Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.325
Peer-review started: October 31, 2019
First decision: November 19, 2019
Revised: December 9, 2019
Accepted: December 13, 2019
Article in press: December 13, 2019
Published online: January 26, 2020
Processing time: 78 Days and 1.1 Hours
False tendon is a common intraventricular anatomical variation. It refers to a fibroid or fibromuscular structure that exists in the ventricle besides the normal connection of papillary muscle and mitral or tricuspid valve. A large number of clinical studies have suggested that there is a significant correlation between false tendons and premature ventricular complexes. However, few studies have verified this correlation during radiofrequency catheter ablation of premature ventricular complexes.
A 45-year-old male was admitted to receive radiofrequency ablation for symptomatic premature ventricular complexes. A three-dimensional model of the left ventricle was established by intracardiac echocardiography using the CartoSoundTM mapping system. In addition to the left anterior papillary muscle, the posterior papillary muscle was mapped. False tendons were found at the base of the interventricular septum, and the other end was connected to the left ventricular free wall near the apex. An irrigated touch force catheter was advanced into the left ventricle via the retrograde approach. The earliest activation site was marked at the interventricular septum attachment of the false tendons and was successfully ablated.
This case verified that false tendons can cause premature ventricular complexes and may be cured by radiofrequency ablation guided by intracardiac echocardiography with the CartoSoundTM system.
Core tip: We report a case of successful radiofrequency catheter ablation of premature ventricular complexes associated with left ventricular false tendons. Intracardiac echocardiography with the CartoSoundTM system was used to demonstrate, for the first time, that the occurrence of ventricular premature complexes was associated with false tendons. No classical Purkinje potential and special potential were observed following interventricular septum attachment of false tendons, the local potential of the target region in this patient, and the ectopic excitability resulting in mechanical traction at the false tendon attachment site of the interventricular septum may be a possible mechanism for these premature ventricular complexes.
- Citation: Yang YB, Li XF, Guo TT, Jia YH, Liu J, Tang M, Fang PH, Zhang S. Catheter ablation of premature ventricular complexes associated with false tendons: A case report. World J Clin Cases 2020; 8(2): 325-330
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/325.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.325
A false tendon is a common intraventricular anatomical variation[1]. It refers to a fibroid or fibromuscular structure that exists in the ventricle besides the normal connection of the papillary muscle and mitral or tricuspid valve. It can be divided into a left ventricular false tendon and right ventricular false tendon. A large number of clinical studies have suggested that there is a significant correlation between false tendons and premature ventricular complexes (PVCs)[1,2]. However, few studies have verified this correlation during radiofrequency catheter ablation of PVCs.
We report a case of successful radiofrequency catheter ablation of PVCs associated with left ventricular false tendons. Intracardiac echocardiography (ICE) with the CartoSoundTM system was used to demonstrate, for the first time, that the occurrence of PVCs was associated with false tendons. No classical Purkinje potential and special potential were observed in the local potential of the target region of the patient, and the ectopic excitability resulting in mechanical traction at the false tendon attachment site of the interventricular septum may be a possible mechanism for these PVC.
This case verified that false tendons can cause premature ventricular beats and may be cured by radiofrequency catheter ablation guided by ICE with the CartoSoundTM system.
A 45-year-old male was admitted to hospital due to “2-mo of intermittent palpitations and shortness of breath”.
Chest pain, convulsion, and nausea were not observed. Occasional vomiting was relieved after a few minutes to several hours of rest, and aggravated under emotional stress and after meals.
The patient denied any previous history of hypertension, coronary heart disease, diabetes, surgical trauma and allergies. No smoking and drinking history, no infectious disease history and no hereditary family history were noted.
On admission, blood pressure was 110/70 mmHg, heart rate was 70 beats/min, premature contraction could be heard, no obvious heart murmur or abnormal heart sound, and no leg swelling were observed. There were no other positive signs.
No obvious abnormalities were found during routine blood, blood biochemistry, coagulation function, thyroid function, and infectious disease screening.
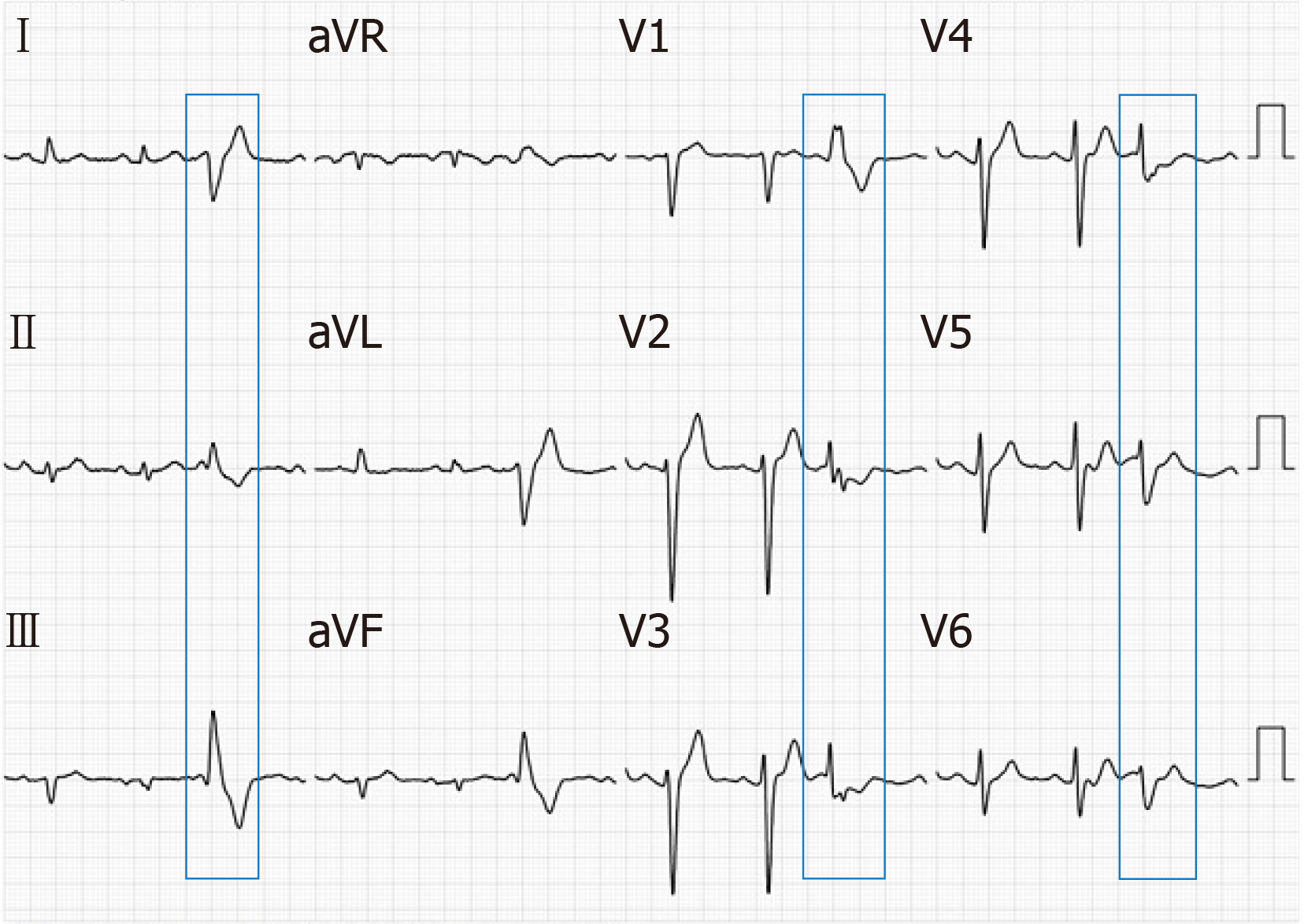
Electrocardiography (ECG) showed sinus rhythm, and frequent PVCs, with right bundle branch block pattern, with lead V1 of R wave, lead V2 and V3 of the rSr wave, and lead V4, V5 and V6 of the RS wave; lead II, III and AVF of the R wave, and the R amplitude in lead III was higher than that of II; lead I, AVL of the rS wave, and S amplitude in lead avL were deeper than that of I (Figure 1). Dynamic 24 h ECG monitoring of the average heart rate showed 69 beats/min, with the slowest at 48 beats/min and the fastest at 104 beats/min. A total of 93926 heart beats with premature ventricular beats were found accounting for 18.1%. Echocardiography showed a left ventricular ejection fraction of 65%; left ventricular end-diastolic diameter of 47 mm; an abnormal muscle bundle at the base of the interventricular septum and the other end was connected to the left ventricular lateral wall near the apex, resulting in a slight stenosis of the left ventricular outflow tract, local thickness of the interventricular septum of approximately 15 mm, and normal thickness of the remaining chamber walls with coordinated movement. Doppler examination demonstrated that the blood flow velocity at the left ventricular outflow tract was approximately 1.8 m/s. It was concluded that an abnormal muscle bundle was present in the left ventricle, and the blood flow velocity was fast.
Frequent PVCs, ventricular tachycardia, second degree SA block, and false tendons.
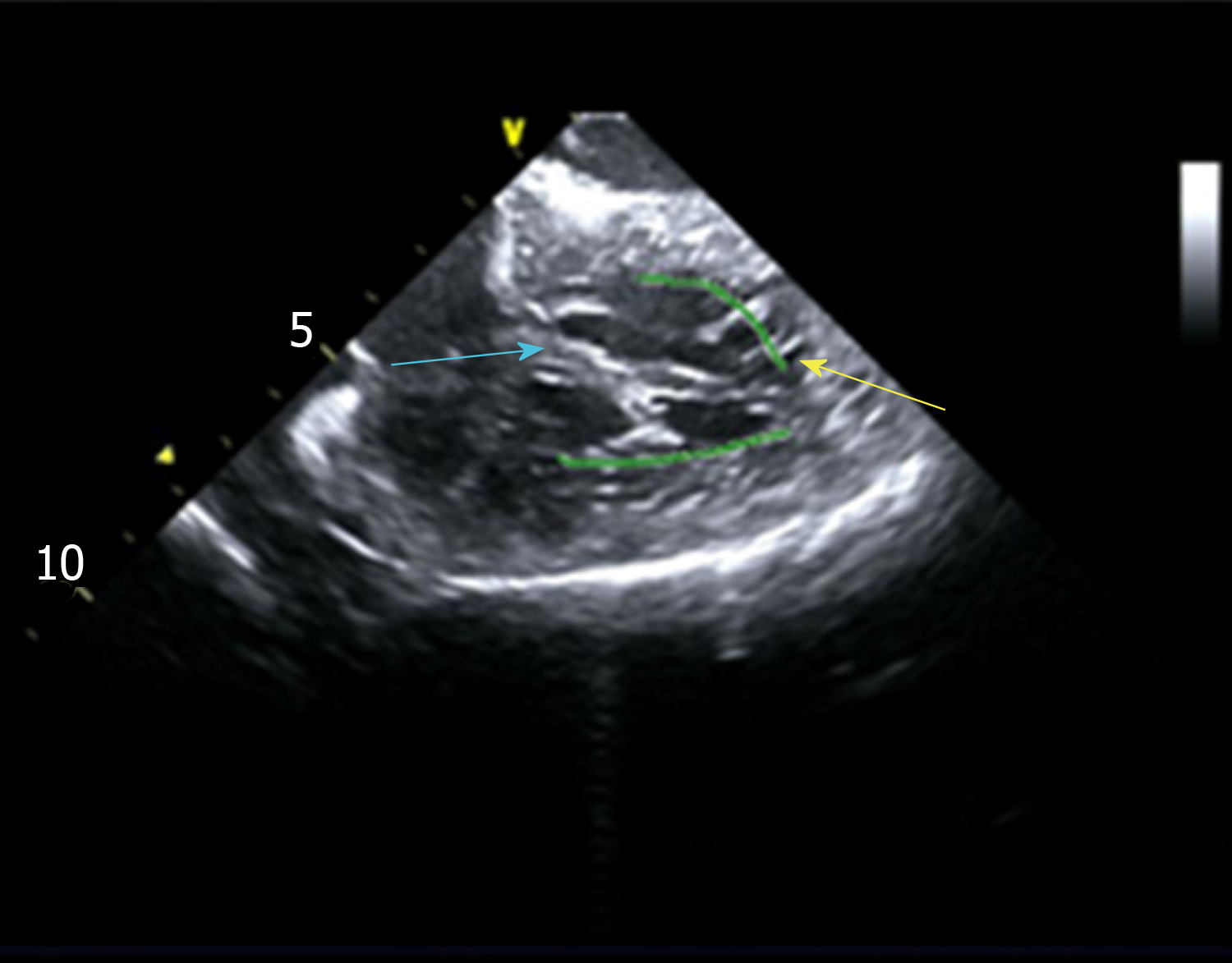
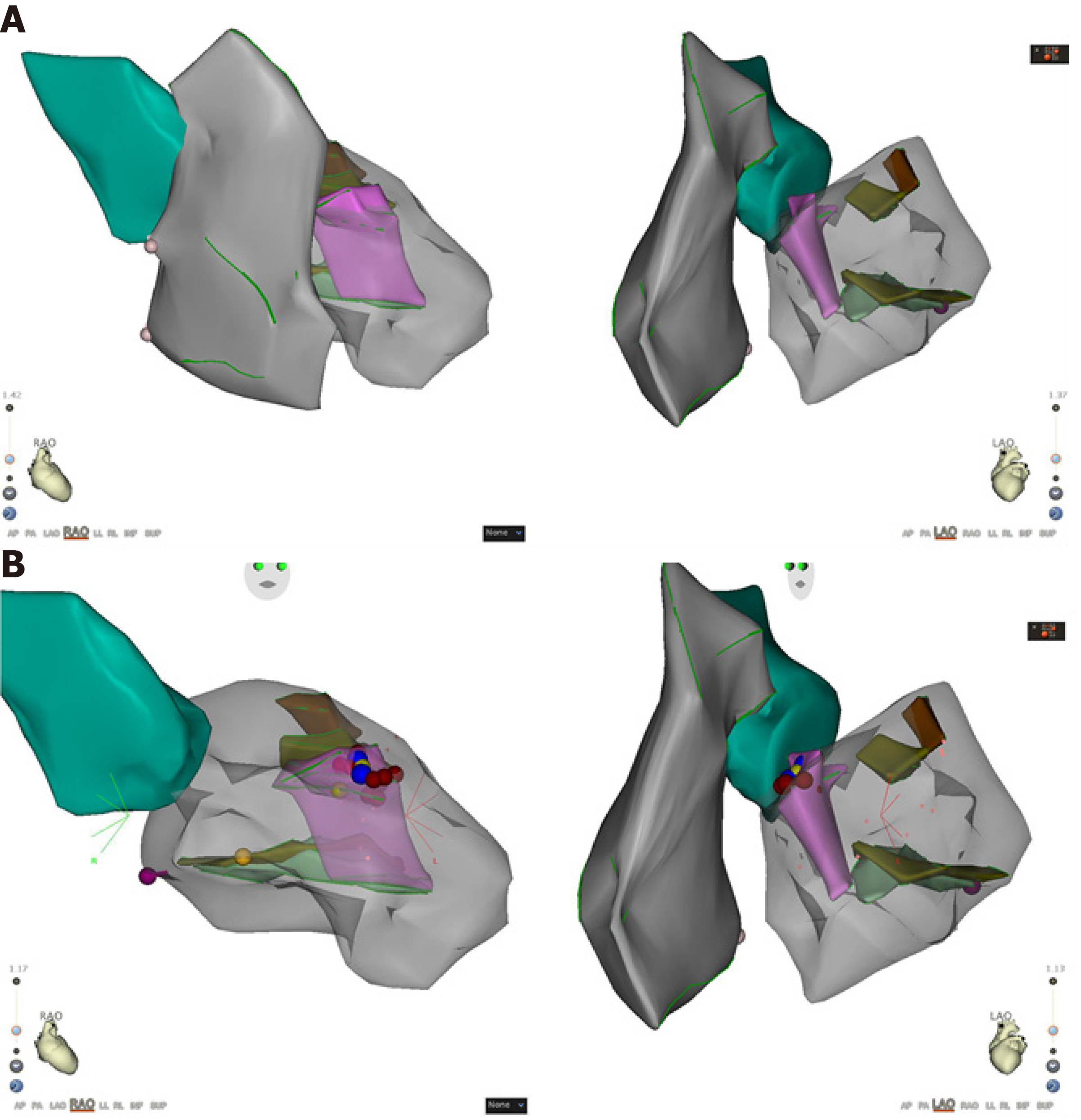
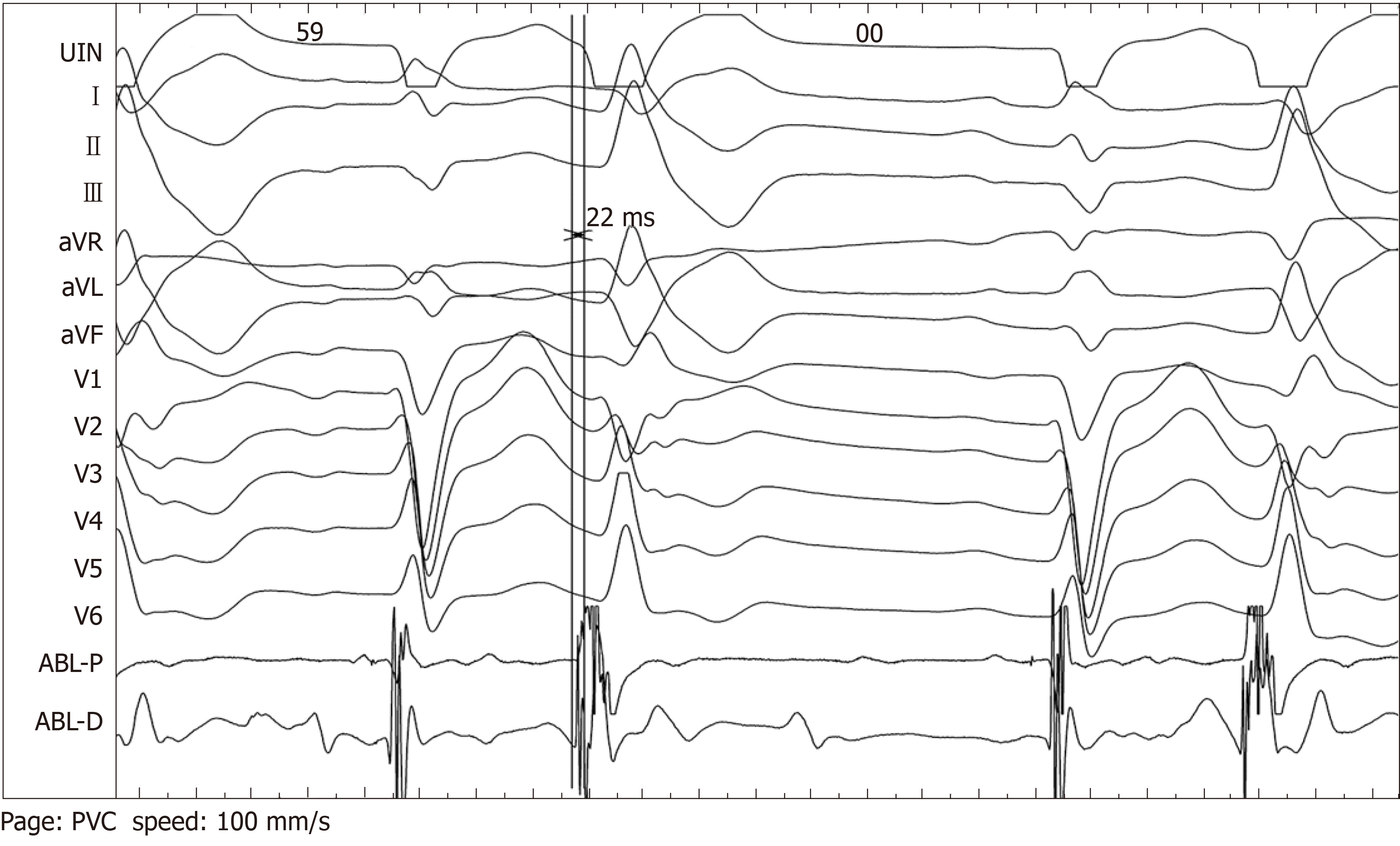
Intracardiac electrophysiological examination and radiofrequency ablation were performed under local anesthesia. A Soundstar catheter (Biosense Webster, Diamond Bar, CA, United States) was inserted into the middle of the right atrium via the left femoral vein and rotated clockwise to the HomeView position. The tricuspid annulus and right ventricular long axis were then established. The Soundstar catheter was placed towards the tricuspid annulus with its sector curved as A for easy insertion into the right ventricle. The curve was then loosened to enable the catheter tip to block the right ventricular outflow tract, and rotated clockwise, left ventricular false tendons were observed by intracardiac two-dimensional echocardiography (Figure 2), a left ventricular structure model was then established (Figure 3A), during which the left anterior papillary muscles, posterior papillary muscles and false tendons were mapped. After the entire left ventricular structure was established by CartoSoundTM, a saline-irrigated Navistar Smarttouch (Biosense Webster, United States) catheter was advanced into the left ventricle using a retrograde approach via the right femoral artery, the PVCs were then mapped in the established structure model (Figure 3B), and the earliest site was located at the interventricular septum attachment of the false tendons, which was 20 ms earlier than in the surface ECG (Figure 4). The PVCs disappeared after 10 s ablation (saline irrigation 17 mL/min, power 30-35 W, temperature 43°C). The ablation lasted for 90-120 s at each point, and 5 points were ablated until no PVCs were observed for 30 min under repeated ventricular stimulation and static isoproterenol.
ECG monitoring showed no PVCs on the first postoperative day. In addition, no complications, such as pericardial tamponade, atrioventricular block and hematoma of the lower extremity were observed. Twenty-four hour dynamic ECG three months later in the out-patient department demonstrated that sinus rhythm, second degree SA blocks, and less than 1000 beats of PVCs. The patient fell well with no relapse of heart palpitations and shortness of breath.
The CartoSoundTM system can provide electroanatomical three-dimensional mapping for arrhythmia[3,4], but the CartoSoundTM alone cannot provide accurate anatomical information, especially for PVCs and ventricular tachycardia arising from abnormal anatomical conditions due to the complexity and large variation in the anatomy[5]. ICE can provide real-time intracardiac ultrasound images[6,7], and the 3D modeling CartoSoundTM catheter and analysis software developed on the basis of ICE can provide a 3D anatomical reconstruction of the heart before insertion of the mapping catheter, and interface seamlessly with electroanatomic mapping, thereby providing accurate anatomical positioning for complex arrhythmia treatments, reducing operating time, increasing ablation success rates, and reducing X-ray exposure[8,9]. In this case, the intracardiac three-dimensional ultrasound catheter technique was used to confirm that the earliest activation site mapped in the ventricle was located at the attachment of false tendons near the basal side of the interventricular septum, and radiofrequency catheter ablation succeeded in curing the PVCs. These techniques can provide direct evidence of PVCs, which can be induced by false tendons and treated by radiofrequency ablation. The mechanisms of PVCs arising from left ventricular false tendons may include the following: High automaticity of Purkinje cells in the muscle fibers, increased ectopic excitability resulting from repeated mechanical traction of the false tendon attachment, and circle reentry from false tendons, normal myocardium, to conductive tissue[10]. No classical Purkinje potential was observed in the local potential of the target region of this patient, and the ectopic excitability resulting in mechanical traction at the false tendon attachment site of the interventricular septum may be a possible mechanism for these PVCs.
This case report verified that false tendons can cause premature ventricular beats, and the ectopic excitability resulting in mechanical traction at the false tendon attachment site may be a possible mechanism for these PVCs which may be cured by radiofrequency ablation guided by ICE with the CartoSoundTM system.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ueda H S-Editor: Zhang L L-Editor: Webster JR E-Editor: Liu MY
| 1. | Suwa M, Hirota Y, Kaku K, Yoneda Y, Nakayama A, Kawamura K, Doi K. Prevalence of the coexistence of left ventricular false tendons and premature ventricular complexes in apparently healthy subjects: a prospective study in the general population. J Am Coll Cardiol. 1988;12:910-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Philip S, Cherian KM, Wu MH, Lue HC. Left ventricular false tendons: echocardiographic, morphologic, and histopathologic studies and review of the literature. Pediatr Neonatol. 2011;52:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Bertagnolli L, Torri F, Richter S, Dinov B, Müssigbrodt A, Arya A, Sommer P, Bollmann A, Hindricks G, Hilbert S. [Three-dimensional mapping: Special aspects and new features of CARTO®]. Herzschrittmacherther Elektrophysiol. 2018;29:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Borlich M, Sommer P. Cardiac Mapping Systems: Rhythmia, Topera, EnSite Precision, and CARTO. Card Electrophysiol Clin. 2019;11:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Hussein A, Jimenez A, Ahmad G, Mesubi O, Klein T, Gurm G, Beck H, Shams O, See V, Saliaris A, Shorofsky S, Dickfeld T. Assessment of ventricular tachycardia scar substrate by intracardiac echocardiography. Pacing Clin Electrophysiol. 2014;37:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Enriquez A, Saenz LC, Rosso R, Silvestry FE, Callans D, Marchlinski FE, Garcia F. Use of Intracardiac Echocardiography in Interventional Cardiology: Working With the Anatomy Rather Than Fighting It. Circulation. 2018;137:2278-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 7. | Basman C, Parmar YJ, Kronzon I. Intracardiac Echocardiography for Structural Heart and Electrophysiological Interventions. Curr Cardiol Rep. 2017;19:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Kimura M, Sasaki S, Owada S, Horiuchi D, Sasaki K, Itoh T, Ishida Y, Kinjo T, Okumura K. Validation of accuracy of three-dimensional left atrial CartoSound™ and CT image integration: influence of respiratory phase and cardiac cycle. J Cardiovasc Electrophysiol. 2013;24:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Kean AC, Gelehrter SK, Shetty I, Dick M 2nd, Bradley DJ. Experience with CartoSound for arrhythmia ablation in pediatric and congenital heart disease patients. J Interv Card Electrophysiol. 2010;29:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Sadek MM, Benhayon D, Sureddi R, Chik W, Santangeli P, Supple GE, Hutchinson MD, Bala R, Carballeira L, Zado ES, Patel VV, Callans DJ, Marchlinski FE, Garcia FC. Idiopathic ventricular arrhythmias originating from the moderator band: Electrocardiographic characteristics and treatment by catheter ablation. Heart Rhythm. 2015;12:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |