lung involvement and the myocardial injury of SARS-CoV-2 infection. On the other hand, children with complete and successful surgical correction of the CHD and without the need for cardiac medications could be managed as healthy children. This approach requires individual assessment and adjustment for consistency with current local recommendations. The specialized evaluation by the pediatric cardiology team could be essential for the adequate selection of cases that will benefit from hospitalization and more therapies than supplemental oxygen, such as heart failure drugs.
| High risk of poor outcomes with COVID-19 | Low risk1 of poor outcomes with COVID-19 | ||
| Physiological stage B | Physiological stage A | Physiological stage A | NYHA FC I symptoms |
| Mild hemodynamic squeal | No hemodynamic or anatomic squeal | ||
| Mild valvular disease | No arrhythmias | ||
| Trivial or small shunt | Normal exercise capacity | ||
| Arrhythmia not requiring treatment | Normal renal/hepatic/pulmonary function | ||
| Abnormal objective cardiac limitation to exercise | |||
| Physiological stage C | NYHA FC III symptoms | ||
| Significant valvular disease moderate or greater ventricular dysfunction | |||
| Moderate aortic enlargement | |||
| Venous or arterial stenosis. | |||
| Mild-moderate hypoxemia/cyanosis | |||
| Hemodynamically significant shunt | |||
| Arrhythmias controlled with treatment | |||
| Mild-Moderate Pulmonary hypertension | |||
| End-organ dysfunction that is responsive to therapy. | |||
| Physiological stage D | NYHA FC IV symptoms | ||
| Severe aortic enlarge | |||
| Arrhythmias refractory to treatment | |||
| Severe hypoxemia (associated with cyanosis) | |||
| Severe pulmonary hypertension | |||
| Eisenmenger syndrome | |||
| Refractory end-organ dysfunction | |||
In addition to the increased risk for severe infection, CHD patients are facing the tremendous impact of the pandemic on outpatient visits and surgical programs around the world[169-172]. To minimize SARS-CoV-2 spread, extensive preventive measures are essential for these patients. Similar to the general population, children with CHD and their careers must adopt physical and social distancing measures, meticulous hygiene with frequent hand washing, and use appropriately of facemasks. Nevertheless, social distancing can be particularly challenging for pediatric CHD patients, especially newborns and infants, who are a fragile population in need of immediate and continuing care. Due to the declared lockout to restrict physical contact, the many sick leaves for healthcare professionals, and the reallocation of CHD specialists to general care facilities to deal with these sick leaves, the volume of CHD outpatient visits may have to be reduced to essential visits only. Likewise, a profound reorganization with prioritization of emergent and urgent procedures, and the cessation of all-elective surgical activity has been adopted (Table 9). Therefore, there is an expanding increase of the waiting lists, leading to a delay of the diagnosis and adequate management of CHD complications and a delay of the optimal timings for corrections of some stable CHD, with a potential direct effect on morbidity and mortality. This problem is particularly significant in newborns and infants, who are a fragile population in need of close and continuing care and often require surgery during a narrow window of time to avoid death and provide for optimal outcomes. To avoid preventable complications due to canceled visits and diagnostic or therapeutic procedures, pediatric cardiology specialists should review outpatient appointments and ensure that high-risk patients are prioritized. For most patients listed on class IA, scheduled clinic visits should be converted to telehealth visits whenever possible, to maintain social distancing to avoid disease spread[173,174]. However, pediatric cardiology teleservices could be not enough for complex chronic conditions. In these patients, the risk of SARS-CoV-2 exposure must be weighed against the needful in-person visits case-by-case. It is still unclear the real impact of this reorganization in terms of increased morbidity and mortality in this population. Of note, it would be essential to catching up with all surgical interventions and outpatient visits deferred during the pandemic at the same time that timely diagnosis and surgical corrections of all new patients are being carried out.
| Emergency cases (Not delay more than 24-48 h) | Urgent cases (Not delay more than days to weeks) | Elective cases (Delay > 2 mo) |
| Surgical or catheter procedures | ||
| ECMO in hemodynamically unstable patient | Transposition of great vessels | |
| PDA stent in unstable patient on prostaglandin treatment | Norwood procedure for hypoplastic left heart syndrome | Valvular regurgitations managed medically |
| Thrombosed shunt | Truncus arteriosus | Slow progressive aortic stenosis scheduled for Ross procedure |
| Pericardial tamponade | Obstructive lesions stabilized with prostaglandins | Pre-Fontan catheterization with adequate saturations on room air (> 75%) |
| Rashkind procedure | Glenn procedure with decreasing saturations (< 75%) | |
| Heart transplant | Persistent heart failure in shunts on maximal anti congestive therapy | |
| Obstructed total anomalous pulmonary venous return | Endocarditis in hemodynamically stable patient | |
| ALCAPA | ||
| Stenotic right ventricle-Pulmonary artery conduit with severe ventricular dysfunction | ||
| Electrophysiological procedures | ||
| Emergency cases (Not delay more than 24-48 h) | Urgent cases (Not delay more than days to weeks) | Elective cases (Delay > 2 mo) |
| Cardiac arrest in association with pre-excited atrial fibrillation | Primary prevention defibrillator implants after life-threatening ventricular arrhythmia | Tilt-table test |
| Arrhythmia causing need for ECMO | Cardiac resynchronization therapy | Implantable loop recorder implants |
| Incessant arrhythmia with severe ventricular dysfunction | Ablation for medically refractory ventricular tachycardia | Ablation of stable arrhythmias adequate managed with drugs without cardiomyopathy |
| Pacemaker insertion for advanced AV-block | Ablation of SVT though to contribute to cardiomyopathy | Upgrades of devices |
| Defibrillator implant for secondary prevention of sudden death | Generator replacements with > 6 wk of battery remaining | |
| Pacemaker generator replacement for pacing-dependent patients | Pacemaker implant for sinus node dysfunction, non-high-grade AV block and tachy-brady syndrome in middle symptomatic patients | |
| Defibrillator generator replacement for patients with appropriate defibrillator therapies. | ||
| Ablation of supra ventricular arrhythmias causing hemodynamic deterioration and WPW syndrome associated with cardiac arrest | ||
| Transvenous lead extraction |
Genetic heart diseases: Genetic heart diseases (GHD) represent a very heterogeneous group of congenital disorders affecting the heart muscle (cardiomyopathies) or the electrical system (channelopathies). Of them, the most prevalent conditions in children are long QT syndrome (LQTS), catecholaminergic polymorphic ventricular tachycardia (CPVT), Brugada syndrome, dilated cardiomyopathy (DCM), and hypertrophic cardiomyopathy (HCM)[175]. These rare conditions are often the underlying cause of life-threatening arrhythmias, myocardial dysfunction, heart failure, and sudden cardiac death in children and adolescents. The role of SARS-CoV-2 infection in these diseases is still not well known. The experience with COVID-19 in these patients is even scarcer than the existent of CHD patients. To date, we only have found 1 case report of a 5-mo-old infant with a personal history of DCM that presented a worsening of his cardiac status during SARS-CoV-2 infection, leading to cardiac arrest and death (Table 7)[162]. Also, a case of Brugada syndrome uncovered by fever has been reported in the adult population[176].
As mentioned above, SARS-CoV-2 infection can produce myocardial dysfunction and ventricular arrhythmia through direct myocardial damage and hyper-inflammatory state. Also, as a viral infection, COVID-19 can provoke situations that can act as triggers for myocardial dysfunction and ventricular arrhythmias such as fever, hyper-adrenergic states, increased energetic and oxygen consumption, dehydration, ion alterations (especially potassium, calcium, and magnesium), metabolic crises with lactic acidosis… Finally, some drugs used against SARS-CoV-2 (hydroxychloroquine, Remdesivir, azithromycin, tocilizumab) could be deleterious[177-180]. Therefore, patients with genetic heart diseases may be at an increased risk in the setting of COVID-19, necessitating additional precautions, and specialized management. As a very heterogeneous group, disease-specific recommendations and precautions should be employed. In the absence of enough evidence, recommendations for the management of these rare diseases are based on previous experience with other viral infections and following expert panel recommendations (Table 10)[181,182]. For example, fever must be managed aggressively in Brugada syndrome; potassium levels must be higher than expected in the case of LQTS. Situations of dehydration can be dangerous in the context of hypertrophic cardiomyopathy. In the case of DCM, there is an increased risk of metabolic decompensation or lactic acidosis with associated severe myocardial dysfunction. The increased adrenergic tone may be harmful in almost all cases, overall HCM, LQTS, and CPVT. Finally, Covid-19 infection in patients with cardiomyopathies represents a substantial risk of worsening patient clinical status, particularly in those who experienced previous heart failure, arrhythmic events, or myocardial dysfunction on echocardiography requiring medications.
| Disease | Recommendations for management during COVID-19 pandemic |
| General recommendations | Preventive measures to minimize SARS-CoV-2 infection: Social distancing, hand-washing, facial mask. Limit outpatient clinic visits and electrophysiological and surgical procedures to life-threatening arrhythmias requiring immediate treatment, non-deferrable treatments and urgent diagnostic devices. Rule-out the presence of ventricular arrhythmia or heart failure when common overlapping COVID-19 symptoms appear: Dyspnea, syncope, cough, fatigue. Aggressive management of fever, diarrhoea and adrenergic stress as the main triggers for cardiac complications. Balance fluid and electrolyte intake according to clinical status. Influenza, pneumococcal and respiratory syncytial virus vaccination are recommended to reduce the possibility of co-infection of COVID-19. Consider at home management as first option whenever possible. Consider initial hospitalization for closely monitoring and intensive treatment in high-risk patients for heart failure or sudden cardiac death episodes. Pediatric cardiologist evaluation is highly advised when hospitalization is required. Careful use of specific COVID-19 treatment (antivirals and immunomodulatory drugs). Not discontinue usual cardiac basal. |
| LQTS | Avoid hyper-adrenergic states as triggers of Ventricular Tachycardia and Torsade de Pointes. Fever is not a main issue in LQTS. Aggressive control of fever is only recommended for LQTS type-2 cases. Beta-blocker therapy must be continued. QT prolonging drugs should be avoided. Flecainide can interact with antivirals but must not be discontinued. Avoid and correct dehydration states with ion alterations, overall potassium). Check serum electrolyte levels (especially potassium) in case of vomiting and diarrhoea. Keep potassium level above 4mEq/l with potassium supplements. Consider hospitalization in high-risk patients: Previous syncope. High-risk mutation. Infants younger than 1 year-old. Whenever an in-hospital admission is needed, a careful QT monitoring and a telemetric system should be used. Specific therapies for COVID-19 that are known to prolong the QT interval, specially hydroxychloroquine, azithromycin and ritonavir, should be avoided or used with caution. |
| Brugada | Aggressive management of Fever is the main issue. All patients should self-treat with paracetamol immediately if they develop signs of fever and stay at home. Consider hospitalization in high-risk patients: Children without an ICD and with previous syncope, spontaneous Brugada type-1 pattern on ECG, persistent fever despite paracetamol treatment at home, presence of palpitations or syncope. Management in the hospital should include monitoring of ECG abnormalities and arrhythmia as well as efforts to reduce the body temperature. If an ECG shows the type 1 Brugada ECG pattern, then the patient will need to be observed until fever and/or the ECG pattern resolves. If all ECGs show no sign of the type 1 Brugada ECG pattern, then they can go home. Specific drugs for COVID-19 do not influence on Brugada syndrome patients. |
| CPVT | At present, there are no data suggesting that patients with CPVT are at increased risk of infection with COVID-19. Avoid hyper-adrenergic states as triggers of Ventricular Tachycardia. Whenever possible, avoid the use of adrenaline in situations of ventricular tachycardia/ventricular fibrillation (VT/VF). Adrenaline is contraindicated in the event of cardiac arrest. Beta-blocker therapy must be continued. QT prolonging drugs should be avoided. Flecainide can interact with antivirals but must not be discontinued. An increased heart rate alone (pacing-induced), as an important symptom of fever or stressful circumstances, does not appear to be sufficient for the induction of ventricular arrhythmias. The antiviral or immunomodulatory therapy proposed for COVID-19 is not expected to influence on CPVT patients. |
| Cardiomyopathies | Avoid hyper-adrenergic and dehydration states that can provoke or increase left ventricular outflow obstruction leading to syncope and sudden cardiac death in HCM. Avoid hyper-adrenergic states with increased energetic and oxygen consumption leading to a worsening the myocardial function and decompensated heart failure in DCM. Consider hospitalization in high risk patients: Basal left ventricular outflow tract obstruction, end stage cardiomyopathies, decompensated HF with no response to intensification of oral treatment at home, syncope Hospital management include balance fluid and electrolyte intake according to the clinical status. Predisposition to Pulmonary edema. Negative hydric balance in case of pulmonary edema in DCM. Positive hydric balance in case of LVOTO in HCM. ECG monitoring watching for VA. QT monitoring, especially in patients on disopyramide and COVID-19 therapies). Echocardiography is mandatory to assess LVOTO and myocardial function. |
Patients with GHD are suitable for multiple outpatient clinic visits, hospitalizations, and surgical or electrophysiological interventions to have the reasonable control of the disease. During the pandemic, similarly to those patients with CHD, only urgent surgical procedures and electrophysiological studies are performed, delaying all elective procedures (Table 9) following expert panel recommendations[181,182]. When possible, most patients should at first be offered telehealth consultation, outpatient clinic visits must be warranted for all the new patients with a history likely of a life-threatening condition and the follow-up of patients with known genetic heart disease when treatment cannot be optimized remotely. Patients with high-risk GHD and SARS-CoV-2 infection could benefit from hospitalization for close monitoring and management of cardiovascular complications. Again, expert evaluation of a pediatric cardiologist may be essential in determining how best to minimize the risk of cardiac events during hospitalization.
Issues with cardiac medication during SARS-CoV-2 infection: Many children with underlying heart diseases are under treatment with cardiac medications during the pandemic. Concern exists about the potential risks of deleterious effects of these drugs when they are administered during SARS-CoV-2 infection. Anti-inflammatory drugs (Aspirin, Ibuprofen, colchicine, steroids) are used in cases of pericarditis and KD[102, 183, 184]. They have been widely used in a safe and effective way in the treatment of PMIS cases. Beta-blockers are commonly used in pediatric cardiology, mainly as a treatment for heart failure in CHD or DCM, minimizing left ventricular outflow tract obstruction in HCM patients, and preventing VT in LQTS and CPVT. Amiodarone, sotalol, and flecainide do have interactions with other QT interval-prolonging drugs such as ritonavir/Lopinavir and chloroquine but is an important enough therapy not to discontinue in these particularly stressful circumstances where paroxysms of tachycardia could occur[185]. Digoxin and diuretics should be used cautiously. Digoxin action depends on potassium/calcium levels and toxicity may occur in case of vomiting or diarrhea during COVID-19. Diuretics can provoke alterations on the electrolyte imbalance and dehydration with deleterious effects for some patients[186]. As there is no evidence for the harmful effects of most of these drugs in the setting of COVID-19, the general recommendation is to continue on these medications unless contraindications or side effects appear.
Only the safety of Renin-angiotensin-aldosterone system (RAAS) inhibitors has been questioned early after the beginning of the pandemic. RAAS is widely used in children with CHD and heart failure as an agent to minimize afterload and avoid the abnormal myocardial remodulation[185,186]. It has been argued that RAAS inhibitors and angiotensin receptor blockers may up-regulate of angiotensin-converting enzyme 2 (ACE2), hereby increasing susceptibility to the virus and hence may cause a more severe infection[40-42,187]. Anyway, treatment with RAAS inhibitors may down-regulate the expression of ACE2, but it seems not to have a significant effect on its activity. Thus, the data is currently insufficient to provide any definitive recommendation to discontinue these medications in children taking them for indications for which the agents are known to be beneficial. Therefore, the leading international cardiovascular scientific societies recommend maintaining or initiating the ACEIs/ARB's treatment in patients with heart diseases when indicated, irrespective of SARS-CoV-2 infection.
Cardiovascular effects of drugs used for COVID-19 treatment: Once a patient with underlying cardiac disease is diagnosed with COVID-19, the management of the infection is similar to the general population, with a particular focus in the surveillance of clinical signs of heart failure and ventricular arrhythmia. Currently, the primary treatment for COVID-19 disease is supportive care, ensuring adequate oxygenation, symptomatic relief with antipyretics, and nutritional support for the patient[12,188,189]. Most children can be managed conservatively and do not require specific treatment. In the case of cardiovascular complications, the management should be done following current guidelines and local protocols for each manifestation. In severe cases of COVID-19, specific treatment with antiviral drugs (lopinavir/ritonavir, chloroquine/hydroxychloroquine) to control viral replication at early stages or immunomodulatory drugs (steroids or tocilizumab) to control the hyper-inflammatory response at later stages of the disease may be considered[190]. However, these drugs are not considered the standard of care at this point. They are mostly off-label and investigational drugs with only positive pre-clinical data. Of note, many specific COVID-19 therapies have potential cardiovascular side effects, which can further increase the risk of myocardial dysfunction and arrhythmias associated with SARS-CoV-2 infection (Table 11).
| Drugs | Cardiovascular adverse effect | Cardiovascular monitoring |
| Hydroxychloroquine | Vomiting with low-potassium levels. QT-prolonging and TdP. Conduction abnormalities; Heart block. Myocardial injury and Cardiomyopathy. | Monitor QTc interval, specially when using in combination with other QT-prolonging drugs and CYP3A4-inhibiting drugs. Monitor myocardial function by echocardiography and pro-BNP levels. |
| Azithromycin | QT-prolonging and TdP. Moderate CYP3A4 inhibitor. | Monitor QTc interval, specially when using in combination with other QT-prolonging drugs and CYP3A4-inhibiting drugs. |
| Lopinavir/Ritonavir | Vomiting with low-potassium levels. PR-prolonging. QT-prolonging and TdP. Major CYP3A4 inhibitor. | Monitor QTc interval, specially when using in combination with other QT-prolonging drugs and CYP3A4-inhibiting drugs. |
| Remdesivir | Limited data. Severe Hypotension and cardiac arrest after loading dose in Ebola patients. Possible CYP3A4 inducer. | Monitor Hemodynamics with infusion. Carful with unstable patients. |
| Steroids | Exacerbation of Lymphopenia. Can induce hypertension. | Monitor Hemodynamics with infusion. Monitor LVOTO in HCM by echocardiography. Careful in HCM. |
| Tocilizumab | Hypertension. Volume retention. Hypersensitivity. Increased lipid profile. | Monitor Hemodynamics with infusion. Monitor myocardial ischemia (ECG; Troponin). Careful in patients with myocardial dysfunction, chamber dilation or pulmonary edema. |
Early in the pandemic, concerns have appeared about the potential of pro-arrhythmic events due to the use of prolonging-qt drugs that have raised, particularly the combined use of chloroquine (CQ), HCQ and azithromycin[147-149,191,192]. The authors of a small single-center, the retrospective study found no risk of QT prolongation or TdP in children with COVID-19 under treatment with HCQ[150]. However, the patients included were not severely affected by the disease, and none of them required intensive care, had cardiovascular comorbidity, and were taking corrected QT-prolonging medications, nor had ionic alterations. Therefore, caution must be used when applying them to patients with pre-existing heart disease. In the absence of clear benefit and safety data, therapies associated with greater QT prolongation and arrhythmic risk should be avoided, particularly if they are administered in combination with CYP3A4-inhibiting drugs. If they are considered to be beneficial, a step-by-step approach with the specialized supervision of the pediatric cardiology team must be made to minimize the occurrence of cardiac toxicity (Table 12)[193].
| Step-by-step approach to administer prolonging-etc drugs during SARS-CoV-2 infection | |
| 1 | QTc intervals should be monitored at baseline and at 4 h after the administration of any QTc-prolonging drug. |
| 2 | QTc interval monitoring previously to combine any drugs prolonging the QTc interval or CYP3A4-inhibiting drugs. |
| 3 | QTc interval monitoring in patients with Known LQTS, acquired QT prolongation, or conditions associated with acquired QT prolongation (e.g, use of other QT-prolonging drugs, underlying heart disease, bradycardia, liver and renal disease electrolyte alterations…) |
| 4 | Serum potassium, calcium and magnesium should be evaluated at baseline and monitored and optimized daily. |
| 5 | Avoiding hypokalaemia is not enough. Patients with acquired LQTS or patients using a combination of QT-prolonging drugs should have a high serum potassium level (5 mEq/L). |
| If QTc increases by > 60 milliseconds or absolute QTc > 500 milliseconds (or > 530-550 milliseconds if QRS > 120 milliseconds) is observed | |
| 1 | Consultation with a pediatric cardiologist (“QT specialist”) for guidance in case of important QT prolongation. A careful balance of pros and cons should guide the decision to discontinue therapy. |
| 2 | Intensified ECG monitoring |
| 3 | Raising potassium levels |
| 4 | Correct QT-prolonging factors (calcium, magnesium, potassium…) |
| 5 | Consider to increase beta-blocker dosage |
In this review, we have summarized the actual evidence about the cardiovascular involvement in children with COVID-19. Although respiratory illness is the dominant clinical manifestation of COVID-19, cardiovascular issues are emerging as one of the most significant complications of SARS-CoV-2 infection in pediatric patients. As described above, patients with pre-existing cardiovascular diseases and with PMIS accounted respectively for 18% and 12% of pediatric deaths associated with COVID-19. Remarkably, PMIS has approximately a ten-fold mortality rate regarding the rest of pediatric COVID-19 cases. These data indicate that prognosis is worse when the cardiovascular system is impaired during SARS-CoV-2 infection in children.
Those patients with underlying heart diseases seem to be a vulnerable population at high risk if they contract COVID-19. Both, the new-onset acute cardiac complications and worsening of the basal state in patients with a low basal cardiopulmonary reserve or high-risk pro-arrhythmic profile, can occur in these children. Of note, not all patients with pre-existing heart diseases have the same risk for complications, and therefore, the hospital admission and anti-COVID drugs administration must be overweighed with the risk of nosocomial infections and side effects through a case by case approach. Furthermore, the delay of timely elective procedures and the radical decrease of in-person visits for the adequate follow-up at outpatient clinics may provoke those not-expected complications before the pandemic occur. To avoid preventable complications due to canceled visits and diagnostic or therapeutic procedures, pediatric cardiology specialists should review outpatient appointments and ensure that high-risk patients are prioritized.
Remarkably, a not negligible number of previously healthy infected children have experienced severe cardiovascular acute events in the form of the new PMIS, arrhythmia, pulmonary hypertension, heart failure, and even fulminant myocarditis. The commonest complication in this population was PMIS, which affects mostly previously healthy school-aged children and adolescents presenting with Kawasaki disease-like features and multiple organ failure with a focus on the heart. They frequently presented cardiogenic shock (53%), ECG alterations (27%), myocardial dysfunction (52%), and coronary artery dilation (15%). Most cases required PICU admission (75%) and inotropic support (57%), with the rare need for extracorporeal membrane oxygenation (4%). Almost all of these children wholly recovered in a few days, although rare deaths have been reported (2%). These observations point out that, although infrequent, no child is free of the occurrence of cardiovascular events during the SARS-CoV-2 infection.
Failure to accurately identify and timely manage cardiovascular complications could lead to increased morbidity and mortality of these patients. Therefore, we recommend that those pediatric COVID-19 cases who are ill enough to require hospitalization should undergo a workup to the screening of cardiovascular issues (Figure 2). Also, close cardiovascular monitoring during hospitalization to avoid misdiagnosing these life-threatening entities should be warranted. The pediatric cardiology team should be involved in the management of any children with a pre-existing cardiac condition and any previously healthy children with abnormalities on the screening workup. In absence of enough evidence, the management of cardiac complications once they are identified should be based on the current guidelines, local protocols and the physician´s experience for each individual condition[194].
There is still scarce data about the role of cardiovascular involvement in COVID-19 in children. Based on our review, the cardiovascular involvement seems to be a relevant factor of SARS-CoV-2 infection in children. Patients with pre-existing heart diseases constitute a high-risk population for development of a severe acute COVID-19 and for decompensation of cardiac conditions. In previously healthy children, PMIS has emerged as a novel and severe condition during the pandemic. Furthermore, COVID-19 can also produce fulminant myocarditis, ventricular arrhythmias and pulmonary hypertension in previously healthy children. Data suggest that prognosis is worse when the cardiovascular system is impaired during pediatric SARS-CoV-2 infection, and that the vast majority of cardiac complications present a full recovery with a timely support. As the early identification and treatment of this entities is crucial, the performance of a cardiac workup and close cardiovascular monitoring of children with severe SARS-CoV-2 infection is highly recommended.
Since the beginning of the pandemic, coronavirus disease-2019 (COVID-19) in children has shown milder cases and a better prognosis than adults. Cardiovascular involvement is emerging as one of the most significant and life-threatening complications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in adults.
To investigate if cardiovascular involvement could be a significant risk factor for severe COVID-19 in children.
We aimed to summarize the current knowledge about the potential cardiovascular involvement in pediatric COVID-19, in order to give a perspective on how to take care of them during the current pandemic emergency.
A literature search to identify publications from January 1st, 2020 until July 31st, 2020. was conducted using PubMed and MEDLINE. Also, the websites of the health organizations including World Health Organization and Centers for Disease Control and Prevention and the website of the Johns Hopkins Coronavirus Resource Center were reviewed to provide up to date numbers and infection control recommendations. The included studies were categorized by whether the study involved previously healthy patients or patients with pre-existing cardiac conditions. For pediatric multisystemic inflammatory syndrome (PMIS) temporally associated with COVID-19, multiple meta-analyses were conducted to summarize the pooled mean proportion of different cardiovascular variables in this population in pseudo-cohorts of observed patients. All the statistical analyses were performed using the STATA 14.0 (StataCorp. College Station, TX, United States).
We included a total of 193 articles in this review. The most relevant articles were 16 studies with size > 10 patients and with complete data about cardiovascular involvement in children with PMIS, 10 articles reporting sporadic cases of myocarditis, pulmonary hypertension and cardiac arrythmias in previously healthy children, and another 10 studies reporting patients with pre-existing heart diseases. The meta-analysis of 16 studies with size > 10 patients and with complete data about cardiovascular involvement in children with PMIS showed that PMIS affects mostly previously healthy school-aged children and adolescents presenting with Kawasaki disease-like features and multiple organ failure with a focus on the heart, accounting for most cases of pediatric COVID-19 mortality. Out of PMIS cases we identified 10 articles reporting sporadic cases of myocarditis, pulmonary hypertension and cardiac arrythmias in previously healthy children. We also found another 10 studies reporting patients with pre-existing heart diseases. Most cases consisted in children with severe COVID-19 infection with full recovery after intensive care support, but cases of death were also identified. There is an increasing concern about the delay of the diagnosis and adequate management of congenital heart diseases (CHD) complications and a delay of the optimal timings for corrections of some stable CHD due to the pandemic, with a potential direct effect on morbidity and mortality.
There is still scarce data about the role of cardiovascular involvement in COVID-19 in children. Based on our review, all children (previously healthy or with pre-existing heart disease) with acute COVID-19 requiring hospital admission should undergo a cardiac workup and close cardiovascular monitoring to identify and treat timely life-threatening cardiac complications. The management of the different cardiac conditions should be based on the correspondent clinical guidelines, expert panel recommendations and physician´s experience.
Although cardiovascular involvement seems to be a crucial risk factor associated with severe pediatric SARS-CoV-2 infection, more evidence in the form of multicenter collaborative studies is necessary to elucidate this association.
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luqman Z S-Editor: Zhang H L-Editor: A P-Editor: Zhang YL
| 1. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14131] [Article Influence: 2826.2] [Reference Citation Analysis (1)] |
| 2. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 3. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4633] [Article Influence: 926.6] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 -- 11 March 2020. [cited 01 May 2020] Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. |
| 5. | Johns Hopkins Center for Systems Science and Engineering. Coronavirus resource center: COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). [cited 01 May 2020] Available from: https://systems.jhu.edu/. |
| 6. | Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020;39:355-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 714] [Cited by in RCA: 696] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 7. | Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020;323:1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 656] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 8. | Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1701] [Cited by in RCA: 1489] [Article Influence: 297.8] [Reference Citation Analysis (1)] |
| 9. | Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, Zhang J, Dong C, Na R, Zheng L, Li W, Liu Z, Ma J, Wang J, He S, Xu Y, Si P, Shen Y, Cai C. A systematic review and meta-analysis of children with Coronavirus Disease 2019 (COVID-19). J Med Virol. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 253] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 10. | Ding Y, Yan H, Guo W. Clinical Characteristics of Children With COVID-19: A Meta-Analysis. Front Pediatr. 2020;8:431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, Sanmartín JL, Fernández-García A, Cruz I, Fernández de Larrea N, Molina M, Rodríguez-Cabrera F, Martín M, Merino-Amador P, León Paniagua J, Muñoz-Montalvo JF, Blanco F, Yotti R; ENE-COVID Study Group. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1200] [Cited by in RCA: 1194] [Article Influence: 238.8] [Reference Citation Analysis (0)] |
| 12. | Escosa-García L, Aguilera-Alonso D, Calvo C, Mellado MJ, Baquero-Artigao F. Ten key points about COVID-19 in children: The shadows on the wall. Pediatr Pulmonol. 2020;55:2576-2586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Parri N, Magistà AM, Marchetti F, Cantoni B, Arrighini A, Romanengo M, Felici E, Urbino A, Da Dalt L, Verdoni L, Armocida B, Covi B, Mariani I, Giacchero R, Musolino AM, Binotti M, Biban P, Fasoli S, Pilotto C, Nicoloso F, Raggi M, Miorin E, Buonsenso D, Chiossi M, Agostiniani R, Plebani A, Barbieri MA, Lanari M, Arrigo S, Zoia E, Lenge M, Masi S, Barbi E, Lazzerini M; CONFIDENCE and COVID-19 Italian Pediatric Study Networks. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur J Pediatr. 2020;179:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 14. | Guo CX, He L, Yin JY, Meng XG, Tan W, Yang GP, Bo T, Liu JP, Lin XJ, Chen X. Epidemiological and clinical features of pediatric COVID-19. BMC Med. 2020;18:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 15. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11507] [Article Influence: 2301.4] [Reference Citation Analysis (0)] |
| 16. | Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145:e20200702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2413] [Article Influence: 482.6] [Reference Citation Analysis (0)] |
| 17. | Rodríguez-González M, Alonso-Ojembarrena A, Castellano-Martínez A, Estepa-Pedregosa L, Benavente-Fernández I, Lubián López SP. [Heart murmur in children less than 2 years-old: looking for a safe and effective referral strategy]. An Pediatr (Barc). 2018;89:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Tagarro A, Epalza C, Santos M, Sanz-Santaeufemia FJ, Otheo E, Moraleda C, Calvo C. Screening and Severity of Coronavirus Disease 2019 (COVID-19) in Children in Madrid, Spain. JAMA Pediatr. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 19. | Kim L, Whitaker M, O'Halloran A, Kambhampati A, Chai SJ, Reingold A, Armistead I, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Openo KP, Weigel A, Ryan P, Monroe ML, Fox K, Kim S, Lynfield R, Bye E, Shrum Davis S, Smelser C, Barney G, Spina NL, Bennett NM, Felsen CB, Billing LM, Shiltz J, Sutton M, West N, Talbot HK, Schaffner W, Risk I, Price A, Brammer L, Fry AM, Hall AJ, Langley GE, Garg S; COVID-NET Surveillance Team. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 20. | Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1712] [Cited by in RCA: 1699] [Article Influence: 339.8] [Reference Citation Analysis (0)] |
| 21. | Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr. 2020;174:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 778] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 22. | DeBiasi RL, Song X, Delaney M, Bell M, Smith K, Pershad J, Ansusinha E, Hahn A, Hamdy R, Harik N, Hanisch B, Jantausch B, Koay A, Steinhorn R, Newman K, Wessel D. Severe Coronavirus Disease-2019 in Children and Young Adults in the Washington, DC, Metropolitan Region. J Pediatr 2020; 223: 199-203. e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 23. | Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, Ushay HM, Cabana MD, Medar SS. Clinical Characteristics and Outcomes of Hospitalized and Critically Ill Children and Adolescents with Coronavirus Disease 2019 at a Tertiary Care Medical Center in New York City. J Pediatr 2020; 223: 14-19. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 24. | Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, Heidemann SM, Kleinman LC, Sen AI, Hall MW, Priestley MA, McGuire JK, Boukas K, Sharron MP, Burns JP; International COVID-19 PICU Collaborative. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020;174:868-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 707] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 25. | Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U, Lo Vecchio A, Shingadia D, Soriano-Arandes A, Melendo S, Lanari M, Pierantoni L, Wagner N, L'Huillier AG, Heininger U, Ritz N, Bandi S, Krajcar N, Roglić S, Santos M, Christiaens C, Creuven M, Buonsenso D, Welch SB, Bogyi M, Brinkmann F, Tebruegge M; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 931] [Cited by in RCA: 848] [Article Influence: 169.6] [Reference Citation Analysis (1)] |
| 26. | Brodin P. Why is COVID-19 so mild in children? Acta Paediatr. 2020;109:1082-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 27. | Balasubramanian S, Rao NM, Goenka A, Roderick M, Ramanan AV. Coronavirus Disease 2019 (COVID-19) in Children - What We Know So Far and What We Do Not. Indian Pediatr. 2020;57:435-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 28. | Lingappan K, Karmouty-Quintana H, Davies J, Akkanti B, Harting MT. Understanding the age divide in COVID-19: why are children overwhelmingly spared? Am J Physiol Lung Cell Mol Physiol. 2020;319:L39-L44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Jia R, Wang X, Liu P, Liang X, Ge Y, Tian H, Chang H, Zhou H, Zeng M, Xu J. Mild Cytokine Elevation, Moderate CD4+ T Cell Response and Abundant Antibody Production in Children with COVID-19. Virol Sin. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Ruggiero A, Attinà G, Chiaretti A. Additional hypotheses about why COVID-19 is milder in children than adults. Acta Paediatr. 2020;109:1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Molloy EJ, Bearer CF. COVID-19 in children and altered inflammatory responses. Pediatr Res. 2020;88:340-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, Banker SL, Giordano M, Manice CS, Diamond R, Sewell TB, Schweickert AJ, Babineau JR, Carter RC, Fenster DB, Orange JS, McCann TA, Kernie SG, Saiman L; Columbia Pediatric COVID-19 Management Group. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children's Hospital in New York City, New York. JAMA Pediatr. 2020;e202430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 352] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 33. | Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: A systematic review. Pediatr Pulmonol. 2020;55:2565-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 34. | Meena J, Yadav J, Saini L, Yadav A, Kumar J. Clinical Features and Outcome of SARS-CoV-2 Infection in Children: A Systematic Review and Meta-analysis. Indian Pediatr. 2020;57:820-826. [PubMed] |
| 35. | Azevedo RB, Botelho BG, Hollanda JVG, Ferreira LVL, Junqueira de Andrade LZ, Oei SSML, Mello TS, Muxfeldt ES. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 36. | Sanna G, Serrau G, Bassareo PP, Neroni P, Fanos V, Marcialis MA. Children's heart and COVID-19: Up-to-date evidence in the form of a systematic review. Eur J Pediatr. 2020;179:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 37. | Imazio M, Klingel K, Kindermann I, Brucato A, De Rosa FG, Adler Y, De Ferrari GM. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 38. | Veer M, Kumar AM, Ivanova V. COVID-19 and the Cardiovascular System. Crit Care Nurs Q. 2020;43:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14274] [Article Influence: 2854.8] [Reference Citation Analysis (0)] |
| 40. | Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D'Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1004] [Cited by in RCA: 931] [Article Influence: 186.2] [Reference Citation Analysis (0)] |
| 41. | Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 121] [Reference Citation Analysis (0)] |
| 42. | Li X, Pan X, Li Y, An N, Xing Y, Yang F, Tian L, Sun J, Gao Y, Shang H, Xing Y. Cardiac injury associated with severe disease or ICU admission and death in hospitalized patients with COVID-19: a meta-analysis and systematic review. Crit Care. 2020;24:468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 43. | González Cortés R, García-Salido A, Roca Pascual D, Slöcker Barrio M, de Carlos Vicente JC; SECIP Study Group on SARS-CoV-2 in Critically Ill Pediatric Patients. A multicenter national survey of children with SARS-CoV-2 infection admitted to Spanish Pediatric Intensive Care Units. Intensive Care Med. 2020;46:1774-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1565] [Cited by in RCA: 1769] [Article Influence: 353.8] [Reference Citation Analysis (0)] |
| 45. | Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020;324:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1333] [Article Influence: 266.6] [Reference Citation Analysis (0)] |
| 46. | Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1578] [Cited by in RCA: 1670] [Article Influence: 334.0] [Reference Citation Analysis (0)] |
| 47. | Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, Udo T, Kumar J, Pulver W, Smith L, Hutton B, Blog D, Zucker H; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 2020;383:347-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 864] [Cited by in RCA: 985] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
| 48. | Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1858] [Article Influence: 371.6] [Reference Citation Analysis (0)] |
| 49. | de Farias ECF, Pedro Piva J, de Mello MLFMF, do Nascimento LMPP, Costa CC, Machado MMM, Rodrigues TDS, Carvalho RDFP, Alves MCB, Aires LFQ, Cotta MLM, Pedreira ARG, Saraty SB, Lima MC, Justino MCA. Multisystem Inflammatory Syndrome Associated With Coronavirus Disease in Children: A Multi-centered Study in Belém, Pará, Brazil. Pediatr Infect Dis J. 2020;39:e374-e376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. [cited 22 May 2020]. Available from: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem- inflammatory-syndrome-associated-covid-19. |
| 51. | World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. 2020 May 15 [cited 22 May 2020] Available from: https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. |
| 52. | Centers for Disease Control and Prevention. Emergency preparedness and response: health alert network. 2020 May 14 [cited 22 May 2020]. Available from: https://emergency.cdc.gov/han/2020/han00432.asp. |
| 53. | Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, Roguski K, Wallace B, Prezzato E, Koumans EH, Lee EH, Geevarughese A, Lash MK, Reilly KH, Pulver WP, Thomas D, Feder KA, Hsu KK, Plipat N, Richardson G, Reid H, Lim S, Schmitz A, Pierce T, Hrapcak S, Datta D, Morris SB, Clarke K, Belay E; California MIS-C Response Team. COVID-19-Associated Multisystem Inflammatory Syndrome in Children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 557] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 54. | Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, Gillen JK, Perez MM, Soshnick SH, Conway EE Jr, Bercow A, Seiden HS, Pass RH, Ushay HM, Ofori-Amanfo G, Medar SS. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-institutional Study from New York City. J Pediatr. 2020;224:24-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 286] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 55. | Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, Fitzgerald JC, Topjian A, John ARO. Multisystem Inflammatory Syndrome in Children During the Coronavirus 2019 Pandemic: A Case Series. J Pediatric Infect Dis Soc. 2020;9:393-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 273] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 56. | Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L, Bordet J, Di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B, Moceri P, Houyel L, Renolleau S, Bonnet D. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 865] [Article Influence: 173.0] [Reference Citation Analysis (1)] |
| 57. | Ebina-Shibuya R, Namkoong H, Shibuya Y, Horita N. Multisystem Inflammatory Syndrome in Children (MIS-C) with COVID-19: Insights from simultaneous familial Kawasaki Disease cases. Int J Infect Dis. 2020;97:371-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 58. | Kest H, Kaushik A, DeBruin W, Colletti M, Goldberg D. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with 2019 Novel Coronavirus (SARS-CoV-2) Infection. Case Rep Pediatr. 2020;2020:8875987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Rauf A, Vijayan A, John ST, Krishnan R, Latheef A. Multisystem Inflammatory Syndrome with Features of Atypical Kawasaki Disease during COVID-19 Pandemic. Indian J Pediatr. 2020;87:745-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 60. | Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Posada R, Sordillo EM, Tosi M, Trachtman R, Paniz-Mondolfi A. Multisystem Inflammatory Syndrome in Children (MIS-C) Related to COVID-19: A New York City Experience. J Med Virol. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 61. | Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, Leruez-Ville M, Quartier P, Léger PL, Geslain G, Semaan N, Moulin F, Bendavid M, Jean S, Poncelet G, Renolleau S, Oualha M. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 62. | Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, Debray A, Basmaci R, Salvador E, Biscardi S, Frange P, Chalumeau M, Casanova JL, Cohen JF, Allali S. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 684] [Article Influence: 136.8] [Reference Citation Analysis (0)] |
| 63. | Heidemann SM, Tilford B, Bauerfeld C, Martin A, Garcia RU, Yagiela L, Sarnaik AP. Three Cases of Pediatric Multisystem Inflammatory Syndrome Associated with COVID-19 Due to SARS-CoV-2. Am J Case Rep. 2020;21:e925779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Ng KF, Kothari T, Bandi S, Bird PW, Goyal K, Zoha M, Rai V, Tang JW. COVID-19 multisystem inflammatory syndrome in three teenagers with confirmed SARS-CoV-2 infection. J Med Virol. 2020;92:2880-2886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 65. | Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, Schleien C; Northwell Health COVID-19 Research Consortium; Epstein S; Johnson JC; Kessel A; Misra N; Mitchell E; Palumbo N; Rajan S; Rocker J; Williamson K; Davidson KW. Characteristics, Cardiac Involvement, and Outcomes of Multisystem Inflammatory Syndrome of Childhood Associated with severe acute respiratory syndrome coronavirus 2 Infection. J Pediatr. 2020;224:141-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 66. | Ramcharan T, Nolan O, Lai CY, Prabhu N, Krishnamurthy R, Richter AG, Jyothish D, Kanthimathinathan HK, Welch SB, Hackett S, Al-Abadi E, Scholefield BR, Chikermane A. Paediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS): Cardiac Features, Management and Short-Term Outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol. 2020;41:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 67. | Yozgat CY, Uzuner S, Bursal Duramaz B, Yozgat Y, Erenberk U, Iscan A, Turel O. Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID-19 in a 3-year-old girl. Dermatol Ther. 2020;e13770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal Symptoms as a Major Presentation Component of a Novel Multisystem Inflammatory Syndrome in Children That Is Related to Coronavirus Disease 2019: A Single Center Experience of 44 Cases. Gastroenterology. 2020;159:1571-1574.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 69. | Davies P, Evans C, Kanthimathinathan HK, Lillie J, Brierley J, Waters G, Johnson M, Griffiths B, du Pré P, Mohammad Z, Deep A, Playfor S, Singh D, Inwald D, Jardine M, Ross O, Shetty N, Worrall M, Sinha R, Koul A, Whittaker E, Vyas H, Scholefield BR, Ramnarayan P. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4:669-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 70. | Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, Bensaid P, Pichard S, Kouider H, Morelle G, Craiu I, Pondarre C, Deho A, Maroni A, Oualha M, Amoura Z, Haroche J, Chommeloux J, Bajolle F, Beyler C, Bonacorsi S, Carcelain G, Koné-Paut I, Bader-Meunier B, Faye A, Meinzer U, Galeotti C, Melki I. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 373] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 71. | Dolinger MT, Person H, Smith R, Jarchin L, Pittman N, Dubinsky MC, Lai J. Pediatric Crohn Disease and Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19 Treated With Infliximab. J Pediatr Gastroenterol Nutr. 2020;71:153-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 72. | Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte-Neto AN, Soares Gomes-Gouvêa M, Viu Degaspare N, Figueiredo Delgado A, Montanari Fiorita C, Nunes Leal G, Rodrigues RM, Taverna Chaim K, Rebello Pinho JR, Carneiro-Sampaio M, Mauad T, Ferraz da Silva LF, Brunow de Carvalho W, Saldiva PHN, Garcia Caldini E. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4:790-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 73. | Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, Delacourt C, Iriart X, Ovaert C, Bader-Meunier B, Kone-Paut I, Levy-Bruhl D. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 74. | Bapst T, Romano F, Müller M, Rohr M. Special dermatological presentation of paediatric multisystem inflammatory syndrome related to COVID-19: erythema multiforme. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Greene AG, Saleh M, Roseman E, Sinert R. Toxic shock-like syndrome and COVID-19: A case report of multisystem inflammatory syndrome in children (MIS-C). Am J Emerg Med. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 76. | Hameed S, Elbaaly H, Reid CEL, Santos RMF, Shivamurthy V, Wong J, Jogeesvaran KH. Spectrum of Imaging Findings on Chest Radiographs, US, CT, and MRI Images in Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19. Radiology. 2020;202543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 77. | Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, Schnuriger A, Lorrot M, Guedj R, Ducou le Pointe H. Cardiac MRI of Children with Multisystem Inflammatory Syndrome (MIS-C) Associated with COVID-19: Case Series. Radiology. 2020;Online ahead of print 202288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 78. | Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, Milner JD. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA. 2020;324:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 430] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 79. | Balasubramanian S, Nagendran TM, Ramachandran B, Ramanan AV. Hyper-inflammatory Syndrome in a Child With COVID-19 Treated Successfully With Intravenous Immunoglobulin and Tocilizumab. Indian Pediatr. 2020;57:681-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 80. | Ouldali N, Pouletty M, Mariani P, Beyler C, Blachier A, Bonacorsi S, Danis K, Chomton M, Maurice L, Le Bourgeois F, Caseris M, Gaschignard J, Poline J, Cohen R, Titomanlio L, Faye A, Melki I, Meinzer U. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health. 2020;4:662-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 81. | Schupper AJ, Yaeger KA, Morgenstern PF. Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Childs Nerv Syst. 2020;36:1579-1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 82. | Rivera-Figueroa EI, Santos R, Simpson S, Garg P. Incomplete Kawasaki Disease in a Child with Covid-19. Indian Pediatr. 2020;57:680-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 83. | Waltuch T, Gill P, Zinns LE, Whitney R, Tokarski J, Tsung JW, Sanders JE. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 84. | Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, Montin D. SARS-CoV-2-Induced Kawasaki-Like Hyperinflammatory Syndrome: A Novel COVID Phenotype in Children. Pediatrics. 2020;146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 85. | Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, Nguyen EL, Barsh GR, Maskatia S, Mathew R. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp Pediatr. 2020;10:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 86. | Moraleda C, Serna-Pascual M, Soriano-Arandes A, Simó S, Epalza C, Santos M, Grasa C, Rodríguez M, Soto B, Gallego N, Ruiz Y, Urretavizcaya-Martínez M, Pareja M, Sanz-Santaeufemia FJ, Fumadó V, Lanaspa M, Jordan I, Prieto L, Belda S, Toral-Vázquez B, Rincón E, Gil-Villanueva N, Méndez-Echevarría A, Castillo-Serrano A, Rivière JG, Soler-Palacín P, Rojo P, Tagarro A. Multi-Inflammatory Syndrome in Children related to SARS-CoV-2 in Spain. Clin Infect Dis. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 87. | Kuo HC. Kawasaki-like disease among Italian children in the COVID-19 era. J Pediatr. 2020;224:179-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6752] [Article Influence: 1350.4] [Reference Citation Analysis (0)] |
| 89. | Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, Rajagopal S, Pai AR, Kutty S. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 340] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 90. | Mangalmurti N, Hunter CA. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53:19-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 492] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 91. | Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity and Progression. Physiology (Bethesda). 2020;35:288-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 92. | Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, Acors S, Graham C, Timms E, Kenny J, Neil S, Malim MH, Tibby SM, Shankar-Hari M. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 93. | Lu X, Xiang Y, Du H, Wing-Kin Wong G. SARS-CoV-2 infection in children - Understanding the immune responses and controlling the pandemic. Pediatr Allergy Immunol. 2020;31:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 94. | Most ZM, Hendren N, Drazner MH, Perl TM. The Striking Similarities of Multisystem Inflammatory Syndrome in Children and a Myocarditis-like Syndrome in Adults: Overlapping Manifestations of COVID-19. Circulation. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 95. | Ashraf O, Young M, Malik KJ, Cheema T. Systemic Complications of COVID-19. Crit Care Nurs Q. 2020;43:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Sun B, Feng Y, Mo X, Zheng P, Wang Q, Li P, Peng P, Liu X, Chen Z, Huang H, Zhang F, Luo W, Niu X, Hu P, Wang L, Peng H, Huang Z, Feng L, Li F, Zhang F, Li F, Zhong N, Chen L. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9:940-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 97. | Ronconi G, Teté G, Kritas SK, Gallenga CE, Caraffa Al, Ross R, Conti P. SARS-CoV-2, which induces COVID-19, causes kawasaki-like disease in children: role of pro-inflammatory and anti-inflammatory cytokines. J Biol Regul Homeost Agents. 2020;34:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 98. | Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020; 181: 1036-1045. e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3510] [Cited by in RCA: 3169] [Article Influence: 633.8] [Reference Citation Analysis (0)] |
| 99. | Park A, Iwasaki A. Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020;27:870-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 678] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 100. | Rowley AH. Multisystem Inflammatory Syndrome in Children and Kawasaki Disease: Two Different Illnesses with Overlapping Clinical Features. J Pediatr. 2020;224:129-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 101. | Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 338] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 102. | McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E; American Heart Association Rheumatic Fever; Endocarditis; and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135:e927-e999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 2441] [Article Influence: 305.1] [Reference Citation Analysis (1)] |
| 103. | McCrindle BW, Manlhiot C. SARS-CoV-2-Related Inflammatory Multisystem Syndrome in Children: Different or Shared Etiology and Pathophysiology as Kawasaki Disease? JAMA. 2020;324:246-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 104. | Rodriguez-Gonzalez M, Perez-Reviriego AA, Castellano-Martinez A, Cascales-Poyatos HM. N-terminal probrain natriuretic peptide as biomarker for diagnosis of Kawasaki disease. Biomark Med. 2019;13:307-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Rodríguez-González M, Castellano-Martínez A, Alonso-Ojembarrena A. Usefulness of age-adjusted N-terminal prohormone of brain natriuretic peptide level as a diagnostic marker for incomplete Kawasaki disease in children. Emergencias. 2019;31:111-114. [PubMed] |
| 106. | Rodríguez-González M, Matamala-Morillo MA, Segado-Arenas A. Infliximab as rescue therapy in refractory Kawasaki disease. Ann Pediatr Cardiol. 2014;7:74-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 107. | Katneni UK, Alexaki A, Hunt RC, Schiller T, DiCuccio M, Buehler PW, Ibla JC, Kimchi-Sarfaty C. Coagulopathy and Thrombosis as a Result of Severe COVID-19 Infection: A Microvascular Focus. Thromb Haemost. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 108. | Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1635] [Cited by in RCA: 1597] [Article Influence: 319.4] [Reference Citation Analysis (1)] |
| 109. | Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1577] [Cited by in RCA: 1749] [Article Influence: 349.8] [Reference Citation Analysis (0)] |
| 110. | Liu Y, Gao W, Guo W, Guo Y, Shi M, Dong G, Ge Q, Zhu J, Lu J. Prominent coagulation disorder is closely related to inflammatory response and could be as a prognostic indicator for ICU patients with COVID-19. J Thromb Thrombolysis. 2020;50:825-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 111. | Berthelot JM, Drouet L, Lioté F. Kawasaki-like diseases and thrombotic coagulopathy in COVID-19: delayed over-activation of the STING pathway? Emerg Microbes Infect. 2020;9:1514-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 112. | Song JC, Wang G, Zhang W, Zhang Y, Li WQ, Zhou Z; People’s Liberation Army Professional Committee of Critical Care Medicine; Chinese Society on Thrombosis and Haemostasis. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res. 2020;7:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 113. | Loi M, Branchford B, Kim J, Self C, Nuss R. COVID-19 anticoagulation recommendations in children. Pediatr Blood Cancer. 2020;e28485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 114. | Rodriguez-Gonzalez M, Rodríguez-Campoy P, Sánchez-Códez M, Gutiérrez-Rosa I, Castellano-Martinez A, Rodríguez-Benítez A. New onset severe right ventricular failure associated with COVID-19 in a young infant without previous heart disease. Cardiol Young. 2020;30:1346-1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 115. | Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, Wang L, Chen Y, Liu W, Zhang K, Wu Y, Yang Z, Tao J, Feng J, Liu K, Ye X, Wang R, Zhang X, Zha Y. A 55-Day-Old Female Infant Infected With 2019 Novel Coronavirus Disease: Presenting With Pneumonia, Liver Injury, and Heart Damage. J Infect Dis. 2020;221:1775-1781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 116. | Del Barba P, Canarutto D, Sala E, Frontino G, Guarneri MP, Camesasca C, Baldoli C, Esposito A, Barera G. COVID-19 cardiac involvement in a 38-day old infant. Pediatr Pulmonol. 2020;55:1879-1881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Gnecchi M, Moretti F, Bassi EM, Leonardi S, Totaro R, Perotti L, Zuccaro V, Perlini S, Preda L, Baldanti F, Bruno R, Visconti LO. Myocarditis in a 16-year-old boy positive for SARS-CoV-2. Lancet. 2020;395:e116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 118. | Giacomet V, Manfredini VA, Meraviglia G, Peri CF, Sala A, Longoni E, Gasperetti A, Stracuzzi M, Mannarino S, Zuccotti GV. Acute Inflammation and Elevated Cardiac Markers in a Two-Month-Old Infant with Severe Acute Respiratory Syndrome Coronavirus 2 Infection Presenting with Cardiac Symptoms. Pediatr Infect Dis J. 2020;39:e149-e151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | Kesici S, Aykan HH, Orhan D, Bayrakci B. Fulminant COVID-19-related myocarditis in an infant. Eur Heart J. 2020;41:3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 120. | Samuel S, Friedman RA, Sharma C, Ganigara M, Mitchell E, Schleien C, Blaufox AD. Incidence of arrhythmias and electrocardiographic abnormalities in symptomatic pediatric patients with PCR-positive SARS-CoV-2 infection, including drug-induced changes in the corrected QT interval. Heart Rhythm. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 121. | Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020;55:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 683] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 122. | Craver R, Huber S, Sandomirsky M, McKenna D, Schieffelin J, Finger L. Fatal Eosinophilic Myocarditis in a Healthy 17-Year-Old Male with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2c). Fetal Pediatr Pathol. 2020;39:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 123. | Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, Liu ZS. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 124. | Su L, Ma X, Yu H, Zhang Z, Bian P, Han Y, Sun J, Liu Y, Yang C, Geng J, Zhang Z, Gai Z. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg Microbes Infect. 2020;9:707-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 125. | Rodriguez-Gonzalez M, Sanchez-Codez MI, Lubian-Gutierrez M, Castellano-Martinez A. Clinical presentation and early predictors for poor outcomes in pediatric myocarditis: A retrospective study. World J Clin Cases. 2019;7:548-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 126. | Tissières P, Teboul JL. SARS-CoV-2 post-infective myocarditis: the tip of COVID-19 immune complications? Ann Intensive Care. 2020;10:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 127. | Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT Jr, Chahal CAA. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 128. | Abdelnabi M, Eshak N, Saleh Y, Almaghraby A. Coronavirus Disease 2019 Myocarditis: Insights into Pathophysiology and Management. Eur Cardiol. 2020;15:e51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 129. | Wei X, Fang Y, Hu H. Immune-mediated mechanism in coronavirus fulminant myocarditis. Eur Heart J. 2020;41:1855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 130. | Lara D, Young T, Del Toro K, Chan V, Ianiro C, Hunt K, Kleinmahon J. Acute Fulminant Myocarditis in a Pediatric Patient With COVID-19 Infection. Pediatrics. 2020;146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 131. | Garot J, Amour J, Pezel T, Dermoch F, Messadaa K, Felten ML, Raymond V, Baubillier E, Sanguineti F, Garot P. SARS-CoV-2 Fulminant Myocarditis. JACC Case Rep. 2020;2:1342-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 132. | Pagnesi M, Baldetti L, Beneduce A, Calvo F, Gramegna M, Pazzanese V, Ingallina G, Napolano A, Finazzi R, Ruggeri A, Ajello S, Melisurgo G, Camici PG, Scarpellini P, Tresoldi M, Landoni G, Ciceri F, Scandroglio AM, Agricola E, Cappelletti AM. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106:1324-1331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 133. | Rodriguez-Gonzalez M, Benavente-Fernandez I, Castellano-Martinez A, Lechuga-Sancho AM, Lubian-Lopez SP. NT-proBNP plasma levels as biomarkers for pulmonary hypertension in healthy infants with respiratory syncytial virus infection. Biomark Med. 2019;13:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 134. | Kimura D, McNamara IF, Wang J, Fowke JH, West AN, Philip R. Pulmonary hypertension during respiratory syncytial virus bronchiolitis: a risk factor for severity of illness. Cardiol Young. 2019;29:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 135. | Rodriguez-Gonzalez M, Perez-Reviriego AA, Castellano-Martinez A, Lubian-Lopez S, Benavente-Fernandez I. Left Ventricular Dysfunction and Plasmatic NT-proBNP Are Associated with Adverse Evolution in Respiratory Syncytial Virus Bronchiolitis. Diagnostics (Basel). 2019;9:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Zhou H, Zhang G, Deng X, Jin B, Qiu Q, Yan M, Wang X, Zheng X. Understanding the current status of patients with pulmonary hypertension during COVID-19 outbreak: a small-scale national survey from China. Pulm Circ. 2020;10:2045894020924566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 137. | Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S; Lille ICU Haemostasis COVID-19 Group. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation. 2020;142:184-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 872] [Article Influence: 174.4] [Reference Citation Analysis (0)] |
| 138. | Faggiano P, Bonelli A, Paris S, Milesi G, Bisegna S, Bernardi N, Curnis A, Agricola E, Maroldi R. Acute pulmonary embolism in COVID-19 disease: Preliminary report on seven patients. Int J Cardiol. 2020;313:129-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 139. | Ribes A, Vardon-Bounes F, Mémier V, Poette M, Au-Duong J, Garcia C, Minville V, Sié P, Bura-Rivière A, Voisin S, Payrastre B. Thromboembolic events and Covid-19. Adv Biol Regul. 2020;77:100735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 140. | Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 840] [Article Influence: 168.0] [Reference Citation Analysis (0)] |
| 141. | Blumfield E, Levin TL, Kurian J, Lee EY, Liszewski MC. AJR Am J Roentgenol. 2020;Online ahead of print Imaging Findings in Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 142. | Winant AJ, Blumfield E, Radiology ML, 2020. Thoracic Imaging Findings of Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19: What Radiologists Need to Know Now. Radiology: Cardiothoracic Imaging. 2020;2:e200346. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 143. | Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4313] [Cited by in RCA: 4066] [Article Influence: 813.2] [Reference Citation Analysis (0)] |
| 144. | Romand JA, Donald FA, Suter PM. Cardiopulmonary interactions in acute lung injury: clinical and prognostic importance of pulmonary hypertension. New Horiz. 1994;2:457-462. [PubMed] |
| 145. | Pinsky MR. Cardiopulmonary Interactions: Physiologic Basis and Clinical Applications. Ann Am Thorac Soc. 2018;15:S45-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 146. | Serwer G. Ventricular arrhythmia in children: diagnosis and management. Curr Treat Options Cardiovasc Med. 2008;10:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 147. | Chorin E, Wadhwani L, Magnani S, Dai M, Shulman E, Nadeau-Routhier C, Knotts R, Bar-Cohen R, Kogan E, Barbhaiya C, Aizer A, Holmes D, Bernstein S, Spinelli M, Park DS, Stefano C, Chinitz LA, Jankelson L. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 148. | Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1036-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 487] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 149. | Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, Cingolani E, Cheng S, Marban E, Albert CM, Chugh SS. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring. J Am Heart Assoc. 2020;9:e017144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 150. | Tuncer T, Karaci M, Boga A, Durmaz H, Guven S. QT interval evaluation associated with the use of hydroxychloroquine with combined use of azithromycin among hospitalised children positive for coronavirus disease 2019. Cardiol Young. 2020;30:1482-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 151. | Lazzerini PE, Acampa M, Laghi-Pasini F, Bertolozzi I, Finizola F, Vanni F, Natale M, Bisogno S, Cevenini G, Cartocci A, Giabbani B, Migliacci N, D'Errico A, Dokollari A, Maccherini M, Boutjdir M, Capecchi PL. Cardiac Arrest Risk During Acute Infections: Systemic Inflammation Directly Prolongs QTc Interval via Cytokine-Mediated Effects on Potassium Channel Expression. Circ Arrhythm Electrophysiol. 2020;13:e008627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 152. | Lazzerini PE, Laghi-Pasini F, Bertolozzi I, Morozzi G, Lorenzini S, Simpatico A, Selvi E, Bacarelli MR, Finizola F, Vanni F, Lazaro D, Aromolaran A, El Sherif N, Boutjdir M, Capecchi PL. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart. 2017;103:1821-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 153. | Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 358] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 154. | Dolbec K, Mick NW. Congenital heart disease. Emerg Med Clin North Am. 2011;29:811-827, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 155. | Simpson M, Collins C, Nash DB, Panesar LE, Oster ME. Coronavirus Disease 2019 Infection in Children with Pre-Existing Heart Disease. J Pediatr. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 156. | Linnane N, Cox DW, James A. A case of COVID-19 in a patient with a univentricular heart post total cavopulmonary connection (Fontan) surgery. Cardiol Young. 2020;30:1350-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 157. | Krishnan US, Krishnan SS, Jain S, Chavolla-Calderon MB, Lewis M, Chung WK, Rosenzweig EB. SARS-CoV-2 Infection in Patients with Down Syndrome, Congenital Heart Disease, and Pulmonary Hypertension: Is Down Syndrome a Risk Factor? J Pediatr. 2020;225:246-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 158. | Olfe J, Grafmann M, Kozlik-Feldmann R. A teenager with CHD and coronavirus disease 2019. Cardiol Young. 2020;30:1358-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 159. | Salik I, Mehta B. Tetralogy of Fallot palliation in a COVID-19 positive neonate. J Clin Anesth. 2020;66:109914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 160. | Russell MR, Halnon NJ, Alejos JC, Salem MM, Reardon LC. COVID-19 in a pediatric heart transplant recipient: Emergence of donor-specific antibodies. J Heart Lung Transplant. 2020;39:732-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 161. | Zheng F, Liao C, Fan QH, Chen HB, Zhao XG, Xie ZG, Li XL, Chen CX, Lu XX, Liu ZS, Lu W, Chen CB, Jiao R, Zhang AM, Wang JT, Ding XW, Zeng YG, Cheng LP, Huang QF, Wu J, Luo XC, Wang ZJ, Zhong YY, Bai Y, Wu XY, Jin RM. Clinical Characteristics of Children with Coronavirus Disease 2019 in Hubei, China. Curr Med Sci. 2020;40:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 162. | Climent FJ, Calvo C, García-Guereta L, Rodríguez-Álvarez D, Buitrago NM, Pérez-Martínez A. [Fatal outcome of COVID-19 disease in a 5-month infant with comorbidities]. Rev Esp Cardiol. 2020;73:667-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 163. | Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 653] [Cited by in RCA: 886] [Article Influence: 177.2] [Reference Citation Analysis (0)] |
| 164. | Checchia PA, Paes B, Bont L, Manzoni P, Simões EA, Fauroux B, Figueras-Aloy J, Carbonell-Estrany X. Defining the Risk and Associated Morbidity and Mortality of Severe Respiratory Syncytial Virus Infection Among Infants with Congenital Heart Disease. Infect Dis Ther. 2017;6:37-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 165. | von der Beck D, Seeger W, Herold S, Günther A, Löh B. Characteristics and outcomes of a cohort hospitalized for pandemic and seasonal influenza in Germany based on nationwide inpatient data. PLoS One. 2017;12:e0180920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 166. | Rodriguez-Gonzalez M, Castellano-Martinez A, Perez-Reviriego AA. Atypical Presentation of Incomplete Kawasaki Disease: A Peripheral Facial Nerve Palsy. J Emerg Med. 2018;55:118-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 167. | Hoang A, Chorath K, Moreira A, Evans M, Burmeister-Morton F, Burmeister F, Naqvi R, Petershack M, Moreira A. COVID-19 in 7780 pediatric patients: A systematic review. EClinicalMedicine. 2020;24:100433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 168. | Sabatino J, Ferrero P, Chessa M, Bianco F, Ciliberti P, Secinaro A, Oreto L, Avesani M, Bucciarelli V, Calcaterra G, Calabrò MP, Russo MG, Bassareo PP, Guccione P, Indolfi C, Di Salvo G. COVID-19 and Congenital Heart Disease: Results from a Nationwide Survey. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 169. | Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e637-e697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 170. | Stephens EH, Dearani JA, Guleserian KJ, Overman DM, Tweddell JS, Backer CL, Romano JC, Bacha E. COVID-19: Crisis management in congenital heart surgery. J Thorac Cardiovasc Surg. 2020;160:522-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 171. | Barker PCA, Lewin MB, Donofrio MT, Altman CA, Ensing GJ, Arya B, Swaminathan M. Specific Considerations for Pediatric, Fetal, and Congenital Heart Disease Patients and Echocardiography Service Providers during the 2019 Novel Coronavirus Outbreak: Council on Pediatric and Congenital Heart Disease Supplement to the Statement of the American Society of Echocardiography: Endorsed by the Society of Pediatric Echocardiography and the Fetal Heart Society. J Am Soc Echocardiogr. 2020;33:658-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 172. | Dearani JA, Stephens EH, Guleserian KJ, Overman DM, Backer CL, Romano JC, Louis JDS, Sarris GE, Bacha E, Tweddell JS. COVID-19: FAQs-Congenital Heart Surgery Recovery and Defining a "New Normal". World J Pediatr Congenit Heart Surg. 2020;11:548-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 173. | Stephens EH, Dearani JA, Guleserian KJ, Overman DM, Tweddell JS, Backer CL, Romano JC, Bacha E. COVID-19: Crisis Management in Congenital Heart Surgery. World J Pediatr Congenit Heart Surg. 2020;11:395-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 174. | Wasson JH. Practice Standards for Effective Telemedicine in Chronic Care Management After COVID-19. J Ambul Care Manage. 2020;43:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 175. | Świerad M, Dyrbuś K, Szkodziński J, Zembala MO, Kalarus Z, Gąsior M. Telehealth visits in a tertiary cardiovascular center as a response of the healthcare system to the severe acute respiratory syndrome coronavirus 2 pandemic in Poland. Pol Arch Intern Med. 2020;130:700-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 176. | Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP; Heart Rhythm Society (HRS); European Heart Rhythm Association (EHRA). HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace. 2011;13:1077-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 177. | Chang D, Saleh M, Garcia-Bengo Y, Choi E, Epstein L, Willner J. COVID-19 Infection Unmasking Brugada Syndrome. HeartRhythm Case Rep. 2020;6:237-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 178. | Drożdżal S, Rosik J, Lechowicz K, Machaj F, Kotfis K, Ghavami S, Łos MJ. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updat. 2020;53:100719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 179. | Ohadian Moghadam S. A Review on Currently Available Potential Therapeutic Options for COVID-19. Int J Gen Med. 2020;13:443-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 180. | Salvi R, Patankar P. Emerging pharmacotherapies for COVID-19. Biomed Pharmacother. 2020;128:110267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 181. | Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, Yarbrough A, Abzug MJ, MacBrayne CE, Soma VL, Dulek DE, Vora SB, Waghmare A, Wolf J, Olivero R, Grapentine S, Wattier RL, Bio L, Cross SJ, Dillman NO, Downes KJ, Timberlake K, Young J, Orscheln RC, Tamma PD, Schwenk HT, Zachariah P, Aldrich M, Goldman DL, Groves HE, Lamb GS, Tribble AC, Hersh AL, Thorell EA, Denison MR, Ratner AJ, Newland JG, Nakamura MM. Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 182. | Gray B, Semsarian C, Fatkin D, Ingles J, Atherton JJ, Davis AM, Sanders P, Pachter N, Skinner JR, Stiles MK; CSANZ Cardiovascular Genetic Diseases Council. Patients With Genetic Heart Disease and COVID-19: A Cardiac Society of Australia and New Zealand (CSANZ) Consensus Statement. Heart Lung Circ. 2020;29:e85-e87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 183. | Kumar S, Haqqani H, Wynn G, Pathak RK, Lipton J, Mahajan R, Sanders P, Healey S, Wilsmore B, Mariani JA, Thomas SP, Weerasooriya R, McGavigan A, Gould PA, Weatherley P, Saad N, Cowan M, Turnbull S, Trivic I, Wong M, Tonchev I, Morton JB, Skinner JR, Pflaumer A, McGuire M, Kistler P, Kalman JM; Cardiac Society of Australia and New Zealand (CSANZ) Heart Rhythm Council COVID-19 Pandemic Working Group. Position Statement on the Management of Cardiac Electrophysiology and Cardiac Implantable Electronic Devices in Australia During the COVID-19 Pandemic: A Living Document. Heart Lung Circ. 2020;29:e57-e68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 184. | Schwier NC, Coons JC, Rao SK. Pharmacotherapy update of acute idiopathic pericarditis. Pharmacotherapy. 2015;35:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 185. | Bautista-Hernandez V, Sanchez-Andres A, Portela F, Fynn-Thompson F. Current pharmacologic management of pediatric heart failure in congenital heart disease. Curr Vasc Pharmacol. 2011;9:619-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 186. | Ortmans S, Daval C, Aguilar M, Compagno P, Cadrin-Tourigny J, Dyrda K, Rivard L, Tadros R. Pharmacotherapy in inherited and acquired ventricular arrhythmia in structurally normal adult hearts. Expert Opin Pharmacother. 2019;20:2101-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 187. | Leusveld EM, Kauling RM, Geenen LW, Roos-Hesselink JW. Heart failure in congenital heart disease: management options and clinical challenges. Expert Rev Cardiovasc Ther. 2020;18:503-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 188. | Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is There an Association Between COVID-19 Mortality and the Renin-Angiotensin System? Clin Infect Dis. 2020;71:870-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 189. | Miao H, Li H, Yao Y, Wu M, Lu C, Wang J, Tian M, Li Y, Luo P, Gu J, Yuan B, Wang S, Zhao X, Gan W, Zhao D. Update on recommendations for the diagnosis and treatment of SARS-CoV-2 infection in children. Eur J Clin Microbiol Infect Dis. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 190. | Marraro GA, Spada C. Consideration of the respiratory support strategy of severe acute respiratory failure caused by SARS-CoV-2 infection in children. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 191. | Atal S, Fatima Z. IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy? Pharmaceut Med. 2020;34:223-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 192. | Singh AP, Tousif S, Umbarkar P, Lal H. A Pharmacovigilance Study of Hydroxychloroquine Cardiac Safety Profile: Potential Implication in COVID-19 Mitigation. J Clin Med. 2020;9:1867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 193. | Offerhaus JA, Wilde AAM, Remme CA. Prophylactic (hydroxy)chloroquine in COVID-19: Potential relevance for cardiac arrhythmia risk. Heart Rhythm. 2020;17:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 194. | Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent Guidance for Navigating and Circumventing the QTc-Prolonging and Torsadogenic Potential of Possible Pharmacotherapies for Coronavirus Disease 19 (COVID-19). Mayo Clin Proc. 2020;95:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 294] [Article Influence: 58.8] [Reference Citation Analysis (0)] |