Published online Aug 26, 2020. doi: 10.12998/wjcc.v8.i16.3583
Peer-review started: February 18, 2020
First decision: July 4, 2020
Revised: July 6, 2020
Accepted: July 16, 2020
Article in press: July 16, 2020
Published online: August 26, 2020
Processing time: 189 Days and 3.4 Hours
Pulmonary tumorlets are nodular hyperplastic neuroendocrine cells (NECs) that extend beyond the basement membrane. They often coexist with other lung diseases such as fibrosis and bronchiectasis, but rarely accompanied by pulmonary sclerosing pneumocytoma (PSP), which has not been reported in the literature.
A 54-year-old woman was admitted to the hospital because she had symptoms of bloody sputum for more than 4 mo and hemoptysis for 1 wk. Computed tomography images showed atrophy accompanied by infections in the middle lobe of her right lung. Moreover, numerous nodules were identified in the middle lobe of the right lung. The patient underwent thoracoscopic pneumonectomy of the middle lobe of the right lung, and the resected mass was pathologically confirmed to have bronchiectasis, multifocal NEC hyperplasia accompanied by tumorlet, and PSP.
Our report presents a rare clinical case of bronchiectasis complicated with multifocal NEC hyperplasia, tumorlet, and PSP.
Core tip: Pulmonary tumorlets are nodular hyperplastic neuroendocrine cells that extend beyond the basement membrane. They often coexist with other lung diseases such as fibrosis and bronchiectasis, but rarely accompanied by pulmonary sclerosing pneumocytoma. This paper reports a rare clinical case of bronchiectasis complicated with multifocal nodular hyperplastic neuroendocrine cell hyperplasia, tumorlet, and pulmonary sclerosing pneumocytoma. The patient was followed after computed tomography scanning and received surgical resection. We observed the progression of the tumor that has not yet been reported, which can be of great value in the diagnosis of the disease.
- Citation: Han XY, Wang YY, Wei HQ, Yang GZ, Wang J, Jia YZ, Ao WQ. Multifocal neuroendocrine cell hyperplasia accompanied by tumorlet formation and pulmonary sclerosing pneumocytoma: A case report. World J Clin Cases 2020; 8(16): 3583-3590
- URL: https://www.wjgnet.com/2307-8960/full/v8/i16/3583.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i16.3583
Pulmonary tumorlets are nests of hyperplastic, neuroendocrine cells (NECs) that extend beyond the basement membrane[1]. The diameter of nests differentiates tumorlets (< 5 mm) from carcinoids (≥ 5 mm)[2]. They arise from focal proliferation of bronchial and bronchiolar neuroendocrine Kultschitsky cells, which exceed the basement membrane. They often coexist with other lung diseases, like fifibrosis and bronchiectasis[3-5]. They are observed with particular frequency in bronchiectasis. But it has not been reported that patients with bronchiectasis and pulmonary tumorlets at the same time with pulmonary sclerosing pneumocytoma (PSP). PSP was first described by Liebow et al[6] in 1956. It is a rare neoplasm that is most often encountered in middle-aged women[7]. PSP has four histological types (hemorrhagic, sclerotic, solid, and papillary) and often grows slowly in the lower lobe of the lung[8]. This paper reports the case of a 54-year-old woman with bronchiectasis, in whom the middle lobe of the right lung was resected, and multifocal neuroendocrine cell hyperplasia and tumorlet were observed on microscopic examination among the bronchiectasis. Histologic and immunohistochemical examination revealed findings consistent with diffuse tumorlets and bronchiectasis accompanied by PSP in the lung.
A 54-year-old woman was admitted to the hospital because of blood in the sputum and hemoptysis.
A 54-year-old woman was admitted to the hospital because she had seen blood in her sputum for more than 4 mo and had had hemoptysis for 1 wk. The hemoptysis occurred without a clear reason. The coughed-out blood was bright red in clots and was accompanied by a large quantity of white and nonviscous sputum. The patient did not sweat excessively either during the day or night, did not feel cold or have a fever, did not feel chest tightness or shortness of breath, and had no dizziness or headache.
The patient had undergone surgical removal of gallbladder polyps more than 20 years ago, and had received minimally invasive surgery for breast fibroma more than 2 years ago.
The patient had no history of smoking or consuming alcohol, and there was no family medical history.
On admission, the patient's temperature was 36.7°C, with a heart rate of 78 beats/min, blood pressure of 110/68 mmHg, and respiratory rate of 16 breaths/min. The patient exhibited clear consciousness and compliance throughout the examination. Her trachea was in a neutral position, there was no cyanosis in her mouth or lips, and no swollen lymph nodes were identified in the supraclavicular region of either side. The breathing sound of both lungs was clear; no obvious rale was heard. The heart rhythm was regular; no obvious pathological noise was heard. The patient’s abdomen was flat and soft; no tenderness or rebound pain was identified. Edema in her lower extremities was not evident, and no clear pathological signs were found in the nervous system.
Her hemoglobin level was relatively low at 98 g/L; the remaining routine blood examination results, C-reactive protein level, liver and kidney function indicators, and electrolyte levels were all within the normal range. A laboratory examination indicated that the levels of the tumor indices neuron specific enolase (NSE), carbohydrate antigen (CA)-724, CA-125, CA19-9, carcinoembryonic antigen, and alpha-fetoprotein were in the normal range.
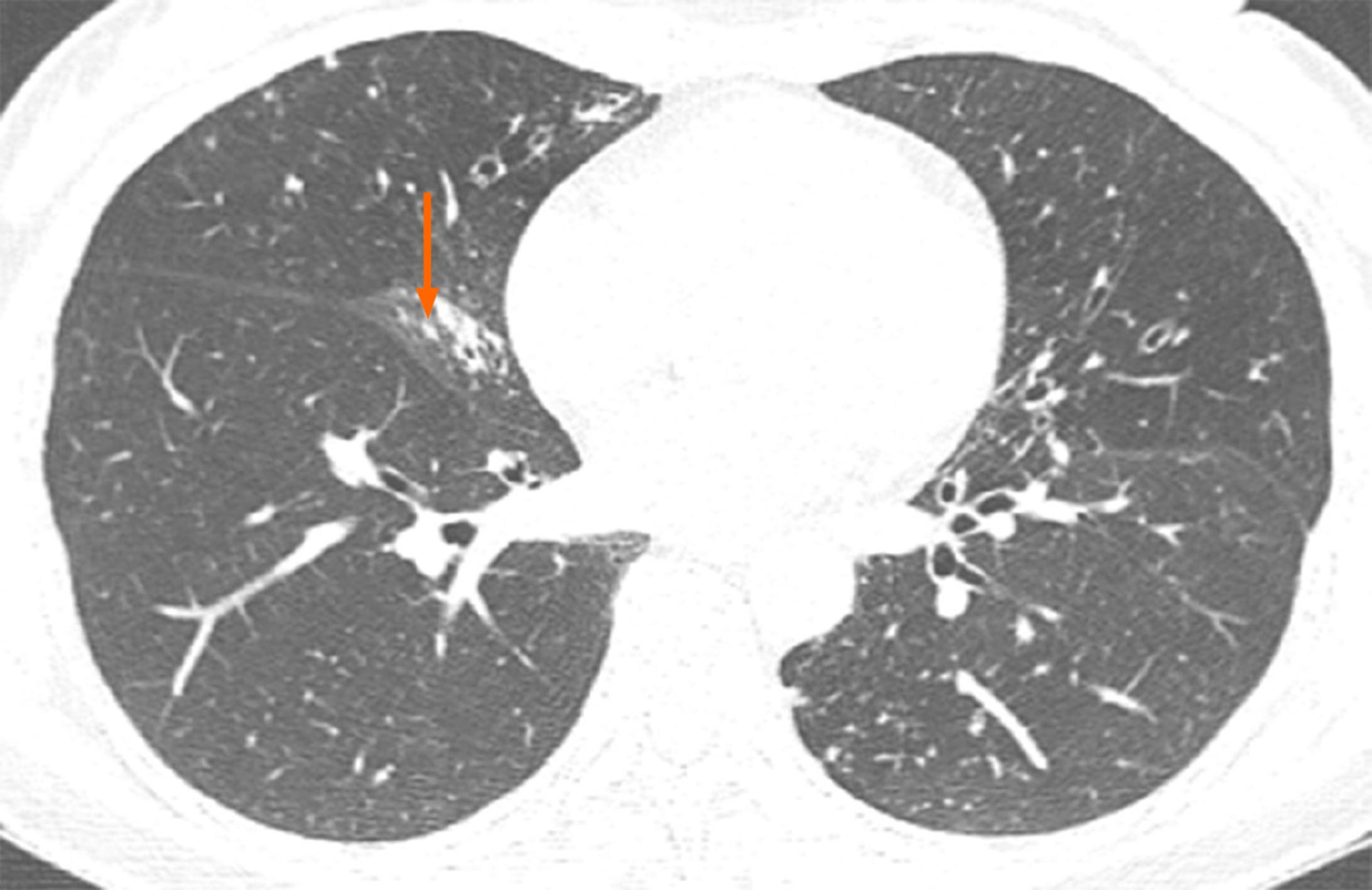
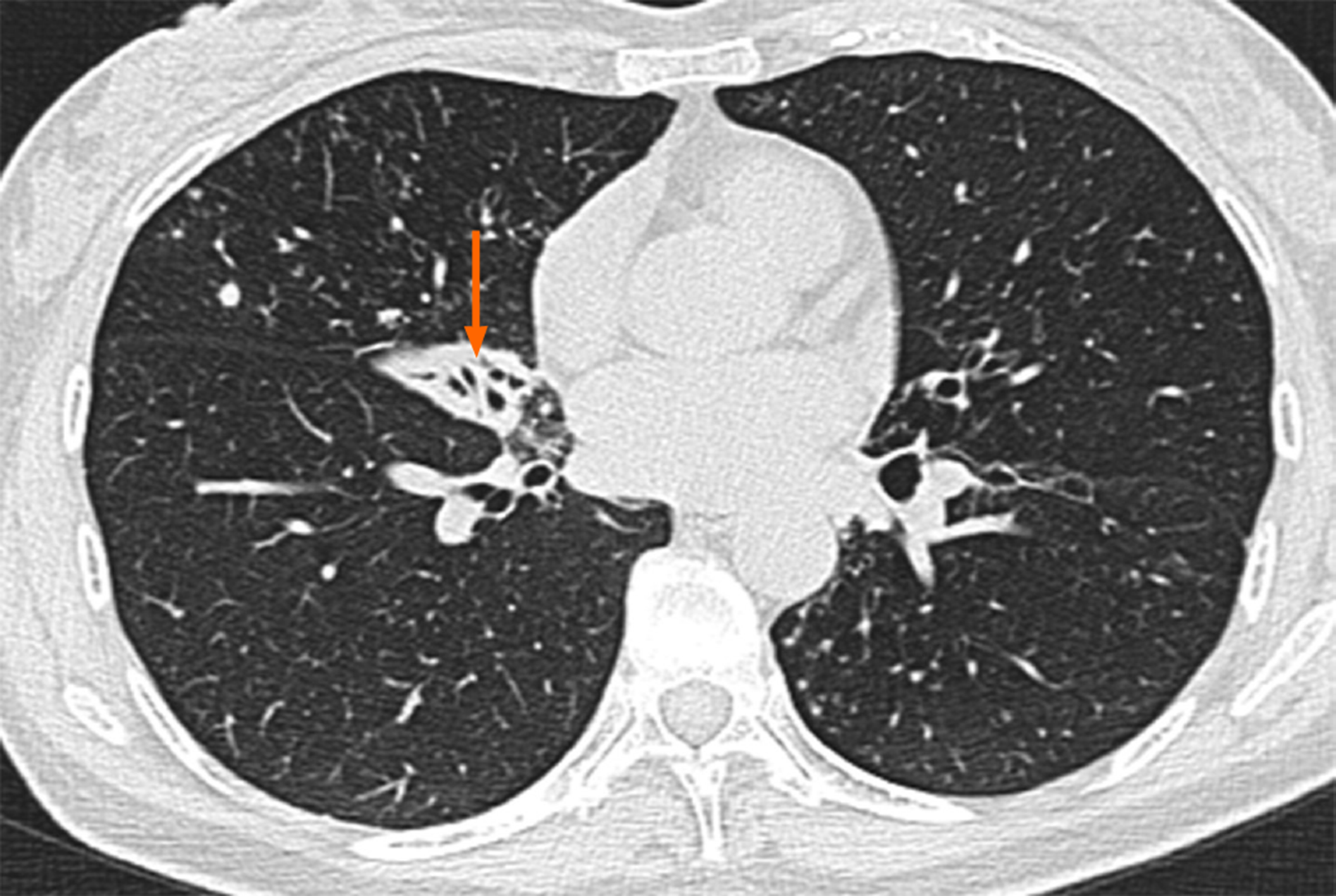
A computer tomography (CT) image of the patient that had been obtained in November 2017 showed atrophy in the middle lobe of her right lung as well as bronchiectasis in the left lung and the middle lobe of the right lung accompanied by infections. Numerous nodules with a diameter of 0.3 to 0.5 cm were identified in the middle lobe of the right lung (Figure 1). Another CT image obtained in March 2018 revealed that the bronchiectasis in the middle lobe of her right lung was accompanied by atelectasis, which was more observable than the atrophy in the same location in the previous CT image (Figure 2).
A final diagnosis of bronchiectasis, multifocal NEC hyperplasia accompanied by tumorlet formation, and pulmonary sclerosing pneumocytoma in the middle lobe of the right lung was made.
Thoracoscopy and pneumonectomy of the middle lobe of the right lung were performed. Considerable atrophy in the middle lobe of the right lung was noted during the surgery.
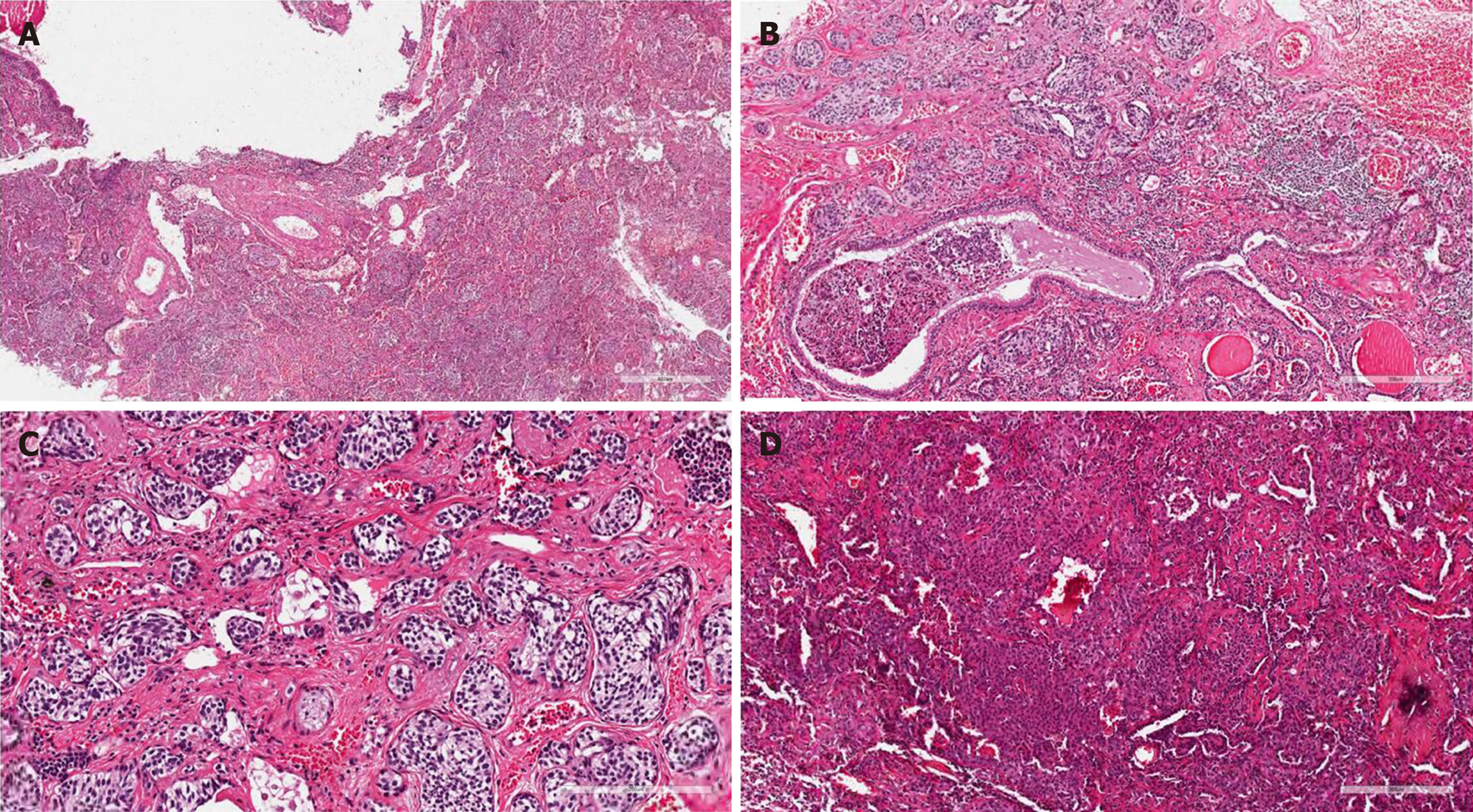
The removed tissue was then sent for pathological examination. Overall, the pathological examination revealed that the lung tissue had the dimensions of 5 cm × 4 cm × 1 cm, was dark red in the cross section, and had numerous greyish-white nodules near the capsule. The largest nodule was 0.3 cm in diameter. The cross section was solid with a medium density. Observation of the issue under a microscope showed inflammatory cell infiltration with lymphocytes, neutrophils, and histiocytes in alveoli. Alveolar septa broke and merged, focal bronchiectasis was observed, and cartilage in the bronchus was damaged (Figure 3A). Multifocal neuroendocrine-like cell nest hyperplasia was found in the surroundings of the expanded bronchus (single focal diameter < 0.5 cm; Figure 3B). The nuclei of hyperplasia cells were polygonal or short fusiform with a uniform size. The nuclear chromatin was delicate and in fine particles. The nucleoli were unclear; no mitotic figures or necrosis was detected (Figure 3C). Solid nodules consisting of two types of cells were discovered in focal lung parenchyma. These cells were single-layered, cuboidal epithelial cells and egg-shaped interstitial cells. Figure 3D shows the papillary arrangement of tumor cells and a sample of vascular cavity. Immunohistochemical staining revealed that neuroendocrine-like cell nests were positive for CgA, SyN, and CD56; egg-shaped interstitial cells positive for Vimentin and TTF-1; single-layered, cuboidal epithelial cells positive for CK7, PCK, and TTF-1; tissue cells positive for CD68 and CD163; and vascular cells positive for ERG and CD34 (+). P53 (−) and Ki-67 (+, < 5%) were also detected.
Appropriate supportive treatments such as infection prevention-oriented hemostasis, pain relief, liver and stomach protection, and phlegm removal were administered to the patient after the surgery. The patient recovered satisfactorily and was discharged. The patient was followed at 6, 12, and 16 mo in the outpatient department. The patient was doing well physically, with no abnormal findings on physical examination. The patient underwent CT reexamination, but no recurrence or metastasis was observed.
The diffuse neuroendocrine system comprises NECs (neuroendocrine cells) distributed all over the body in various organs and systems. Tumors derived from this system are neuroendocrine neoplasms (NENs), previously known as amine precursor uptake and decarboxylation[9]. Pulmonary Kultschisky NECs exist in the epithelium of the normal respiratory tract. They have a paracrine function and are essential for the development and maturation of the lungs. The number of NECs decreases with age. However, people living in high-altitude regions and those with chronic pulmonary fibrosis such as bronchiectasis or interstitial pulmonary fibrosis may have NEC hyperplasia because of hypoxia and structural damage to the lungs. The hyperplasia is temporary and reversible; it does not typically progress into carcinoids[10].
In the 2015 World Health Organization Classification of Lung Tumors[11], diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) was defined as a type of precancerous lesion for pulmonary NEN. DIPNECH is prevalent among older women who do not smoke and middle-aged people. Most of the patients are 50 to 60 years old and seek medical treatment because of airway obstruction symptoms such as coughing and shortness of breath. Pulmonary function tests show that these patients have obstructive or restrictive ventilatory dysfunction, and the outcomes of anti-infective treatments tend to be poor. The patients are at risk of developing a carcinoid. DIPNECH refers to NEC hyperplasia that occurs without noticeable pulmonary diseases. Histologically, this disease is defined as diffuse linear and nested growth of cells along the bronchial mucosal epithelium, with the hyperplasia cells not breaking the basement membrane. A tumorlet (i.e., NEC hyperplasia with a diameter < 0.5 cm) is highly similar to DIPNECH but with some differences. The similarities are as follows[4,12]: (1) Both diseases are prevalent among women aged 50 to 70 years; (2) NEC hyperplasia and tumorlets both manifest as focal NEC hyperplasia under the microscope; the hyperplasia cells can be restricted under the epithelium of the bronchus or that of the fine bronchus, or they can break through the basement membrane to form a nodule-like growth in the interstitial tissue or develop into a tumorlet. A tumorlet can be multifocal and consist of short fusiform or elliptical cells with a relatively consistent size. These cells have less cytoplasm, are eosinophilic, have delicate or finely granular nuclear chromatin, have a nonobvious nucleolus, rarely have mitotic figures, and have no necrosis; and (3) The results of immunohistochemical staining for neuroendocrine markers are positive for these two diseases. Conversely, the differences between the two diseases are as follows[13-15]: (1) Regarding the clinical manifestation, DIPNECH exhibits no specificity and primarily manifests as airway obstruction; by contrast, NEC hyperplasia primarily manifests as bronchiectasis, such as coughing up of phlegm or hemoptysis; (2) The inflammatory response of DIPNECH lesions is mild, and the surrounding lung tissue is relatively normal; however, bronchiectasis accompanied by NEC hyperplasia causes a severe inflammatory response and partial destruction of lung structure; and (3) DIPNECH may develop into a carcinoid (including an atypical carcinoid), whereas NEC hyperplasia does not usually develop into a carcinoid. Research has demonstrated that 5.7% of patients with carcinoids have DIPNECH as a comorbidity and 47.4% of patients with DIPNECH have typical carcinoids, whereas 15.8% have atypical carcinoids. These results suggest that the lesion develops from DIPNECH into a tumorlet and eventually to a carcinoid; thus, DIPNECH is a type of precancerous lesion.
After performing a literature review, we analyzed the case discussed in this study to identify several characteristics: A middle-aged or older woman, no smoking history, and reported coughing up of phlegm and blood in phlegm as clinical symptoms. Observation of the surgical specimen under the microscope revealed bronchial wall damage and alveolar fusion amid diffuse inflammation. Multifocal hyperplasia of neuroendocrine-like cell nests was discovered around the bronchus, with the diameter of single focal cell nests being < 0.5 cm. Hyperplasia neuroendocrine-like cells were found all around the bronchus. The nucleoli of hyperplasia cells were round or egg-shaped with a relatively consistent size. The cells had delicate and finely granular nuclear chromatin, had a nonobvious nucleolus, and did not exhibit mitotic figures or necrosis. The neuroendocrine markers CgA, SyN, and CD56 were all positive on immunohistochemical staining.
Tumorlets are basically identical to typical carcinoids in terms of tissue morphology and immunophenotype. The greatest difference between the two is lesion diameter; lesions with a diameter > 0.5 cm are considered typical carcinoids. Because the diagnostic criteria for DIPNECH are relatively unclear, scholars have proposed in case series studies that DIPNECH should be defined as at least five sites of NEC hyperplasia in at least three independent small airways, accompanied by more than three lesions identified as tumorlets. They emphasized that such lesions are considerably more likely to develop into carcinoids and that the probability of DIPNECH developing into carcinoids is far higher than that of NEC hyperplasia. Most studies have been focused on lung tumors, such as adenocarcinoma and neuroendocrine tumors, and conducted retrospective analyses on lung specimens resected because of tumors. Large-series pioneering studies of lung specimens are still few. Therefore, the data may be limited or biased[15]; more case data and in-depth research are warranted.
NENs in different body parts have dissimilar classification standards. For example, the 2015 World Health Organization Classification of Lung Tumors employs tumor size, mitotic figures, and necrosis as the criteria for dividing NENs into typical carcinoids, atypical carcinoids, small-cell lung cancer, and large-cell neuroendocrine carcinoma; DIPNECH and tumorlet are identified as precancerous lesions. Regarding gastrointestinal and pancreatic NENs, the level of tumor cell differentiation, necrosis, and the Ki-67 index serve as the main criteria for dividing NENs into neuroendocrine tumors [NETs; can be further divided into NETs Level 1 (carcinoid) and NETs Level 2] and neuroendocrine carcinoma. Pulmonary, gastrointestinal, and pancreatic NENs have a preinfiltration (precursor) lesion. We inferred that NEC hyperplasia develops into NENs through a continuous process. Although the lungs, gastrointestinal system, and pancreas all have an intramucosal lesion as the preinfiltration lesion, the diagnostic criteria for lesions in the lungs are different from those for lesions in the gastrointestinal system and pancreas. The preinfiltration lesion of gastrointestinal and pancreatic NENs is an intramucosal tumor < 0.5 mm in size, whereas that of pulmonary NENs is an intramucosal tumor < 0.5 cm in size. In addition, the classification criteria for pulmonary NENs include a distinction between NEC hyperplasia and DIPNECH, which is nonexistent for gastrointestinal NENs. The authors conjectured that similar to gastrointestinal preinfiltration lesions, pulmonary preinfiltration lesions may have the potential to develop into carcinoids regardless of the presence of a primary pulmonary disease.
Another characteristic of the investigated case was a comorbid PSP, which was previously called pulmonary sclerosing hemangioma. PSP can be accompanied by diffuse NEC hyperplasia or a typical carcinoid. Fan et al[16] have recently considered that PSP may originate from primitive respiratory epithelial cells; hence, it has the potential for multidirectional differentiation. Partial expression of neuroendocrine markers has been discovered in some cases, and both have TTF-1 expression; therefore, some scholars have believed that they might be the same. However, in the case investigated, PSP was irrelevant to the location of NEC hyperplasia. They could also be a type of “collision tumor”; in other words, PSP and primary pulmonary lesions break the local airway homeostasis and injure neighboring alveolar epithelial cells, leading to NEC activation and hyperplasia. When PSP is worsened to a certain extent, it can lead to local lung hypoxia, decreased lung compliance, and ventilatory disorders, thereby promoting further hyperplasia and differentiation of NECs. In addition, hypoxia can trigger a series of molecular events that eventually lead to the development of a tumorlet[17,18].
For cases of bronchiectasis for which internal medicine interventions such as anti-infective treatments have proven ineffective, we recommend surgical resection of pulmonary tissue, because the repeated clinical symptoms may be caused by not only bronchiectasis but also airway obstruction, which is caused by the gastrin-releasing peptide precursors secreted by NECs or by interstitial fibrosis induced by the stimulation of NECs. Furthermore, NEC hyperplasia is likely to progress into a tumorlet. Pulmonary CT images often reveal signs of bronchiectasis, pulmonary consolidation, and numerous lung nodules with a diameter ≤ 5 mm. If nodule growth or lesion progression is detected in follow-up visits, the medical team should be cautious about the possibility of the tumorlet developing into a carcinoid. However, clinical diagnosis still relies on postoperative pathological analysis. Because NEC hyperplasia and DIPNECH are usually small in size, obtaining tissue specimens is also a challenge for pathologists. For cases with focal hardness and no clear signs of nodules, the pathologist should obtain sufficient specimens and be cautious about the possibility of NEC hyperplasia and carcinoid development, because these two types of lesions often manifest as focal hardness of pulmonary tissue rather than nodules. In addition, for lung specimens resected from carcinoids, the pathologist should closely examine regions either near or far from the tumor to prevent DIPNECH being missed.
In this case, the patient had symptoms of bloody sputum for more than 4 mo and hemoptysis for 1 wk. After anti-inflammatory treatment, the symptoms have not been alleviated. The patient was then undergoing a CT scan, which revealed multiple nodules in the middle lobe of the right lung with an unclear boundary. After 4-mo CT follow-up, the progression of the lesions was detected, which could not exclude the possibility of a malignant tumor. Therefore, thoracoscopy and pneumonectomy of the middle lobe of the right lung were performed. At present, antibiotics are effective treatments for most of the patients with bronchiectasis, who do not need surgical treatment. The surgical indications include[19]: (1) Active drug treatment is still difficult to control symptoms; and (2) Massive hemoptysis is life-threatening, for which drug and interventional therapy may be ineffective. Given that bronchiectasis with tumorlet and PSP may not usually require treatment, accurate preoperative diagnosis and long-term follow-up are of great importance.
In summary, this paper reports a rare clinical case of bronchiectasis with comorbid multifocal NEC hyperplasia, tumorlet, and PSP. The patient was followed after undergoing CT imaging and received surgical resection. We observed tumor change and progression that have not yet been reported. This report can be of great value in the diagnosis the disease.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bolshakova GB, Turner AM S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Churg A, Warnock ML. Pulmonary tumorlet. A form of peripheral carcinoid. Cancer. 1976;37:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Ranchod M. The histogenesis and development of pulmonary tumorlets. Cancer. 1977;39:1135-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Kallianos A, Velentza L, Zarogoulidis P, Baka S, Kosmidis C, Labaki S, Lazaridis G, Huang H, Hohenforst-Schmidt W, Trakada G. Progressive dyspnea due to pulmonary carcinoid tumorlets. Respir Med Case Rep. 2017;21:84-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Jin L, Wang Z, Qi X. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: Case series and a review of the literature. Medicine (Baltimore). 2018;97:e13806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Marchevsky AM, Wirtschafter E, Walts AE. The spectrum of changes in adults with multifocal pulmonary neuroendocrine proliferations: what is the minimum set of pathologic criteria to diagnose DIPNECH? Hum Pathol. 2015;46:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer. 1956;9:53-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Kuo KT, Hsu WH, Wu YC, Huang MH, Li WY. Sclerosing hemangioma of the lung: an analysis of 44 cases. J Chin Med Assoc. 2003;66:33-38. [PubMed] |
| 8. | Yuan ZQ, Wang Q, Bao M. Symptomatic pulmonary sclerosing hemangioma: a rare case of a solitary pulmonary nodule in a woman of advanced age. J Int Med Res. 2019;47:2302-2308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Uccella S, La Rosa S, Volante M, Papotti M. Immunohistochemical Biomarkers of Gastrointestinal, Pancreatic, Pulmonary, and Thymic Neuroendocrine Neoplasms. Endocr Pathol. 2018;29:150-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Ichiki Y, Matsumiya H, Mori M, Kanayama M, Nabe Y, Taira A, Shinohara S, Kuwata T, Takenaka M, Hirai A, Imanishi N, Yoneda K, Noguchi H, Shimajiri S, Fujino Y, Nakayama T, Tanaka F. Predictive factors of postoperative survival among patients with pulmonary neuroendocrine tumor. J Thorac Dis. 2018;10:6912-6920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Aubry MC, Thomas CF, Jett JR, Swensen SJ, Myers JL. Significance of multiple carcinoid tumors and tumorlets in surgical lung specimens: analysis of 28 patients. Chest. 2007;131:1635-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Ruffini E, Bongiovanni M, Cavallo A, Filosso PL, Giobbe R, Mancuso M, Molinatti M, Oliaro A. The significance of associated pre-invasive lesions in patients resected for primary lung neoplasms. Eur J Cardiothorac Surg. 2004;26:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Davies SJ, Gosney JR, Hansell DM, Wells AU, du Bois RM, Burke MM, Sheppard MN, Nicholson AG. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: an under-recognised spectrum of disease. Thorax. 2007;62:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Marchevsky AM, Walts AE. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH). Semin Diagn Pathol. 2015;32:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Fan X, Lin L, Wang J, Wang Y, Feng A, Nie L, Wu H, Meng F, Xu H. Genome profile in a extremely rare case of pulmonary sclerosing pneumocytoma presenting with diffusely-scattered nodules in the right lung. Cancer Biol Ther. 2018;19:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Wang Y, He Q, Shi W, Wang J, Ji H. A mixture of carcinoid tumors, extensive neuroendocrine proliferation, and multiple pulmonary sclerosing hemangiomas. World J Surg Oncol. 2014;12:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Kim Y, Choi YD, Kim BJ, Oh IJ, Song SY, Nam JH, Park CS. Multiple peripheral typical carcinoid tumors of the lung: associated with sclerosing hemangiomas. Diagn Pathol. 2013;8:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, Murris M, Cantón R, Torres A, Dimakou K, De Soyza A, Hill AT, Haworth CS, Vendrell M, Ringshausen FC, Subotic D, Wilson R, Vilaró J, Stallberg B, Welte T, Rohde G, Blasi F, Elborn S, Almagro M, Timothy A, Ruddy T, Tonia T, Rigau D, Chalmers JD. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 790] [Article Influence: 98.8] [Reference Citation Analysis (0)] |