Published online Jul 6, 2020. doi: 10.12998/wjcc.v8.i13.2727
Peer-review started: January 16, 2020
First decision: April 14, 2020
Revised: April 26, 2020
Accepted: June 29, 2020
Article in press: June 29, 2020
Published online: July 6, 2020
Processing time: 172 Days and 18.6 Hours
Eosinophil counts are a promising guide to systemic steroid administration for chronic obstructive pulmonary disease (COPD).
To study the role of peripheral eosinophilia in hospitalized patients with acute exacerbation of COPD (AECOPD).
From January 2014 to May 2017, patients with AECOPD hospitalized in Taipei Tzu Chi Hospital were retrospectively stratified into two groups according to their peripheral eosinophil count: The EOS group (eosinophil count ≥ 2%) and the non-EOS group (eosinophil count < 2%). Demographics, comorbidities, laboratory data, steroid use, length of hospital stay, and COPD-related readmissions were compared between the groups.
A total of 625 patients were recruited, with 176 patients (28.2%) in the EOS group. The EOS group showed a lower prevalence of infection, lower cumulative doses of prednisolone equivalents, shorter length of hospital stay, and higher number of COPD-related readmissions than the non-EOS group. There were significantly linear correlations between eosinophil percentage and number of readmissions and between eosinophil percentage and length of hospital stay (P < 0.001, Pearson's r = 0.147; P = 0.031, Pearson's r = -0.086, respectively). The EOS group and a lower percent-predicted value of forced expiratory volume in one second (FEV1) were associated with shorter time to first COPD-related readmission [adjusted hazard ratio (adj. HR) = 1.488, P < 0.001; adj. HR = 0.985, P < 0.001, respectively].
The study findings suggest that the EOS group had the features of a shorter length of hospital stay, and lower doses of systemic steroids, but more frequent readmissions. The EOS group and lower percent-predicted FEV1 values were risk factors for shorter time to first COPD-related readmission.
Core tip: This is the first study in Taiwan that comprehensively evaluates the role of eosinophilia in chronic obstructive pulmonary disease (COPD). Eosinophilic COPD exacerbation is associated with a high risk of readmissions and a short length of hospital stay. It has the characteristics of non-infectious inflammation and is a predictor of steroid therapy. A routine survey of the peripheral blood eosinophil count is warranted for acute COPD exacerbations.
- Citation: Wu CW, Lan CC, Hsieh PC, Tzeng IS, Wu YK. Role of peripheral eosinophilia in acute exacerbation of chronic obstructive pulmonary disease. World J Clin Cases 2020; 8(13): 2727-2737
- URL: https://www.wjgnet.com/2307-8960/full/v8/i13/2727.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i13.2727
Chronic obstructive pulmonary disease (COPD) is characterized by airway obstruction that is not completely reversible. It has been predicted to be the third most common cause of death in 2020[1]. COPD exacerbation is associated with significant morbidity and mortality. Systemic steroid therapy is a cornerstone of the treatment of COPD exacerbation, but it can exacerbate hyperglycemia, psychiatric problems, and osteoporosis[2]. Patients with COPD are of old age and have multiple comorbidities; therefore, they are vulnerable to the side effects of systemic steroids. Traditionally, asthma is considered as eosinophilic airway disease, while COPD is considered as neutrophilic airway disease. Bafadhel et al[3] proposed four models of COPD exacerbation: Bacterial (55%), viral (29%), eosinophilic (28%), and pauci-inflammatory. A classification of COPD exacerbation based on the phenotype is required for the development of precision medicine.
Eosinophilia in patients with COPD is a marker of steroid response. The 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend using the peripheral blood eosinophil count (PBEC) to guide the choice of inhalational steroids to prevent COPD exacerbation, and the cut-off values are the absolute values of PBEC (100 and 300 cells/μL)[1]. Mepolizumab, an interleukin-5 antibody, showed slight efficacy for reducing the rate of exacerbations in patients with COPD and eosinophilia[4]. Most studies used 2% of the total white blood cell (WBC) count as the cut-off value to diagnose patients with an eosinophilic or a non-eosinophilic COPD exacerbation. However, only a few studies focused on the impact of PBEC on systemic steroid administration for acute COPD exacerbation. Until recently, two prospective studies showed that PBEC-guided systemic steroid therapy could reduce the steroid exposure and improve the health status of patients but without altering survival[5,6].
The eosinophilic phenotype accounts for 20%-40% of COPD exacerbations[7]. PBEC is a well-established predictor of the length of hospital stay, steroid response, prognosis, and readmission rate[7-13]. Nevertheless, some characteristics of patients with eosinophilic COPD exacerbation, such as demographics, comorbidities, lung function, etc., are inconsistent across studies[14]. Studies on the impact of the PBEC were primarily focused on the Caucasian race. Only a few studies investigated eosinophilic COPD exacerbation in the Asian populations, including patients from China and South Korea[13,15]. The aim of the present study was to elucidate the role of peripheral eosinophilia in hospitalized patients with COPD exacerbation in Taiwan.
We retrospectively screened the medical records for COPD-related hospitalizations in the department of pulmonary and critical care of Taipei Tzu chi Hospital from January 1, 2014 to May 31, 2017. Patients who experienced at least one COPD-related admission during this period were recruited. The index hospitalization was defined as the first COPD-related admission during the study period. Patients with COPD exacerbations were classified into two groups according to their blood eosinophil count at the time of the index hospitalization: The EOS group (PBEC ≥ 2% of the total WBC count) and the non-EOS group (PBEC < 2% of the total WBC count).
Other inclusion criteria were patients aged > 40 years and a post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio < 0.7, in accordance with 2020 GOLD guidelines[1]. We excluded patients with a history of asthma and bronchiectasis, long-term oral steroid use, and those who received systemic steroids within 48 h before the blood test at the index hospitalization.
The protocol of PBEC processing was as follows: (1) The nurses or technicians collected 3 mL of venous blood in a lavender-top tube (ethylenediaminetetraacetic acid); (2) The sample was sent to the automated hematology analyzer Sysmex XN-9000™ (Sysmex Corporation, Kobe, Japan); (3) The complete and differential blood counts were reported within one hour; (4) The PBEC (%) was obtained from the differential count; and (5) If the PBEC (%) was larger than 30%, the technicians manually recalculated it.
The primary outcome was the total number of 12-mo COPD-related readmissions. The secondary outcomes were total cumulative dose of systemic steroids in the index hospitalization, the length of stay in the index hospitalization, time to first COPD-related readmission within 12 mo, risk factors for first COPD-related readmission, and the total number of 12-mo COPD-related admissions before the index hospitalization. We assessed the linear relationship between the percentage of eosinophils with the number of 12-mo COPD-related readmissions and the length of stay in the index hospitalization.
We collected the following data from medical records before the index hospitalization: Demographics, body mass index, smoking history, comorbidities (the neuromuscular disease included stroke, Parkinsonism), the use of home noninvasive ventilation, pulmonary function test, and the respiratory medicines including long-acting anti-muscarinic agents, long-acting beta-agonists, and inhaled corticosteroids. The following data were collected at the index hospitalization: Hemogram, C-reactive protein (CRP) levels, chest X-ray (CXR) findings, length of hospital stay, hospital outcomes including home discharge, death, and respiratory care ward (RCW) discharge, and major in-hospital treatments including systemic steroids and antibiotics.
The study was approved by Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation Institutional Review Board on September 2019 (protocol number 08-X-094).
Data were presented as mean ± standard deviation and number (percentage). The independent samples t-test was used for comparison of continuous data. Chi-squared test was used for comparison of categorical variables. Relationships between the continuous variables were assessed by the Pearson correlation coefficient (r) and simple linear regression analysis. R-values of < 0.3, 0.3-0.7, and > 0.7 were considered to indicate mild, moderate, and strong relationships, respectively. Cox regression analysis was used to clarify the risk factors for the time to first COPD-related readmission. We used the Kaplan-Meier method to plot the time to first COPD-related readmission curves of the two groups and the log-rank test to analyze the difference between the two groups. A P value < 0.05 was considered to be statistically significant. SPSS software version 25 (IBM Corporation, Armonk, NY, United States) was used for all statistical analyses.
A total of 625 patients were enrolled. The EOS group and the non-EOS group included 176 (28.2%) and 449 (71.8%) patients, respectively. Table 1 shows the comparison of demographics, smoking history, hemogram data, infection status, use of home noninvasive ventilation, comorbidities, pulmonary function test and inhaled medications before admission between the two groups. The mean percentage of eosinophils in the EOS group was greater than in the non-EOS group (6.47% vs 0.79%, P < 0.001). In contrast, the percentage of neutrophils and the neutrophil to lymphocyte ratio (NLR) were significantly lower in the EOS group than in the non-EOS group (both P < 0.001). The EOS group had significantly lower burden of infectious inflammation (assessed by CXR infiltrate, fever, CRP level, and antibiotic administration) than the non-EOS group. There were no significant differences in the prevalence of comorbidities between the two groups, with the exception of neuromuscular disease (14.2% in the EOS group vs 7.1% in the non-EOS group, P = 0.009). Most of the pulmonary function test parameters showed no significant difference between the groups. However, the FVC was higher in the EOS group than in the non-EOS group (2.14 L[1] vs 1.94 L, P = 0.002). Both groups received similar inhaled medications before admission.
| Variables | EOS group (n = 176) | Non-EOS group (n = 449) | P value |
| Male (%) | 166 (94.3) | 384 (85.5) | 0.004 |
| BMI | 23.52 ± 4.19 | 23.23 ± 4.23 | 0.441 |
| Age, yr | 74.90 ± 11.74 | 76.89 ± 10.09 | 0.049 |
| Smoking, n (%) | 0.054 | ||
| Never | 45 (25.6) | 142 (31.6) | |
| Current | 54 (30.7) | 98 (21.8) | |
| Ex-smoker | 77 (43.8) | 209 (46.5) | |
| Hemogram values | |||
| Leukocyte count, 109/ L | 8723.92 ± 3447.99 | 11002.72 ± 4311.44 | 0.000 |
| Neutrophil, % | 64.50 ± 9.89 | 77.74 ± 9.86 | 0.000 |
| Monocyte, % | 6.96 ± 2.20 | 6.71 ± 3.05 | 0.253 |
| Lymphocyte, % | 21.30 ± 8.18 | 13.13 ± 8.50 | 0.000 |
| Eosinophil count, % | 6.47 ± 3.81 | 0.79 ± 0.84 | 0.000 |
| Basophil, % | 0.40 ± 0.30 | 0.19 ± 0.28 | 0.000 |
| Hemoglobin, g/dL | 13.25 ± 2.31 | 13.24 ± 2.11 | 0.942 |
| Hematocrit, % | 39.31 ± 6.33 | 39.37 ± 5.83 | 0.909 |
| MCV, fL | 88.58 ± 7.58 | 89.35 ± 6.99 | 0.228 |
| Platelet count, 103/µL | 228.14 ± 86.15 | 206.59 ± 70.09 | 0.001 |
| Mean platelet volume, fL | 9.63 ± 0.76 | 9.75 ± 0.81 | 0.106 |
| Neutrophil count, 109/L | 5715.46 ± 2872.96 | 8718.93 ± 3962.78 | 0.000 |
| Lymphocyte count, 109/ L | 1770.57 ± 822.48 | 1294.99 ± 840.47 | 0.000 |
| Eosinophil count, 109/ L | 543.18 ± 351.54 | 76.16 ± 82.77 | 0.000 |
| Neutrophil-to-lymphocyte ratio | 4.39 ± 6.29 | 10.59 ± 13.11 | 0.000 |
| Infection status, n (%) | |||
| CXR infiltrate | 18 (10.2) | 83 (18.5) | 0.016 |
| Fever | 9 (5.1) | 51 (11.4) | 0.026 |
| Antibiotics | 145 (82.4) | 404 (90.0) | 0.013 |
| Inflammatory markers | |||
| CRP | 2.08 ± 3.76 | 4.64 ± 5.75 | 0.000 |
| Home noninvasive ventilation, n (%) | 18 (10.2) | 43 (9.6) | 0.923 |
| Comorbidities, n (%) | |||
| Neuromuscular disease | 25 (14.2) | 32 (7.1) | 0.009 |
| Ischemic heart disease | 35 (19.9) | 92 (20.5) | 0.954 |
| Cancer | 8.0 (14) | 30 (6.7) | 0.705 |
| ESRD | 0 (0) | 3 (0.6) | 0.370 |
| Hypertension | 82 (46.6) | 208 (46.3) | 0.715 |
| Diabetes mellitus | 28 (15.9) | 65 (14.5) | 0.743 |
| Liver cirrhosis | 3 (1.7) | 3 (0.6) | 0.238 |
| Autoimmune disease | 1 (0.6) | 9 (2.0) | 0.327 |
| Pulmonary function tests | |||
| FVC (L) | 2.14 ± 0.77 | 1.94 ± 0.65 | 0.002 |
| FVC % predicted | 75.66 ± 27.52 | 73.16 ± 22.76 | 0.285 |
| FEV1(L) | 1.07 ± 0.43 | 1.02 ± 0.42 | 0.247 |
| FEV1 % predicted | 47.20 ± 17.70 | 48.55 ± 17.69 | 0.393 |
| FEV1/FVC % | 50.90 ± 12.70 | 53.12 ± 13.02 | 0.054 |
| Bronchodilator response, n (%) | 80 (45.5) | 170 (37.9) | 0.099 |
| Inhaled medications before admission, n (%) | |||
| LAMA | 13 (7.4) | 32 (7.1) | 0.910 |
| LAMA + LABA | 4 (2.3) | 13 (2.9) | 0.667 |
| ICS + LABA | 43 (24.4) | 112 (24.9) | 0.894 |
| LAMA + LABA + ICS | 68 (38.6) | 155 (34.5) | 0.334 |
Table 2 shows the comparison of the length of hospital stay, systemic steroid use, histories of admissions and readmissions, and hospital outcomes. We found that the mean prednisolone equivalent was 302.65 mg in the EOS group, and it was significantly lower than that of the non-EOS group (373.35 mg) (P = 0.021). There was no significant difference between the groups regarding major treatments including antibiotics and steroids (P = 0.095). The mean number of COPD-related readmissions within 12 mo was higher in the EOS group than in the non-EOS group (2.14 vs 1.53, P = 0.002). The EOS group had a higher proportion of patients with at least one COPD-related readmission within 12 mo than the non-EOS group (71.0% vs 55.9%, P = 0.001). The number of admissions in the previous 12 mo was also higher in the EOS group compared with the non-EOS group (2.05 vs 1.44, P = 0.004). In the EOS group, the mean length of hospital stay was 8.81 d. It was significantly shorter than that of the non-EOS group (10.00 d) (P = 0.049). No significant differences were observed in discharge outcomes evaluated by home discharge, RCW discharge, and death between the two groups (P = 0.204).
| Variables | EOS group (n = 176) | Non-EOS group (n = 449) | P value |
| Length of stay (d) | 8.81 ± 5.95 | 10.00 ± 7.12 | 0.049 |
| Prednisolone equivalent, mg | 302.65 ± 341.35 | 373.35 ± 409.25 | 0.021 |
| Major treatment (%) | 0.095 | ||
| None | 4 (2.3) | 6 (1.3) | |
| Antibiotics only | 25 (14.2) | 36 (8) | |
| Steroid only | 27 (15.3) | 75 (16.7) | |
| Antibiotics + steroid | 176 (68.2) | 332 (73.9) | |
| Hospitalization before admission within 12 mo, n (%) | 111 (63.1) | 242 (53.9) | 0.047 |
| No. of hospitalizations within 12 mo before admission | 2.05 ± 2.41 | 1.44 ± 2.12 | 0.004 |
| Hospitalizations after admission within 12 mo, n (%) | 125 (71.0) | 251 (55.9) | 0.001 |
| First readmission (days after discharge) | 83.13 ± 90.01 | 73.85 ± 77.13 | 0.301 |
| No. of readmissions within 12 mo | 2.14 ± 2.34 | 1.53 ± 2.14 | 0.002 |
| Discharge outcome, n (%) | 0.204 | ||
| Home discharge | 176 (100.0) | 441 (98.2) | |
| RCW | 0 (0) | 4 (0.9) | |
| Death | 0 (0) | 4 (0.9) |
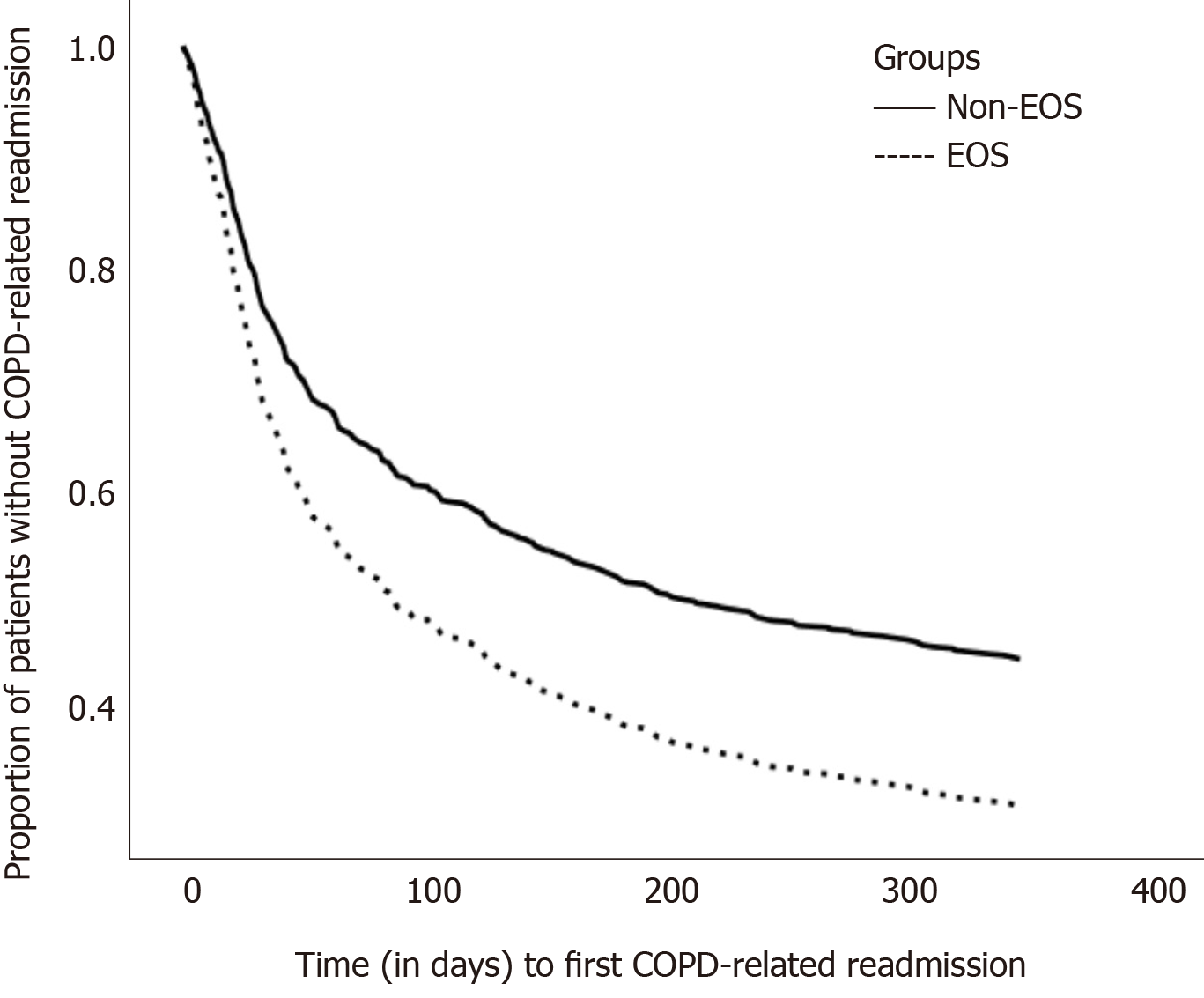
Table 3 integrates the risk factors for first COPD-related readmission by the Cox regression model. After adjustment, phenotype (EOS vs non-EOS), the percent-predicted value of FEV1, and age were significant risk factors. The adjusted hazard ratio (adj. HR) of the EOS group versus the non-EOS group was 1.488 [95% confidence interval (CI): 1.195-1.854, P < 0.001]. Higher percent-predicted value of FEV1 was associated with reduced risk of first COPD-related readmission (adj. HR = 0.985, 95%CI = 0.979-0.991, P < 0.001). Time to first COPD-related readmission of the EOS group and the non-EOS group is plotted in Figure 1.
| Variables | Hazard ratio (adjusted) | 95%CI: Lower–upper limit | P value |
| Group (EOS vs non-EOS) | 1.488 | 1.195-1.854 | < 0.001 |
| FEV1 % predicted value | 0.985 | 0.979-0.991 | < 0.001 |
| Age | 1.010 | 1.000-1.020 | 0.049 |
| BMI | 0.985 | 0.961-1.009 | 0.208 |
| Gender (male vs female) | 0.951 | 0.692-1.305 | 0.754 |
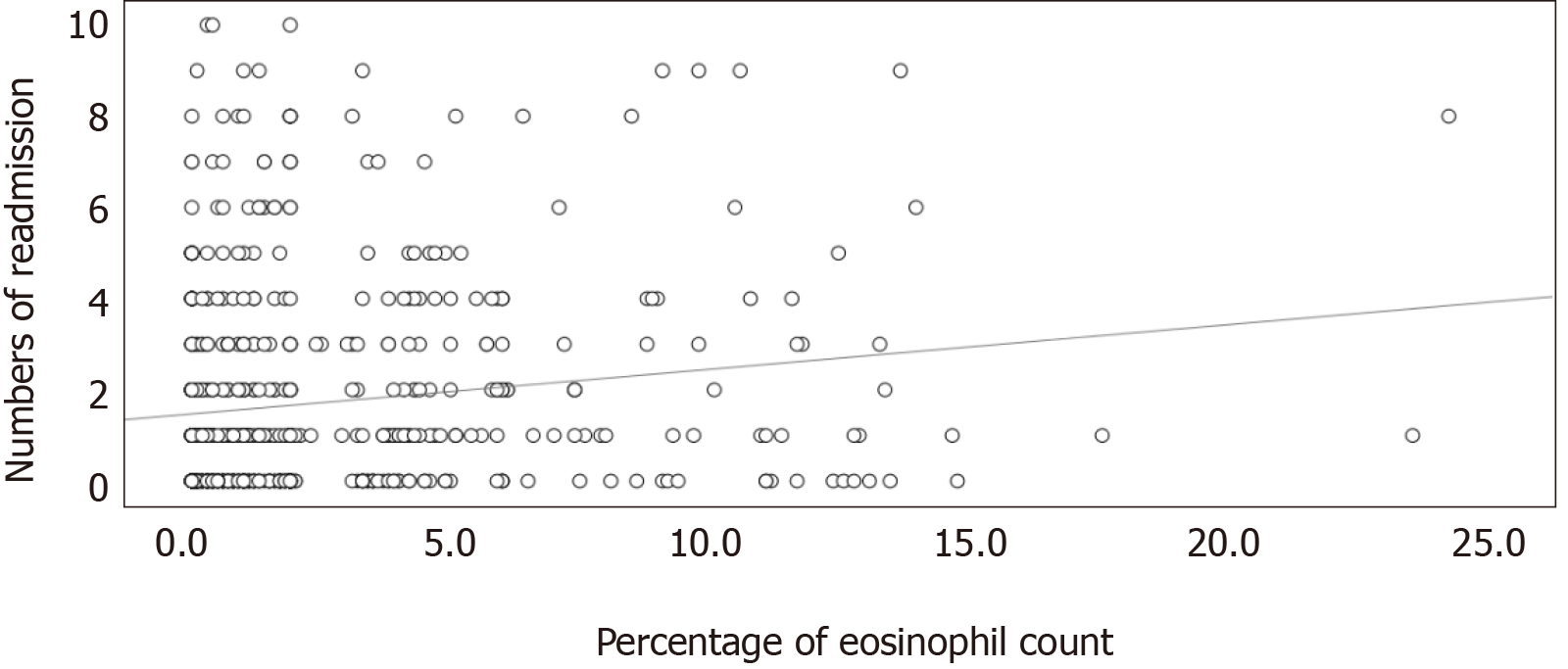
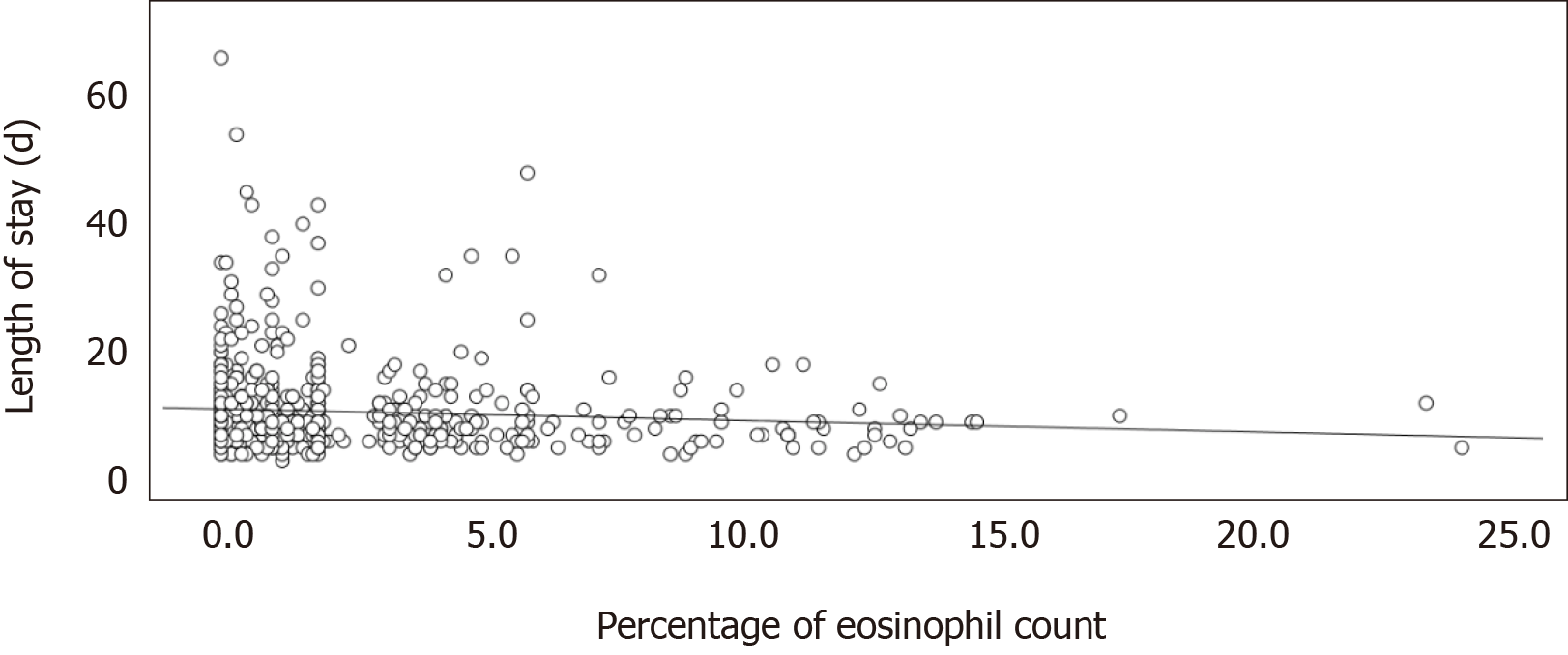
There was a significantly mild linear correlation between the percentage of eosinophils and number of readmissions within 12 mo (Pearson's r = 0.147; P < 0.001) (Figure 2). The linear relationship between the percentage of eosinophils and the length of hospital stay in the index hospitalization showed a significantly mild correlation (Pearson's r = -0.086; P = 0.031) (Figure 3).
In the present study, we comprehensively evaluated the role of eosinophils in COPD exacerbation. We found that the EOS group (PBEC ≥ 2%) was associated with a shorter length of hospital stay, a lower dose of steroids, a shorter time to first COPD-related readmission, a higher number of COPD-related readmissions, and a tendency of non-infectious inflammation. Although most findings were consistent with previous literature, there are several novel findings in this study: (1) This was the first study in Taiwan; and (2) The PBEC had a significantly linear correlation with the number of readmissions and length of hospital stay.
Our findings supported the results of previous studies on the tendency of non-infectious inflammation in the EOS group (PBEC ≥ 2%). The hemogram and antibiotic administration in our study are consistent with those of a previous Chinese study by Xue et al[15]. Duman et al[9] reported that the non-EOS group (PBEC ≤ 2%) had higher NLR and CRP levels in a the Turkish population. Saltürk et al[16] reported similar results for hemogram and CRP levels in the non-EOS group (PBEC ≤ 2%) in the intensive care unit (ICU) population.
Our study revealed that the EOS group (PBEC ≥ 2%) showed higher absolute values of FVC. Similarly, Singh reported that the EOS group (PBEC ≥ 2%) was characterized by higher absolute values of FEV1 and FVC[17]. Kang et al[13] also observed that the EOS group (PBEC > 2%) had higher absolute values of FEV1 and FVC in the Korean population. The aforementioned findings suggest that patients with eosinophilic COPD exacerbation have better lung function. However, a meta-analysis by Wu et al[14] revealed that the percentage of predicted FEV1 value showed no significant difference between the EOS (PBEC > 2%) and non-EOS groups (PBEC ≤ 2%).
In the present study, the EOS group (PBEC ≥ 2%) showed a shorter time to first COPD-related readmission. Previous studies that defined the EOS group by 2% eosinophilia or ≥ 200 cells/μL in the Caucasian[8,10] and Asian races[15] showed results similar to those of our study. Couillard et al[8] stated that the EOS group (PBEC ≥ 200 cells/μL and/or ≥ 2%) had a higher risk of COPD-related readmissions within 12 mo and shorter time to the first COPD-related readmission within 12 mo. Bélanger et al[10] reported that in infrequent exacerbations (defined as the first exacerbation in previous 5 years), the EOS group (PBEC ≥ 200 cells/μL and/or ≥ 2%) had a higher risk of COPD-related readmissions and shorter time to the first COPD-related readmission. In Asian races, Xue et al[15] revealed that the EOS group (PBEC ≥ 2%) had a higher risk of severe exacerbation.
Eosinophilic COPD exacerbation is a well-known risk factor for COPD-related readmissions. The current study demonstrated a significant linear correlation between the percentage of blood eosinophil and the number of readmissions. Although our finding was intuitively reasonable, we believe it is novel in the current literature.
Among all hospitalized patients with acute COPD exacerbation, the EOS group (PBEC ≥ 2%) required a lower systemic steroid dose compared to the non-EOS group (PBEC < 2%) in the present study, consistent with previous retrospective studies[9,11]. Serafino-Agrusa et al[11] showed that a lower dose of daily systemic steroids was administered in the EOS group (≥ 2%) than in the non-EOS group (PBEC < 2%). Duman et al[9] revealed that a lower proportion of the EOS group (PBEC > 2%) received systemic steroids compared to the non-EOS group (PBEC ≤ 2%).
To the best of our knowledge, only two prospective studies addressed the role of eosinophil on systemic steroids. In a prospective study enrolling outpatients, Bafadhel et al[5] reported that eosinophil-guided therapy (cut-off value: PBEC = 2%) could decrease the proportion of patients receiving steroids (51% vs 100%) compared to the standard treatment, and steroid treatment in the non-EOS group is associated with a poorer health status and higher treatment failure rate (15% vs 2%) compared to placebo. In a prospective study enrolling inpatients by Sivapalan et al[6], eosinophil-guided therapy (cut-off value: Absolute eosinophil count = 300 cells /μL) reduced the duration of steroid treatment (2 d vs 5 d), but there were no differences in the 30-d treatment failure rate (26% vs 26%) and 30-d survival rate (94% vs 96%) compared to the standard treatment.
In real-world practice, physicians in charge adjusted the steroid dose according to the clinical response (i.e., reduced the steroid dose according to the relief of breathlessness). Because eosinophilic COPD exacerbation has a better clinical response to systemic steroids, the EOS group required a lower systemic steroid dose than the non-EOS group in the present study.
The length of hospital stay was shorter in the EOS group (PBEC ≥ 2%) in our study. Many retrospective studies enrolled patients with different in-hospital treatments, such as antibiotics and steroid use, for acute COPD exacerbation. Their findings related to the length of hospital stay are similar and consistent with those of our study[9,11,18]. Duman et al[9] reported that the EOS group (PBEC > 2%) had a shorter length of stay than the non-EOS group (PBEC ≤ 2%). In a study by Serafino-Agrusa et al[11], the EOS group (PBEC ≥ 2%) had a shorter length of stay compared to the non-EOS group (PBEC < 2%). Bafadhel et al[18] revealed that the length of stay was shorter in the EOS group (PBEC ≥ 200 cells/μL and/or ≥ 2%) than in the non-EOS group.
Furthermore, Xue et al[15] pointed out that the EOS group (PBEC ≥ 2%) showed a better steroid response after evaluation with the COPD assessment test (CAT) than the non-EOS group (PBEC < 2%). Shorter lengths of hospital stay, and better CAT responses are probably due to the fact that the use of steroids had a rationale only in the EOS group.
We found no difference in the discharge outcomes between the EOS (PBEC ≥ 2%) and the non-EOS groups (PBEC < 2%) in this study. Because eosinophilia is a risk factor for COPD-related readmissions, we could reasonably infer that eosinophilic COPD exacerbation has higher risks of mortality and morbidity. However, previous studies showed better mortality and morbidity in eosinophilic COPD exacerbation[12,13,16]. Saltürk et al[16] stated that the EOS group (PBEC > 2%) had a shorter median length of ICU stay and lower ICU mortality compared to the non-EOS group. Kang et al[13] showed that the EOS group (PBEC > 2%) had lower rate of ICU admissions and lower mortality rate. Mendy et al[12] reported that after a median follow-up of 3 years, the non-EOS group (PBEC < 2%) was a predictor of long-term COPD mortality.
Patients with long-term oral steroid use imply poor COPD control. In our study, we excluded these patients, and therefore, discharge outcomes may be similar. Additionally, eosinophils play an essential role in innate and adaptive immune response and takes part in the defense against various pathogens, including virus, bacteria, etc[19]. Eosinopenia is associated with sepsis[20]. Eosinophilic COPD exacerbation had a lower risk of pneumonia[21]. These anti-infectious capacities of eosinophil may lead to better mortality and morbidity.
This was a single-center study in Taiwan involving only the Asian race and a relatively small sample size. Moreover, the retrospective design of the study had intrinsic limitations.
Although eosinophilic COPD exacerbation COPD shows better systemic steroid responses, the precise mechanism is unknown. Systemic steroids can reduce the blood eosinophil count by > 50% within the first four hours after administration[22]; thus, the anti-eosinophil capacity of steroids could partially explain this phenomenon.
The linear correlation between the PBEC and the number of readmissions and length of hospital stay is weak. Further studies are required to clarify the relationship.
The EOS group (PBEC ≥ 2%) was associated with a higher number of COPD-related readmissions within 12 mo, shorter time to the first COPD-related readmission, lower dose of systemic steroid use, and shorter length of hospital stay compared to the non-EOS group. Low percent-predicted FEV1 values were observed to be a risk factor for the first COPD-related readmission.
We should strengthen the management of comorbidities and optimization of inhaled medications to reduce the high readmission risk in the EOS group. Routine survey of the PBEC for acute COPD exacerbation is warranted to reduce the side effects of steroids. With meticulous exclusion of possible infections, we could avoid empirical antibiotic therapy since the EOS group has a non-infectious nature.
The eosinophilic phenotype accounts for 20%-40% of chronic obstructive pulmonary disease (COPD) exacerbations. The peripheral blood eosinophil count (PBEC) is a well-established predictor of the length of hospital stay, steroid response, prognosis, and readmission rate. Additionally, previous research supported the tendency of non-infectious inflammation in the eosinophilic group.
Some characteristics of patients with eosinophilic COPD exacerbation, such as demographics, comorbidities, lung function, etc., are inconsistent across studies. Studies on the role of eosinophilia primarily focused on the Caucasian race. A few studies investigated eosinophilic COPD exacerbation in Asian populations.
We aimed to study the role of peripheral eosinophilia in hospitalized patients with COPD exacerbation in Taiwan.
From January 2014 to May 2017, patients with COPD exacerbation hospitalized in Taipei Tzu Chi Hospital were retrospectively stratified into two groups according to their peripheral eosinophil count: The EOS group (eosinophil count ≥ 2%) and the non-EOS group (eosinophil count < 2%).
A total of 625 patients were recruited, with 176 patients (28.2%) in the eosinophilic group. The eosinophilic group showed a lower prevalence of infection, lower cumulative doses of prednisolone equivalents, shorter length of hospital stay, and higher number of COPD-related readmissions than the non-EOS group. There were significantly linear correlations between eosinophil percentage and number of readmissions and between eosinophil percentage and length of hospital stay (P < 0.001, Pearson's r = 0.147; P = 0.031, Pearson's r = -0.086, respectively).
The PBEC had a positive linear correlation with the number of readmissions. Eosinophilia is a predictor of steroid response and non-infectious inflammation.
We should strengthen the management of comorbidities and optimization of inhaled medications to reduce the high readmission risk in the EOS group. Routine survey of PBEC for acute COPD exacerbation is warranted to reduce the notorious side effects of steroids. With meticulous exclusion of possible infections, we could avoid empirical antibiotic therapy since the EOS group has a non-infectious nature. Effective target therapy for eosinophilic COPD exacerbation is urgently required.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rouabhia M, Soriano-Ursúa MA S-Editor: Dou Y L-Editor: A E-Editor: Wang LL
| 1. | Global Initiative for Chronic Obstructive Lung Disease. Gold Reports for Personal Use 2020 GOLD Report. Available from: https://goldcopd.org/gold-reports/. |
| 2. | Walters JA, Tan DJ, White CJ, Gibson PG, Wood-Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;CD001288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, Lindblad K, Patel H, Rugman P, Dodson P, Jenkins M, Saunders M, Newbold P, Green RH, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 790] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 4. | Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S, Lugogo N, Martinot JB, Sagara H, Albers FC, Bradford ES, Harris SS, Mayer B, Rubin DB, Yancey SW, Sciurba FC. Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease. N Engl J Med. 2017;377:1613-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 421] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 5. | Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 469] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 6. | Sivapalan P, Lapperre TS, Janner J, Laub RR, Moberg M, Bech CS, Eklöf J, Holm FS, Armbruster K, Sivapalan P, Mosbech C, Ali AKM, Seersholm N, Wilcke JT, Brøndum E, Sonne TP, Rønholt F, Andreassen HF, Ulrik CS, Vestbo J, Jensen JS. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicentre, randomised, controlled, open-label, non-inferiority trial. Lancet Respir Med. 2019;7:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 7. | Camp J, Cane JL, Bafadhel M. Shall We Focus on the Eosinophil to Guide Treatment with Systemic Corticosteroids during Acute Exacerbations of COPD?: PRO. Med Sci (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Couillard S, Larivée P, Courteau J, Vanasse A. Eosinophils in COPD Exacerbations Are Associated With Increased Readmissions. Chest. 2017;151:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 9. | Duman D, Aksoy E, Agca MC, Kocak ND, Ozmen I, Akturk UA, Gungor S, Tepetam FM, Eroglu SA, Oztas S, Karakurt Z. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. 2015;10:2469-2478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Bélanger M, Couillard S, Courteau J, Larivée P, Poder TG, Carrier N, Girard K, Vézina FA, Vanasse A. Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization. Int J Chron Obstruct Pulmon Dis. 2018;13:3045-3054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Serafino-Agrusa L, Scichilone N, Spatafora M, Battaglia S. Blood eosinophils and treatment response in hospitalized exacerbations of chronic obstructive pulmonary disease: A case-control study. Pulm Pharmacol Ther. 2016;37:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Mendy A, Forno E, Niyonsenga T, Gasana J. Blood biomarkers as predictors of long-term mortality in COPD. Clin Respir J. 2018;12:1891-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Kang HS, Rhee CK, Kim SK, Kim JW, Lee SH, Yoon HK, Ahn JH, Kim YH. Comparison of the clinical characteristics and treatment outcomes of patients requiring hospital admission to treat eosinophilic and neutrophilic exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2467-2473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Wu HX, Zhuo KQ, Cheng DY. Prevalence and Baseline Clinical Characteristics of Eosinophilic Chronic Obstructive Pulmonary Disease: A Meta-Analysis and Systematic Review. Front Med (Lausanne). 2019;6:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Xue J, Cui YN, Chen P, Cai S, Chen L, Dai ZS, Chen Y. [Blood eosinophils: a biomarker of response to glucocorticoids and increased readmissions in severe hospitalized exacerbations of COPD]. Zhonghua Jie He He Hu Xi Za Zhi. 2019;42:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 16. | Saltürk C, Karakurt Z, Adiguzel N, Kargin F, Sari R, Celik ME, Takir HB, Tuncay E, Sogukpinar O, Ciftaslan N, Mocin O, Gungor G, Oztas S. Does eosinophilic COPD exacerbation have a better patient outcome than non-eosinophilic in the intensive care unit? Int J Chron Obstruct Pulmon Dis. 2015;10:1837-1846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R; ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44:1697-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 18. | Bafadhel M, Greening NJ, Harvey-Dunstan TC, Williams JE, Morgan MD, Brightling CE, Hussain SF, Pavord ID, Singh SJ, Steiner MC. Blood Eosinophils and Outcomes in Severe Hospitalized Exacerbations of COPD. Chest. 2016;150:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 19. | Ravin KA, Loy M. The Eosinophil in Infection. Clin Rev Allergy Immunol. 2016;50:214-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 20. | Shaaban H, Daniel S, Sison R, Slim J, Perez G. Eosinopenia: Is it a good marker of sepsis in comparison to procalcitonin and C-reactive protein levels for patients admitted to a critical care unit in an urban hospital? J Crit Care. 2010;25:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Pavord ID, Lettis S, Anzueto A, Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med. 2016;4:731-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 22. | Thorn GW, Renold AE, Wilson DL, Frawley TF, Jenkins D, Garcia-Reyes J, Forsham PH. Clinical studies on the activity of orally administered cortisone. N Engl J Med. 1951;245:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.3] [Reference Citation Analysis (0)] |