Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.398
Peer-review started: October 29, 2019
First decision: November 22, 2019
Revised: December 16, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: January 26, 2020
Processing time: 79 Days and 16.3 Hours
Solid pseudopapillary tumor (SPT) of the pancreas is a rare pancreatic tumor and 10% to 15% of cases are associated with metastasis. Cryoablation is a new method that can induce tumor necrosis, and treatment of tumors by cryoablation can cause anti-tumor immune responses.
A 16-year-old woman with SPT of the pancreas developed liver metastases 5.3 years after complete resection of the primary pancreatic tumor. She was admitted with chief complaints of abdominal pain in the upper abdomen and a weight loss of approximately 5 kg over 4 mo. Carbohydrate antigen (CA) 125, carcinoembryonic antigen, and CA 199 were normal. An abdominal computed tomography scan found multiple nodules in the right lobe of the liver that measured approximately 13.5 cm × 10.8 cm × 21.4 cm. Immunohistochemical staining results showed that CD10 and CD56 were positive, and the patient was diagnosed with SPT of the pancreas with liver metastasis. The patient underwent percutaneous cryoablation and interventional embolization. During the 5-year follow-up, the patient remained disease-free after cryoablation, with relatively normal immune function.
Herein, we for the first time report the treatment of liver metastasis from SPT of the pancreas using cryoablation plus interventional embolization, which could be a promising alternative therapy for pancreatic SPT liver metastasis.
Core tip: In this study, we report the case of a 16-year-old female patient with solid pseudopapillary tumor (SPT) of the pancreas with liver metastasis who obtained a favorable outcome after combined cryoablation and transcatheter arterial embolization treatment. Our study showed that SPT liver metastasis is rare and occurs at various time points after diagnosis. Cryoablation with transcatheter arterial embolization could be utilized as an alternative therapy for pancreatic SPT liver metastasis.
- Citation: Ma YY, Chen JB, Shi JJ, Niu LZ, Xu KC. Cryoablation for liver metastasis from solid pseudopapillary tumor of the pancreas: A case report. World J Clin Cases 2020; 8(2): 398-403
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/398.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.398
Solid pseudopapillary tumor (SPT) of the pancreas is a rare tumor type with low malignant potential. Its incidence accounts for 0.3%-3% of all pancreatic tumors, accounting for 3%-15% of pancreatic cystic tumors; the ratio of male to female is about 1:10[1]. Although SPT shows low malignant potential, 10%-15% of tumors show aggressive behavior, with metastatic involvement of the liver. SPT was first reported by Frantz in 1959, and the number of reported cases has increased[2]. Domestic and foreign scholars have used a variety of different diagnostic names according to their characteristic solid, cystic, and pseudo-papillary structures, such as solid papillary epithelioma, solid cystic tumor, papillary cystic tumor, solid cystic papillary acinar cell tumor, and Frantz tumor[3-5]. The World Health Organization redefined SPT as a low-grade malignant tumor in 2010[6]. Surgery is the preferred treatment for SPT, and most tumors are completely resected and the prognosis is good[7].
Cryoablation is based on the Joule-Thomson principle that uses argon as a refrigerant to rapidly cool down, helium as a heat medium for rapid temperature rise, biosensing, timely monitoring, and other aerospace technology patents[8]. Compared with traditional surgery and other ablation techniques, cryoblation has been accepted by doctors and patients because of its little trauma, visualization of an ice ball, less pain, mild damage to large blood vessels, activation of the immune function in the body, and many other advantages, such as high safety and good curative effect. In tumors of the pancreas, liver, kidney, and other organs, encouraging results have been achieved[8-10].
Transcatheter arterial embolization (TAE) is a technique in which an embolic agent is injected or delivered into a target vessel to occlude the vessel for the intended therapeutic purpose. TAE has become an accepted treatment option for patients[11,12]. The human liver receives double blood supply from the hepatic artery and the portal vein, and when one of the two experiences blood flow obstruction, the blood flow through the other can maintain the normal function of the liver. TAE can reduce tumor blood supply or block tumor blood supply and maintain unaffected normal liver tissue.
Herein, we report the case of a 16-year-old female patient with liver metastasis from SPT of the pancreas who obtained a favorable outcome after combined cryoablation and TAE treatment.
A 21-year-old female patient presented on January 1, 2014 with complaints of persistent abdominal distension and abdominal pain in the upper abdomen, accompanied by edema of the lower extremities. The above symptoms were progressively aggravated with a weight loss of approximately 5 kg over 4 mo (May 2014), and the patient was admitted to our hospital.
Approximately five years ago, the 16-year-old female patient was admitted to a local hospital on March 22, 2009. The patient inadvertently touched a mass in the left middle and upper abdomen. A computed tomography (CT) scan of the abdomen showed a large mass in the patient’s left middle and upper abdominal cavity, and a surgical resection was performed. Pathological results showed SPT of the pancreas. Subsequent radiological studies showed no residual lesions in the pancreas.
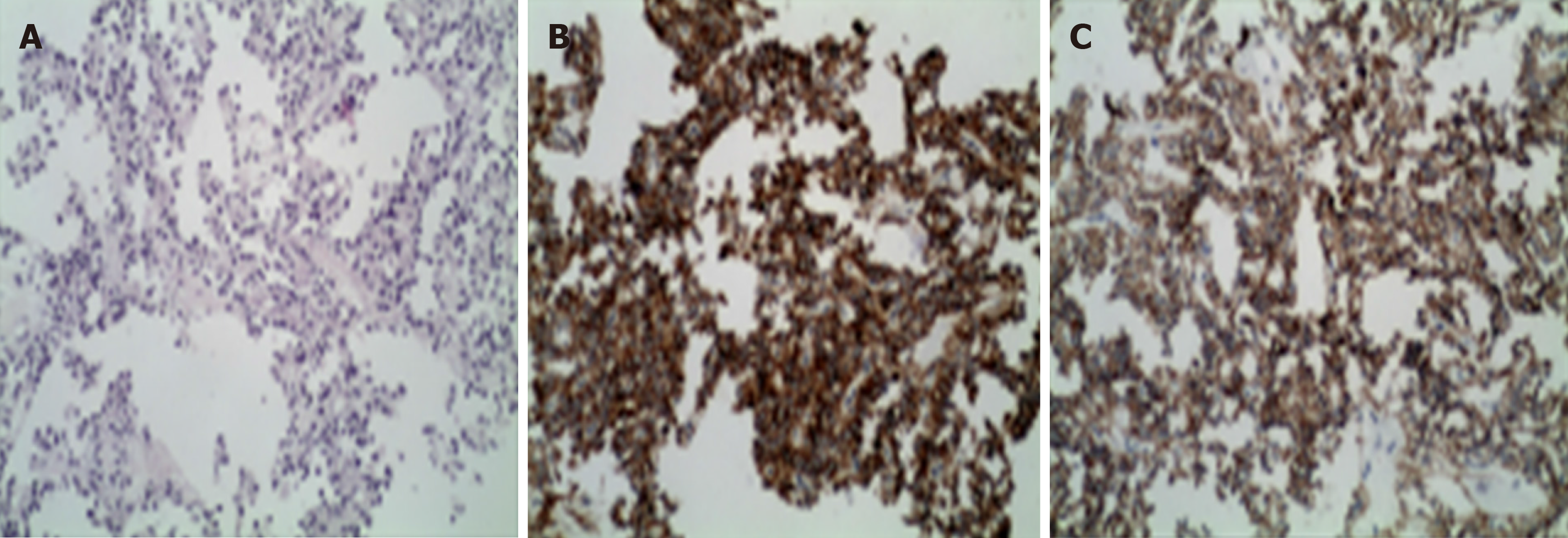
The levels of carbohydrate antigen (CA) 125, carcinoembryonic antigen (CEA), and CA 199 were normal. Percutaneous liver tumor biopsies were performed using ultrasound-guided biopsy needles. Immunohistochemical staining results showed that CD10 and CD56 were positive, and the patient was diagnosed with SPT of the pancreas with liver metastasis (Figure 1).
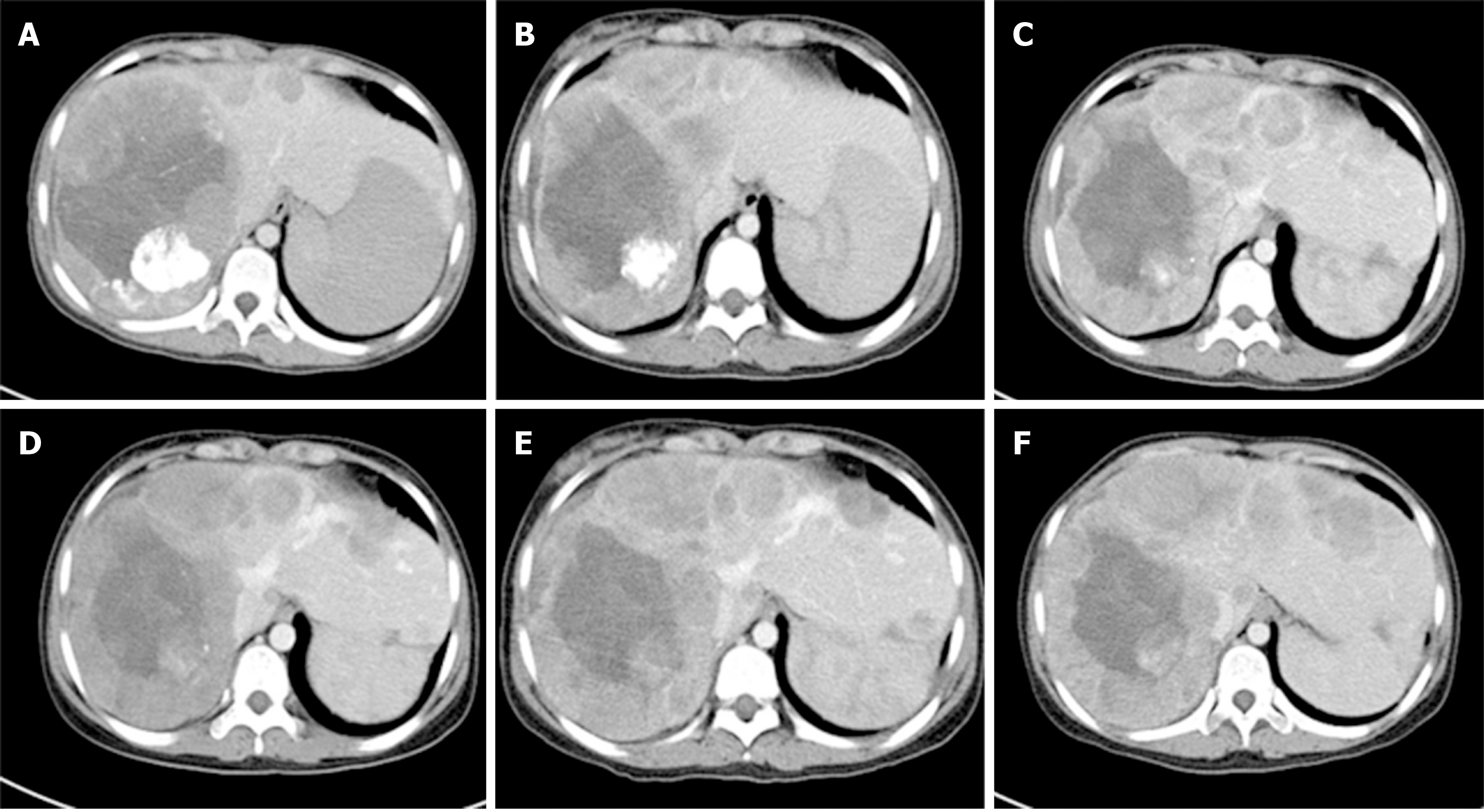
An abdominal CT scan found multiple nodules in the right lobe of the liver that measured approximately 13.5 cm × 10.8 cm × 21.4 cm (Figure 2A).
The patient was diagnosed with SPT of the pancreas with liver metastasis.
Surgery is the first choice for SPT of the pancreas with liver metastases. However, after informed consent, the patient refused surgery due to the risk of surgery and the possibility of incompletely resected tumors. As a less invasive and alternative technique, cryoablation was accepted by the patient and informed consent was obtained for cryoablation treatment.
After the diagnosis of liver metastasis from SPT of the pancreas, the patient underwent TAE of the liver metastases. A total of 7.5 mL of super-liquefied lipiodol and gelatin sponge particles in ten capsules were administered five times in approximately two years. Simultaneously, liver metastasis was treated by cryoablation under general anesthesia on August 21 and December 1, 2014. The patient showed a reduced platelet count and activated partial thromboplastin time, and a prolonged thrombin time post-operation. Fresh frozen plasma infusion was given to correct recurrence of coagulation abnormalities.
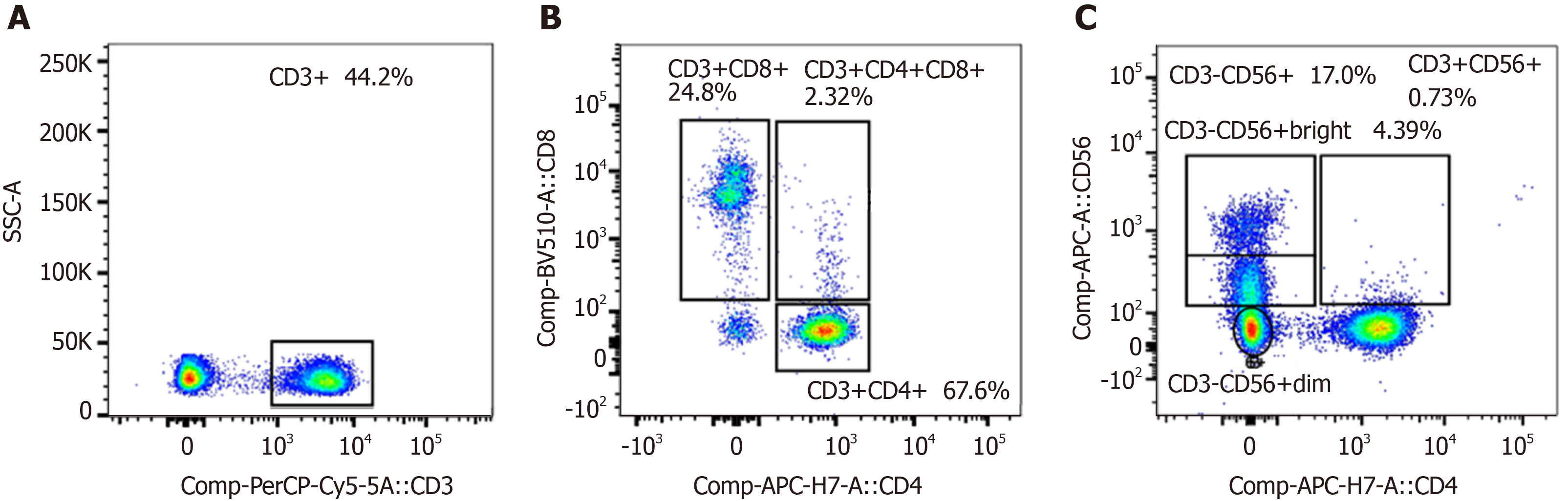
After treatment, serum tumor markers CA199, CEA, and CA125 were within the normal range. During follow-up, abdominal CT scans were performed every 12 mo. The patient remained disease-free 5.1 years after cryoablation (i.e. 10.4 years after removal of the primary pancreatic tumor) (Figure 2). As shown in Figure 3, we analyzed the patient’s peripheral blood lymphocyte immune function on August 12, 2019. The results revealed that the percentages of CD3+, CD4+, and CD8+ T lymphocytes were 44.2%, 67.6%, and 24.8%, respectively (Figure 3A and B). Natural killer cell function analysis showed a 17% percentage of CD56+ T lymphocytes (Figure 3C).
Herein, we report the case of a 16-year-old female patient with SPT of the pancreas who underwent surgery. After 5.3 years, the patient was diagnosed with liver metastasis and received TAE and cryoablation shortly after detection of the liver metastasis. The patient showed a prolonged survival and good health 10.4 years after the initial diagnosis, with normal immune function.
Solid pseudopapilloma is a rare pancreatic inert tumor that accounts for 0.9%-2.7% of all exocrine pancreatic tumors[13,14]. However, previous studies have shown that the incidence of SPT is increasing, and that it usually occurs in young women in the second or third decade of life, as seen in the present study[5]. It is difficult for patients to find SPT. Most patients have normal CA 125, CEA, and CA 199 levels. In the present case, CA 125, CEA, and CA 199 did not increase before and after liver metastasis, suggesting that laboratory examination was not meaningful for this patient.
SPT is usually considered a low malignant tumor and has a good overall prognosis. Therefore, surgical resection has been considered an option for treatment[15,16]. The specific procedure depends on the location and size of the tumor and whether there is adjacent invasion or distant metastasis. Most tumors are completely resected and the prognosis is good[7]. Owing to the rarity and generally indolent biology of SPT of the pancreas, optimal management of liver metastasis is not well defined. Approximately 6% of all SPTs are reported to invade surrounding organs and about 10%-15% develop distant metastases[17]. The most common metastatic organ is the liver; tumor cells may metastasize to the liver through the superior mesenteric vein and portal vein. Law et al[18] analyzed data from 2744 SPT patients who underwent surgery from 1961 to 2012 and found that 4.4% (86 patients) had recurrence, with 50.5 mo as the median time to recurrence. Our case presented with liver metastasis five years after surgical resection. Currently, the longest time to liver metastasis is 15.8 years after surgical resection[19]. The World Health Organization defines malignant SPT as tumors with surrounding tissue invasion, perineural invasion, vascular invasion on microscopic pathology, and metastasis[20].
Systemic chemotherapy with gemcitabine and cisplatin or 5-fluorouracil for the treatment of SPT remains controversial, and there has been no obvious response to radiation therapy[21]. Compared with traditional surgery, cryoablation is less traumatic, with slight adverse reactions, short recovery time, and short hospital stay. Ravindranath et al[22] have shown that anti-tumor-related ganglioside antibodies are significantly increased in patients with metastatic liver cancer treated with argon-helium cryoablation compared with surgical treatment and radiofrequency ablation. Therefore, argon-helium cryoablation results in the release of this antibody into the blood. In theory, it can effectively induce a tumor-specific immune response. Radiofrequency ablation causes cell membrane melting and protein denaturation due to high temperature and no tumor antigen is released into the blood. Therefore, it is impossible to stimulate immune enhancement using radiofrequency ablation, which could instead increase the risk of complications. During the 5-year follow-up, the patient remained disease-free after cryoablation, with relatively normal immune function.
In conclusion, SPT liver metastasis is rare and occurs at various time points after diagnosis. Cryoablation with TAE could be utilized as an alternative therapy for pancreatic SPT liver metastasis.
The authors thank the patient for participating in the study and for agreeing to the publication of this report.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Damaskos C S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 2. | Lin MY, Stabile BE. Solid pseudopapillary neoplasm of the pancreas: a rare and atypically aggressive disease among male patients. Am Surg. 2010;76:1075-1078. [PubMed] |
| 3. | Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Soudack M, Ben-Nun A, Malkin L, Hashmonai M. [Solid and papillary pancreatic neoplasm]. Harefuah. 2000;138:105-107, 174. [PubMed] |
| 5. | Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: a typically cystic carcinoma of low malignant potential. Semin Diagn Pathol. 2000;17:66-80. [PubMed] |
| 6. | Ugras N, Yerci Ö, Coşkun SK, Ocakoğlu G, Sarkut P, Dündar HZ. Retrospective analysis of clinicopathological features of solid pseudopapillary neoplasm of the pancreas. Kaohsiung J Med Sci. 2016;32:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Campanile M, Nicolas A, LeBel S, Delarue A, Guys JM, de Lagausie P. Frantz's tumor: is mutilating surgery always justified in young patients? Surg Oncol. 2011;20:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Wu S, Hou J, Ding Y, Wu F, Hu Y, Jiang Q, Mao P, Yang Y. Cryoablation Versus Radiofrequency Ablation for Hepatic Malignancies: A Systematic Review and Literature-Based Analysis. Medicine (Baltimore). 2015;94:e2252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Kim GM, Won JY, Kim MD, Park SI, Lee do Y, Shin W, Shin M, Han KH, Kim do Y, Kim SU. Cryoablation of Hepatocellular Carcinoma with High-Risk for Percutaneous Ablation: Safety and Efficacy. Cardiovasc Intervent Radiol. 2016;39:1447-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Gunn AJ, Joe WB, Salei A, El Khudari H, Mahmoud KH, Bready E, Keasler EM, Patten PP, Gordetsky JB, Rais-Bahrami S, Abdel Aal AK. Percutaneous Cryoablation of Stage T1b Renal Cell Carcinoma: Safety, Technical Results, and Clinical Outcomes. Cardiovasc Intervent Radiol. 2019;42:970-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Hoshino J, Ubara Y, Suwabe T, Sumida K, Hayami N, Mise K, Hiramatsu R, Hasegawa E, Yamanouchi M, Sawa N, Takei R, Takaichi K. Intravascular embolization therapy in patients with enlarged polycystic liver. Am J Kidney Dis. 2014;63:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Hoshino J, Suwabe T, Hayami N, Sumida K, Mise K, Kawada M, Imafuku A, Hiramatsu R, Yamanouchi M, Hasegawa E, Sawa N, Takei R, Takaichi K, Ubara Y. Survival after arterial embolization therapy in patients with polycystic kidney and liver disease. J Nephrol. 2015;28:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 14. | Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. 2006;7:131-136. [PubMed] |
| 15. | Reddy S, Cameron JL, Scudiere J, Hruban RH, Fishman EK, Ahuja N, Pawlik TM, Edil BH, Schulick RD, Wolfgang CL. Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. J Am Coll Surg. 2009;208:950-7; discussion 957-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Cai Y, Ran X, Xie S, Wang X, Peng B, Mai G, Liu X. Surgical management and long-term follow-up of solid pseudopapillary tumor of pancreas: a large series from a single institution. J Gastrointest Surg. 2014;18:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Chung YE, Kim MJ, Choi JY, Lim JS, Hong HS, Kim YC, Cho HJ, Kim KA, Choi SY. Differentiation of benign and malignant solid pseudopapillary neoplasms of the pancreas. J Comput Assist Tomogr. 2009;33:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, Dal Molin M, Moran RA, Khashab MA, Ahuja N, Goggins M, Hruban RH, Wolfgang CL, Lennon AM. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 19. | Gomez P, Yorke R, Ayala AG, Ro JY. Solid-pseudopapillary neoplasm of pancreas with long delayed liver metastasis. Ann Diagn Pathol. 2012;16:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Liszka L, Mrowiec S, Pająk J, Kostrząb-Zdebel A, Lampe P, Kajor M. Limited usefulness of histopathological features in identification of a clinically aggressive solid-pseudopapillary neoplasm of the pancreas. Pol J Pathol. 2014;65:182-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Matsunou H, Konishi F. Papillary-cystic neoplasm of the pancreas. A clinicopathologic study concerning the tumor aging and malignancy of nine cases. Cancer. 1990;65:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Ravindranath MH, Wood TF, Soh D, Gonzales A, Muthugounder S, Perez C, Morton DL, Bilchik AJ. Cryosurgical ablation of liver tumors in colon cancer patients increases the serum total ganglioside level and then selectively augments antiganglioside IgM. Cryobiology. 2002;45:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |