Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2510
Peer-review started: February 24, 2020
First decision: March 24, 2020
Revised: April 9, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: June 26, 2020
Processing time: 121 Days and 2.4 Hours
The diagnostic specificity of conventional ultrasound for breast non-mass lesions (NMLs) is low at approximately 21%-43%. Shear wave elastography (SWE) can distinguish benign from malignant lesions by evaluating the internal and peripheral stiffness. SWE has good reproducibility and high diagnostic efficacy. However, there are very few independent studies on the diagnostic value of SWE in breast NMLs.
To determine the value of SWE in the differential diagnosis of breast NMLs.
This study enrolled a total of 118 patients with breast NMLs who underwent SWE examinations in the Beijing Shijitan Hospital Affiliated to Capital Medical University and The Second Hospital of Shandong University from January 2019 to January 2020. The internal elastic parameters of the lesions were recorded, including maximum (Emax), mean (Emean) and minimum elastic values and the standard deviation. The following peripheral parameters were noted: Presence of a “stiff rim” sign; Emax, and Emean elasticity values within 1 mm, 1.5 mm, 2 mm, 2.5 mm and 3 mm from the edge of NMLs. The receiver operating characteristic curve of each parameter was drawn, and the areas under the curve were calculated.
Emax, Emean and elastic values, and the standard deviation of the internal elastic values in malignant NMLs were significantly higher than those in benign NMLs (P < 0.05). The percentage with the “stiff rim” sign in malignant NMLs was significantly higher than that in the benign group (P < 0.05), and Emax and Emean at the shell of 1 mm, 1.5 mm, 2 mm, 2.5 mm and 3 mm in the malignant group were all higher than those in the benign group (P < 0.05). Of the surrounding elasticity values, Emax of the shell at 2.5 mm in malignant NMLs had maximum areas under the curve of 0.900, and the corresponding sensitivity and specificity were 94.57% and 85.86%, respectively.
The “stiff rim” sign and multiple quantitative elastic values within and around the lesion had good diagnostic performance in the differential diagnosis of breast NMLs. Emax in peripheral tissue had better diagnostic efficiency than other parameters.
Core tip: This study is the first to qualitatively and quantitatively analyze the stiffness in and around breast non-mass lesions. We found that the evaluation of stiffness around the breast non-mass lesions had a better differential diagnostic reference value.
- Citation: Xu P, Wu M, Yang M, Xiao J, Ruan ZM, Wu LY. Evaluation of internal and shell stiffness in the differential diagnosis of breast non-mass lesions by shear wave elastography. World J Clin Cases 2020; 8(12): 2510-2519
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2510.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2510
Breast cancer is the most common malignant tumor in women and one of the most common causes of death[1]. Timely and accurate diagnosis is crucial for treatment and prognosis[2]. Breast lesions can be classified as mass lesions and non-mass lesions (NMLs)[3]. NMLs are characterized by no clear boundary and no space occupying effect in two or more different scanning sections on gray-scale ultrasonography. The diagnostic specificity of conventional ultrasound is low at approximately 21%-43%[4-6]. Shear wave elastography (SWE) can distinguish benign lesions from malignant lesions by evaluating the stiffness of breast masses, SWE has good reproducibility[7] and high diagnostic efficacy[8-10]. However, there are very few independent studies on the diagnostic value of SWE in breast NMLs[5,11]. Studies have shown that the hardest areas of malignant lesions were located in the periphery rather than the interior of breast lesions, which means that elasticity analysis of the periphery may have good diagnostic value[12]. Qualitative and quantitative analysis of elasticity in the surrounding tissues of NMLs was performed in our study innovatively[13]. In the present study, 118 patients with NMLs underwent SWE examination to analyze the elasticity characteristics and their clinical diagnostic values.
From January 2019 to January 2020, 118 patients with breast NMLs were enrolled in this study in Beijing Shijitan Hospital and The Second Hospital of Shandong University. All patients were female, aged 24 to 68 years. All patients who underwent breast NMLs biopsy or surgery signed an informed consent.
Inclusion criteria: (1) Conventional ultrasonography shows the characteristics of NMLs[3]; (2) Diagnosis was confirmed by pathological results; and (3) Conventional ultrasonography and SWE examination were performed before surgery.
Exclusion criteria: (1) Having accepted radiotherapy or endocrine therapy before examination; (2) Surgical scar within the lesion area; (3) Cystic or cystic-solid mixed mass[14]; (4) Lesion maximum diameter > 4.0 cm; and (5) Massive calcification within the lesion area.
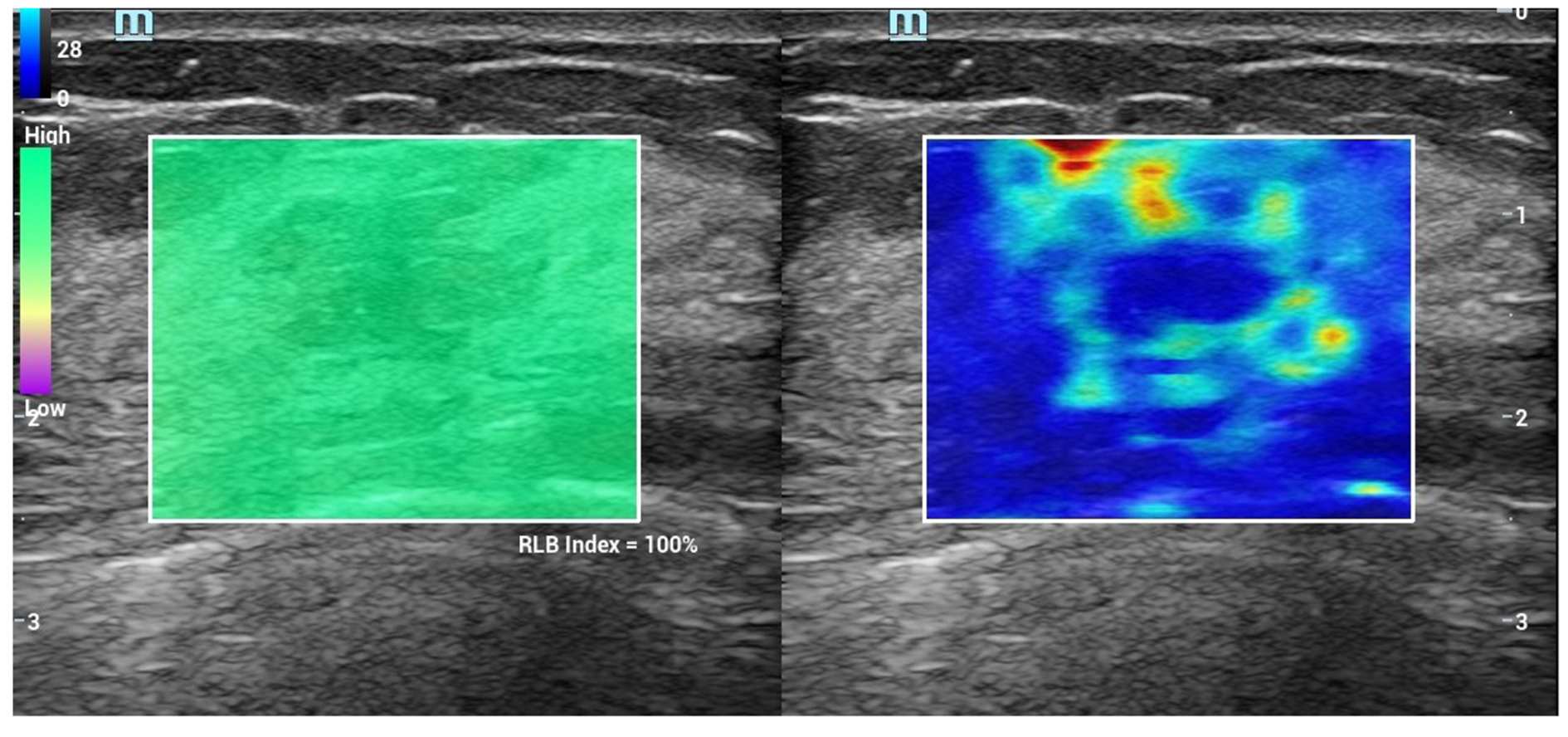
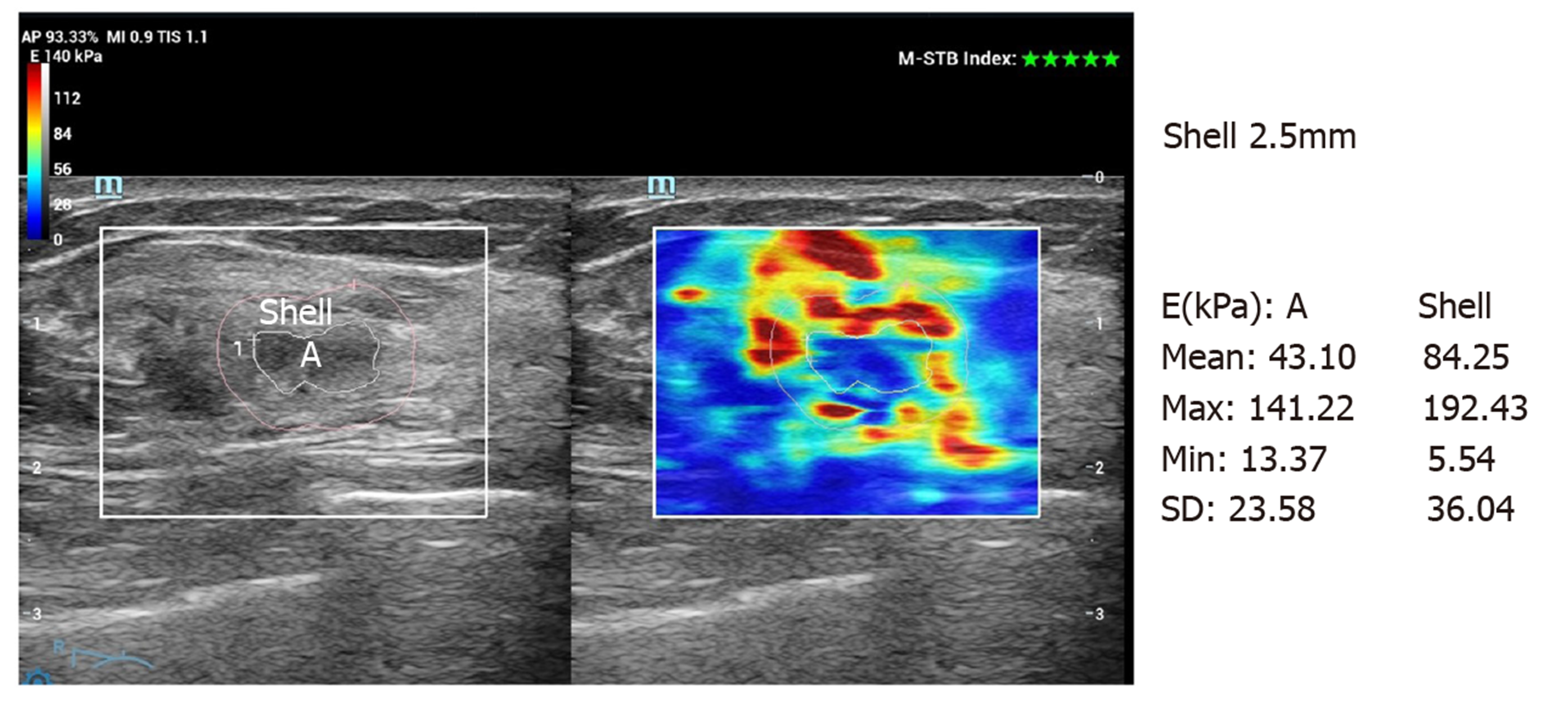
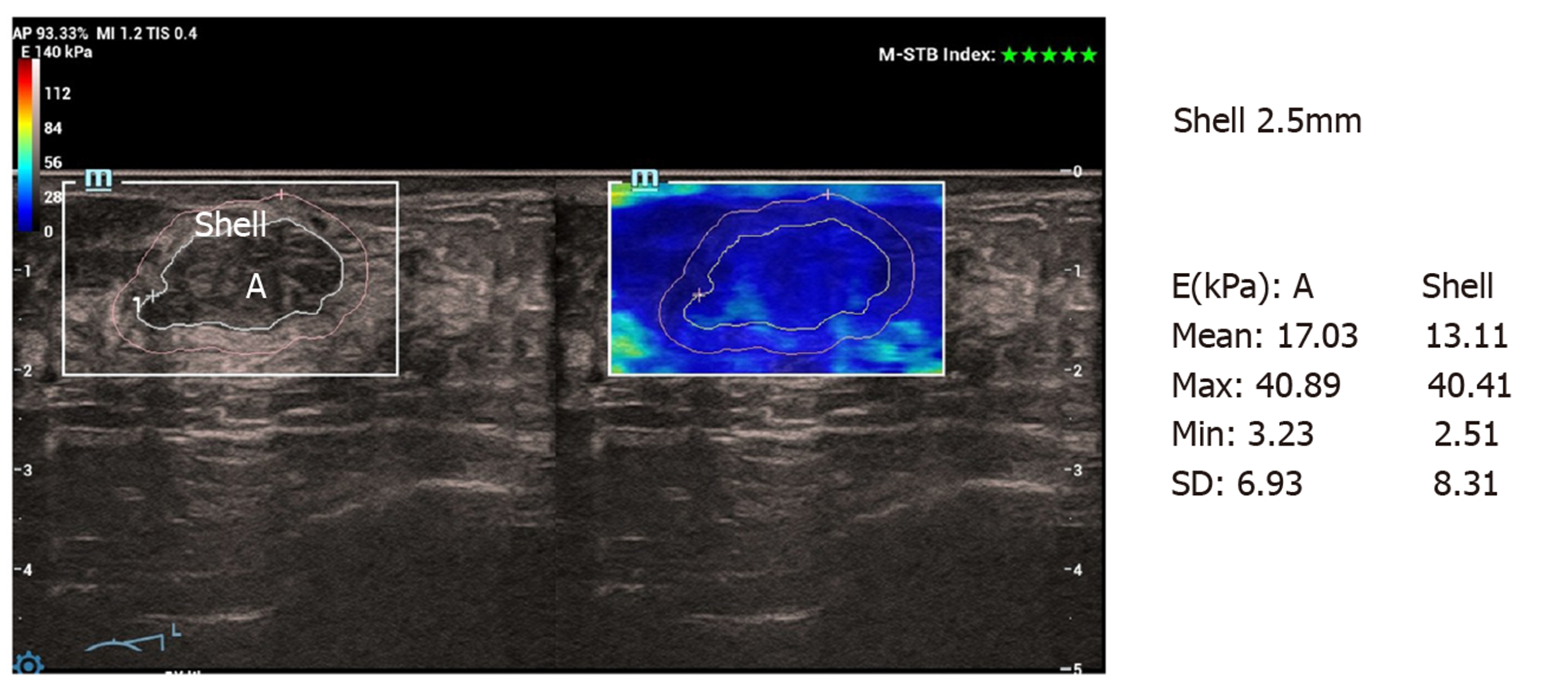
Conventional ultrasound and SWE examination were performed using Ultrasonic System Resona 7 (Mindray, Shenzhen, China), with a line array transducer (L14-5W), frequency 4.0-14.0 MHz. Examination method: Conventional ultrasound examination of the breast was performed first, and then switched to SWE mode. The section with the largest lesion was selected, and the lesion was placed in the center of the sampling box. SWE mass-velocity dual dynamic pattern was used to perform the examination. Patients were asked to hold their breath. A good quality “stiff rim” sign was observed, which showed that the mass diagram on the left of the screen had a homogeneous green background without an obvious pseudo-image in purple[15] (Figure 1). In the right image on the screen, red indicated “hard tissue” and blue indicated “soft tissue”. The lesion with a red or orange ring was seen as the “stiff rim” sign, indicating that the lesion was malignant. The lesion without a “stiff rim” sign was thought to be benign. Tracing the outer edge of the lesion on gray-scale image, the internal elastic parameters, including maximum (Emax), mean (Emean), minimum and standard deviation (Esd) of NMLs were calculated automatically in gray-scale-velocity dual pattern. Then, Emax and Emean elasticity values at shells of 1 mm, 1.5 mm, 2 mm, 2.5 mm and 3 mm from the edge of NMLs were measured, respectively (Figure 2 and 3). All images and data were recorded on the internal hard disk of the ultrasonic system. All examinations were performed by two radiologists both with more than 10 years of experience on breast diagnoses. They analyzed the ultrasonography independently. If their opinions differed, an agreement was reached by discussion.
Pathological results were the gold standard. SPSS22.0 software was used for statistical analysis. Quantitative parameters of SWE are shown as mean ± standard deviation and comparisons between groups were evaluated using the t test. Qualitative parameters are shown as frequency or percentage and comparisons between groups were evaluated using the χ2 test. A receiver operating characteristic (ROC) curve was plotted and then the areas under the curve, Youden indices (sensitivity + specificity -1), cutoff values and the corresponding sensitivity, specificity and accuracy of each parameter were calculated. P < 0.05 was considered statistically significant.
There were no significant differences in age, maximum diameter, location, amenorrhea and history of lactation between the two groups (Table 1).
| Group | Age (yr) | Maximum diameter (cm) | Location | Amenorrhea | Lactation history | |||
| Left | Right | Yes | No | Yes | No | |||
| Benign (n = 66) | 49.70 ± 10.38 | 2.10 ± 1.52 | 38 | 28 | 17 | 49 | 55 | 11 |
| Malignant (n = 52) | 50.56 ± 9.27 | 2.38 ± 1.64 | 27 | 25 | 13 | 39 | 44 | 8 |
| t/χ2 value | -0.205 | -0.959 | 0.376 | 0.009 | 0.035 | |||
| P value | 0.419 | 0.339 | 0.540 | 0.925 | 0.851 | |||
Of the 118 patients, 66 were diagnosed with benign lesions and 52 with malignant lesions. There were 30 cases of intraductal carcinoma in situ, 19 cases of invasive ductal carcinoma, 2 cases of invasive lobular carcinoma and 1 case of lymphoma in the malignant group. There were 32 cases of adenosis, 9 cases of intraductal papilloma, 8 cases of breast inflammation, 7 cases of atypical ductal hyperplasia, 6 cases of fibroadenoma and 4 cases of sclerosing adenosis in the benign group.
Comparison of internal elasticity parameters in benign and malignant NMLs: Emax, Emean and Esd in malignant NMLs were significantly higher than those in benign lesions (P < 0.05), and there was no significant difference in minimum value between the two groups (Table 2).
| Internal parameter | Benign (n = 66) | Malignant (n = 52) | t value | P value |
| Emax | 42.43 ± 20.62 | 97.95 ± 23.56 | -13.634 | 0.000 |
| Emean | 18.34 ± 7.16 | 32.62 ± 14.98 | -6.823 | 0.000 |
| Emin | 4.26 ± 2.05 | 5.00 ± 2.47 | -1.778 | 0.078 |
| Esd | 6.34 ± 3.48 | 15.39 ± 4.17 | -12.848 | 0.000 |
Comparison of elasticity parameters around NMLs: The percentage of “stiff rim” signs in malignant NMLs was significantly higher than that in benign lesions (P < 0.05). Emax and Emean of the shell at 1 mm, 1.5 mm, 2 mm, 2.5 mm and 3 mm in the malignant group were also higher than those in the benign group (P < 0.05) (Table 3).
| Peripheral parameter | Benign (n = 66) | Malignant (n = 52) | t/χ2 value | P value | |
| “Stiff rim” sign | 6 | 26 | 24.628 | 0.000 | |
| Shell at 1 mm | E1 max | 45.53 ± 22.81 | 125.41 ± 35.68 | -14.765 | 0.000 |
| E1 mean | 24.94 ± 7.92 | 45.49 ± 17.71 | -8.425 | 0.000 | |
| Shell at 1.5 mm | E1.5 max | 46.61 ± 22.39 | 136.40 ± 38.39 | -15.889 | 0.000 |
| E1.5 mean | 25.36 ± 8.52 | 49.64 ± 18.98 | -9.281 | 0.000 | |
| Shell at 2 mm | E2 max | 50.43 ± 24.62 | 153.95 ± 35.56 | -18.655 | 0.000 |
| E2 mean | 26.34 ± 7.16 | 54.62 ± 17.98 | -11.668 | 0.000 | |
| Shell at 2.5 mm | E2.5 max | 48.43 ± 25.62 | 167.95 ± 37.56 | -20.506 | 0.000 |
| E2.5 mean | 29.34 ± 7.16 | 69.62 ± 19.98 | -15.200 | 0.000 | |
| Shell at 3 mm | E3 max | 42.43 ± 20.62 | 153.95 ± 23.56 | -27.386 | 0.000 |
| E3 mean | 26.34 ± 7.16 | 58.62 ± 14.98 | -15.424 | 0.000 | |
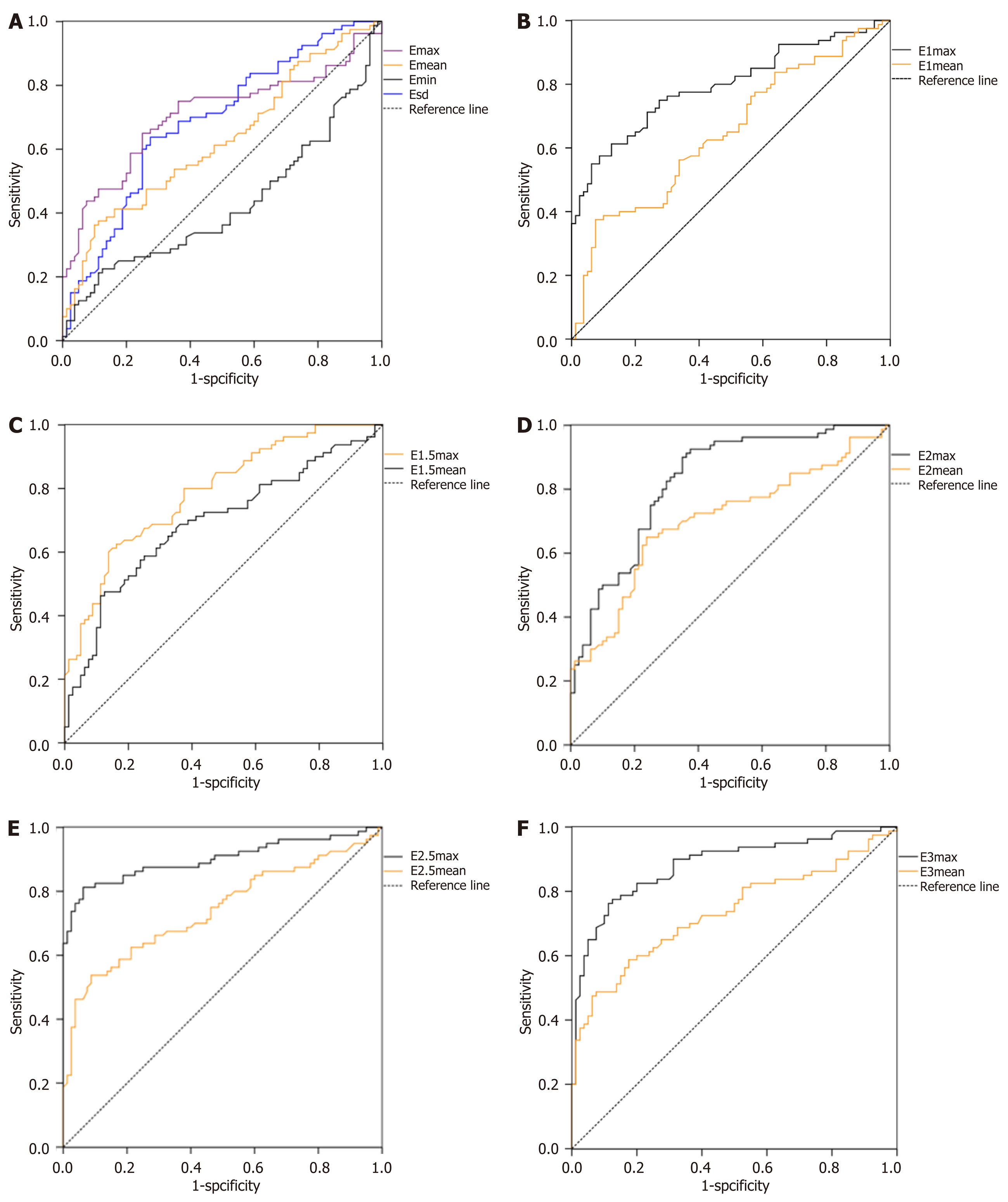
ROC curves of each parameter and corresponding sensitivity, specificity, accuracy, cutoff value and the area under the curve of each parameter: The ROC curve of each parameter are shown in Figure 4. The sensitivity, specificity, accuracy, cutoff value and the areas under the curve (AUC) are presented in Table 4. Emax at the shell of 2.5 mm in NMLs had the maximum AUC of 0.900, and the corresponding sensitivity, specificity accuracy and cutoff value were 94.57%, 85.86%, 87.44% and 94.62 kPa, respectively.
| Parameter | Sensitivity (%) | Specificity (%) | Accuracy (%) | Cutoff value | AUC |
| “Stiff rim” sign | 50.00 | 90.91 | 72.88 | - | - |
| Emax | 74.33 | 66.80 | 67.05 | 67.61 | 0.707 |
| Emean | 54.95 | 68.47 | 59.86 | 28.05 | 0.626 |
| Emin | 36.15 | 49.60 | 43.64 | 4.96 | 0.425 |
| Esd | 70.18 | 63.67 | 68.93 | 11.30 | 0.687 |
| E1 max | 83.61 | 75.01 | 75.04 | 84.65 | 0.795 |
| E1 mean | 68.29 | 57.94 | 63.69 | 33.08 | 0.647 |
| E1.5 max | 83.00 | 74.18 | 77.00 | 95.60 | 0.789 |
| E1.5 mean | 74.48 | 63.95 | 65.24 | 36.24 | 0.692 |
| E2 max | 86.36 | 76.04 | 80.06 | 100.50 | 0.826 |
| E2 mean | 75.53 | 66.11 | 68.51 | 33.97 | 0.704 |
| E2.5 max | 94.57 | 85.86 | 87.44 | 94.62 | 0.900 |
| E2.5 mean | 79.05 | 70.04 | 80.51 | 36.17 | 0.746 |
| E3 max | 90.52 | 81.83 | 84.03 | 88.56 | 0.881 |
| E3 mean | 76.82 | 68.11 | 76.86 | 35.48 | 0.734 |
Previous research reported that both benign and malignant lesions could present as NMLs[3,16,17], accounting for approximately 9.2% of all breast lesions[18]. The diagnostic specificity of conventional ultrasound is low in distinguishing malignant lesions from benign lesions[4]. Some studies[5,19] have shown that SWE had better diagnostic performance than conventional ultrasound in the diagnosis of benign and malignant breast NMLs; however, these studies mainly focused on the internal elasticity characteristics of the lesions. In addition, some studies[13] suggested that the stiffness evaluation of surrounding tissues of breast lesions might have good differential diagnostic value. The elasticity characteristics and diagnostic value of the stiffness of the surrounding tissues in NMLs are still not clear, and there is no independent studies on the qualitative and quantitative evaluation of these parameters.
In the present study, we found that the internal and peripheral Emax and Emean of malignant NMLs were higher than those of benign NMLs, which is consistent with a previous study on breast mass lesions[20]. However, the mean values of internal elasticity parameters of NMLs were lower than those of mass lesions in the malignant group, and so was the diagnostic efficiency of internal parameters. This was mainly related to the high percentage of intraductal carcinoma in situ in malignant NMLs. Our results indicate that evaluation of the surrounding tissues may be more helpful in the differential diagnosis of NMLs than internal SWE elasticity. The hardest tissue with pathological changes was not located inside the lesion but on the peripheral tissue, which was mainly because malignant lesions tend to seep into the surrounding tissue[12]. Cancer cells infiltrate and diffuse to the peripheral interstitial tissue, and this dynamic reaction leads to the formation of the mixed zone. The mixed zone may consist of cell proliferation, lymphocytic infiltration, fibrosis, and angiogenesis of the tumor[13], which could increase the peripheral stiffness around malignant lesions up to 2-10 times higher[21]. Another study[22] reported that “stiff rim” sign was caused by attenuation of the energy of the shear wave of the peritumoral region of the lesion.
Qualitative and quantitative analyses of the stiffness parameters in the tissues surrounding NMLs, including the qualitative parameter (“stiff rim” sign) and quantitative parameters (elasticity values of the shell at 1 mm, 1.5 mm, 2 mm, 2.5 mm and 3 mm around the lesion) were performed. Using shell function, we found that the presence of the “stiff rim” sign in the malignant group was significantly higher than that in the benign group. In peripheral stiffness quantitative analysis, Emax and Emean of the shell within 1-3 mm were significantly higher in the malignant group than in the benign group. These results showed that elasticity analysis of the surrounding tissues of NMLs may have the potential ability to distinguish benign lesions from malignant lesions. The “stiff rim” sign and Emax/Emean of the shell within 1-3 mm have high diagnostic value, and the AUC of Emax at the shell of 2.5 mm has the largest AUC of 0.900. Huang et al[13] studied the surrounding tissues of breast mass lesions and found that Emax of the shell at 3 mm had the best diagnostic value. Possible reasons for this are as follows: (1) In our study, there was a large percentage of intraductal carcinoma in situ, which has mild invasion ability to the surrounding region; and (2) Our study thins out the shell layer. Of all the quantitative elasticity parameters, Emax had better diagnostic efficacy than the others, which was similar to previous studies[15,23]. This was mainly because Emax, Emean and Esd reflected different pathological anatomy changes. Emean reflects the average stiffness in the region of interest (ROI), and Esd represents the heterogeneity of various tissue textures in the ROI. Cancers are histologically heterogeneous due to the heterogeneity of the tumor cell population and the cancer microenvironment. Emean and Esd were more likely affected by internal liquefaction, necrosis, calcification, collagen etc. Emax reflects the maximum stiffness of the hardest tissue in the ROI, which is rarely affected by other factors. Huang et al[24] reported that Esd can improve the diagnosis sensitivity of malignant lesions. The different results obtained in different studies might be due to the different instruments used and the ROI type. Although Emean, Esd and Emax are all stiffness parameters, the selection of appropriate parameters is important, as they reflect different pathological formations.
SWE had good diagnostic value in NMLs; however, the false negative and false positive results should not be ignored. Many studies[6,25,26] have shown that multiple clinical and ultrasonic factors are associated with false negative and false positive results. In malignant NMLs, calcification, small lesion size and the appearance of in situ carcinoma were associated with false negative results. In benign NMLs, short distance to the nipple was the main reason for false positive results[6]. To reduce both false positive and false negative findings, it is necessary to make a diagnosis using a combination of other imaging techniques, for instance, mammography is necessary in cases with calcification in NMLs.
As the internal and peripheral parameters reflect different pathological formations of the lesion, combined application of imaging techniques could further improve diagnostic accuracy in NMLs. The combination of qualitative and quantitative analyses of stiffness in the circumjacent area around NMLs was applied in this study, which showed that Emax yielded a better performance than Emean as well as the elasticity parameters of surrounding tissues. Our study still has some limitations. The sample size was small. Qualitative and quantitative analyses of stiffness in the circumjacent area of NMLs by SWE still requires further investigation in a multicenter trial with a large sample size.
In conclusion, the “stiff rim” sign and other quantitative parameters within and around the breast NMLs have good diagnosis accuracy. The Emax of peripheral shells had the best evaluation efficiency. Combining qualitative and quantitative analyses of stiffness may further improve the accuracy of diagnosis.
Shear wave elastography can help the differential diagnosis of breast lesions by evaluating the hardness of breast lesions; however, there are few studies on the evaluation of the elasticity of breast non-mass lesions. In recent years, studies have shown that the evaluation of peripheral elasticity of breast lesions may have better diagnostic efficacy than other parameters. However, there has been no study on the internal and peripheral elasticity of breast non-mass lesions.
The aim of this study was to evaluate the internal and peripheral elasticity of breast non-mass lesions using shear wave elastography.
To determine the value of shear wave elastography in the differential diagnosis of non-mass breast lesions.
The peripheral (the shell of 1 mm, 1.5 mm, 2 mm, 2.5 mm and 3 mm around the lesions) and internal elasticity of non-mass breast lesions in 118 cases were evaluated. ROC curves of each parameter were drawn and the diagnostic efficacy of each parameter was compared.
The “stiff rim” sign and other quantitative parameters within and around the breast NMLs had good diagnostic efficiency. Emax of peripheral shells had better evaluation efficiency.
Combining qualitative and quantitative analyses of both internal and shell stiffness may further improve the diagnostic efficiency of breast non-mass lesions.
In this study, we focused on the influence of the stiffness of the peripheral shell in breast non-mass lesions on diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ehrhardt M, Kawai HF, McCarthy M S-Editor: Wang J L-Editor: Webster JR E-Editor: Zhang YL
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21354] [Article Influence: 2135.4] [Reference Citation Analysis (3)] |
| 2. | Are C, Rajaram S, Are M, Raj H, Anderson BO, Chaluvarya Swamy R, Vijayakumar M, Song T, Pandey M, Edney JA, Cazap EL. A review of global cancer burden: trends, challenges, strategies, and a role for surgeons. J Surg Oncol. 2013;107:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Ko KH, Hsu HH, Yu JC, Peng YJ, Tung HJ, Chu CM, Chang TH, Chang WC, Wu YC, Lin YP, Hsu GC. Non-mass-like breast lesions at ultrasonography: feature analysis and BI-RADS assessment. Eur J Radiol. 2015;84:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Wang ZL, Li N, Li M, Wan WB. Non-mass-like lesions on breast ultrasound: classification and correlation with histology. Radiol Med. 2015;120:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Choi JS, Han BK, Ko EY, Ko ES, Shin JH, Kim GR. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol. 2016;26:3542-3549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Park SY, Choi JS, Han BK, Ko EY, Ko ES. Shear wave elastography in the diagnosis of breast non-mass lesions: factors associated with false negative and false positive results. Eur Radiol. 2017;27:3788-3798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Zhang L, Dong YJ, Zhou JQ, Jia XH, Li S, Zhan WW. Similar Reproducibility for Strain and Shear Wave Elastography in Breast Mass Evaluation: A Prospective Study Using the Same Ultrasound System. Ultrasound Med Biol. 2020;46:981-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Fujioka T, Mori M, Kubota K, Kikuchi Y, Katsuta L, Kasahara M, Oda G, Ishiba T, Nakagawa T, Tateishi U. Simultaneous comparison between strain and shear wave elastography of breast masses for the differentiation of benign and malignant lesions by qualitative and quantitative assessments. Breast Cancer. 2019;26:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Han J, Li F, Peng C, Huang Y, Lin Q, Liu Y, Cao L, Zhou J. Reducing Unnecessary Biopsy of Breast Lesions: Preliminary Results with Combination of Strain and Shear-Wave Elastography. Ultrasound Med Biol. 2019;45:2317-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Suvannarerg V, Chitchumnong P, Apiwat W, Lertdamrongdej L, Tretipwanit N, Pisarnturakit P, Sitthinamsuwan P, Thiravit S, Muangsomboon K, Korpraphong P. Diagnostic performance of qualitative and quantitative shear wave elastography in differentiating malignant from benign breast masses, and association with the histological prognostic factors. Quant Imaging Med Surg. 2019;9:386-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Ko KH, Jung HK, Kim SJ, Kim H, Yoon JH. Potential role of shear-wave ultrasound elastography for the differential diagnosis of breast non-mass lesions: preliminary report. Eur Radiol. 2014;24:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, Jordan L, Baker L, Thompson A. Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res. 2010;12:R104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 13. | Huang L, Ma M, Du Z, Liu Z, Gong X. Quantitative evaluation of tissue stiffness around lesion by sound touch elastography in the diagnosis of benign and malignant breast lesions. PLoS One. 2019;14:e0219943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Xu HX, Zhao CK, Li XL, Bo XW, He YP, Liu BJ, Wang D, Ren WW. Complex cystic and solid breast lesions: Diagnostic performance of conventional ultrasound, strain imaging and point shear wave speed measurement. Clin Hemorheol Microcirc. 2018;69:355-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Zhou J, Zhan W, Chang C, Zhang X, Jia Y, Dong Y, Zhou C, Sun J, Grant EG. Breast lesions: evaluation with shear wave elastography, with special emphasis on the "stiff rim" sign. Radiology. 2014;272:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Uematsu T. Non-mass-like lesions on breast ultrasonography: a systematic review. Breast Cancer. 2012;19:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Zhang W, Xiao X, Xu X, Liang M, Wu H, Ruan J, Luo B. Non-Mass Breast Lesions on Ultrasound: Feature Exploration and Multimode Ultrasonic Diagnosis. Ultrasound Med Biol. 2018;44:1703-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Stöblen F, Landt S, Ishaq R, Stelkens-Gebhardt R, Rezai M, Skaane P, Blohmer JU, Sehouli J, Kümmel S. High-frequency breast ultrasound for the detection of microcalcifications and associated masses in BI-RADS 4a patients. Anticancer Res. 2011;31:2575-2581. [PubMed] |
| 19. | Wang ZL, Li Y, Wan WB, Li N, Tang J. Shear-Wave Elastography: Could it be Helpful for the Diagnosis of Non-Mass-Like Breast Lesions? Ultrasound Med Biol. 2017;43:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Ren WW, Li XL, He YP, Li DD, Wang D, Zhao CK, Bo XW, Liu BJ, Yue WW, Xu HX. Two-dimensional shear wave elastography of breast lesions: Comparison of two different systems. Clin Hemorheol Microcirc. 2017;66:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Insana MF, Pellot-Barakat C, Sridhar M, Lindfors KK. Viscoelastic imaging of breast tumor microenvironment with ultrasound. J Mammary Gland Biol Neoplasia. 2004;9:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Barr RG. Shear wave imaging of the breast: still on the learning curve. J Ultrasound Med. 2012;31:347-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Wang ZL, Li JL, Li M, Huang Y, Wan WB, Tang J. Study of quantitative elastography with supersonic shear imaging in the diagnosis of breast tumours. Radiol Med. 2013;118:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Huang Y, Li F, Han J, Peng C, Li Q, Cao L, Liu Y, Zhou J. Shear Wave Elastography of Breast Lesions: Quantitative Analysis of Elastic Heterogeneity Improves Diagnostic Performance. Ultrasound Med Biol. 2019;45:1909-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Yoon JH, Jung HK, Lee JT, Ko KH. Shear-wave elastography in the diagnosis of solid breast masses: what leads to false-negative or false-positive results? Eur Radiol. 2013;23:2432-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Kim MY, Choi N, Yang JH, Yoo YB, Park KS. False positive or negative results of shear-wave elastography in differentiating benign from malignant breast masses: analysis of clinical and ultrasonographic characteristics. Acta Radiol. 2015;56:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |