Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2127
Peer-review started: February 13, 2020
First decision: April 1, 2020
Revised: April 8, 2020
Accepted: May 13, 2020
Article in press: May 13, 2020
Published online: June 6, 2020
Processing time: 115 Days and 17 Hours
Since high-quality evidence on conservative treatment of acute appendicitis using antibiotics has increased, differentiation of patients with complicated appendicitis (CA) from those with simple appendicitis (SA) has become increasingly important. Previous studies have revealed that male gender, advanced age, comorbid conditions, prehospital delay, fever, and anorexia are risk factors of perforated appendicitis. Elevated serum C-reactive protein (CRP) level and hyponatremia have also been reported as predictive biomarkers of CA. However, confounding between various factors is problematic because most previous studies were limited to univariate analysis.
To evaluate non-laboratory and laboratory predictive factors of CA using logistic regression analyses.
We performed an exploratory, single-center, retrospective case-control study that evaluated 198 patients (83.9%) with SA and 38 patients (16.1%) with CA. Diagnoses were confirmed by computed tomography images for all cases. We compared age, sex, onset-to-visit interval, epigastric/periumbilical pain, right lower quadrant pain, nausea/vomiting, diarrhea, anorexia, medical history (of previous non-surgically treated appendicitis, diabetes, hypertension, dyslipidemia, liver cirrhosis, hemodialysis, chronic lung diseases, malignant tumors, immunosuppressant use, and antiplatelet use), vital signs, physical findings, and laboratory data to select the explanatory variates for logistic regression. Based on the univariate comparisons, we performed logistic regression for clinical differentiation between CA and SA using only non-laboratory factors and also including both non-laboratory and laboratory factors.
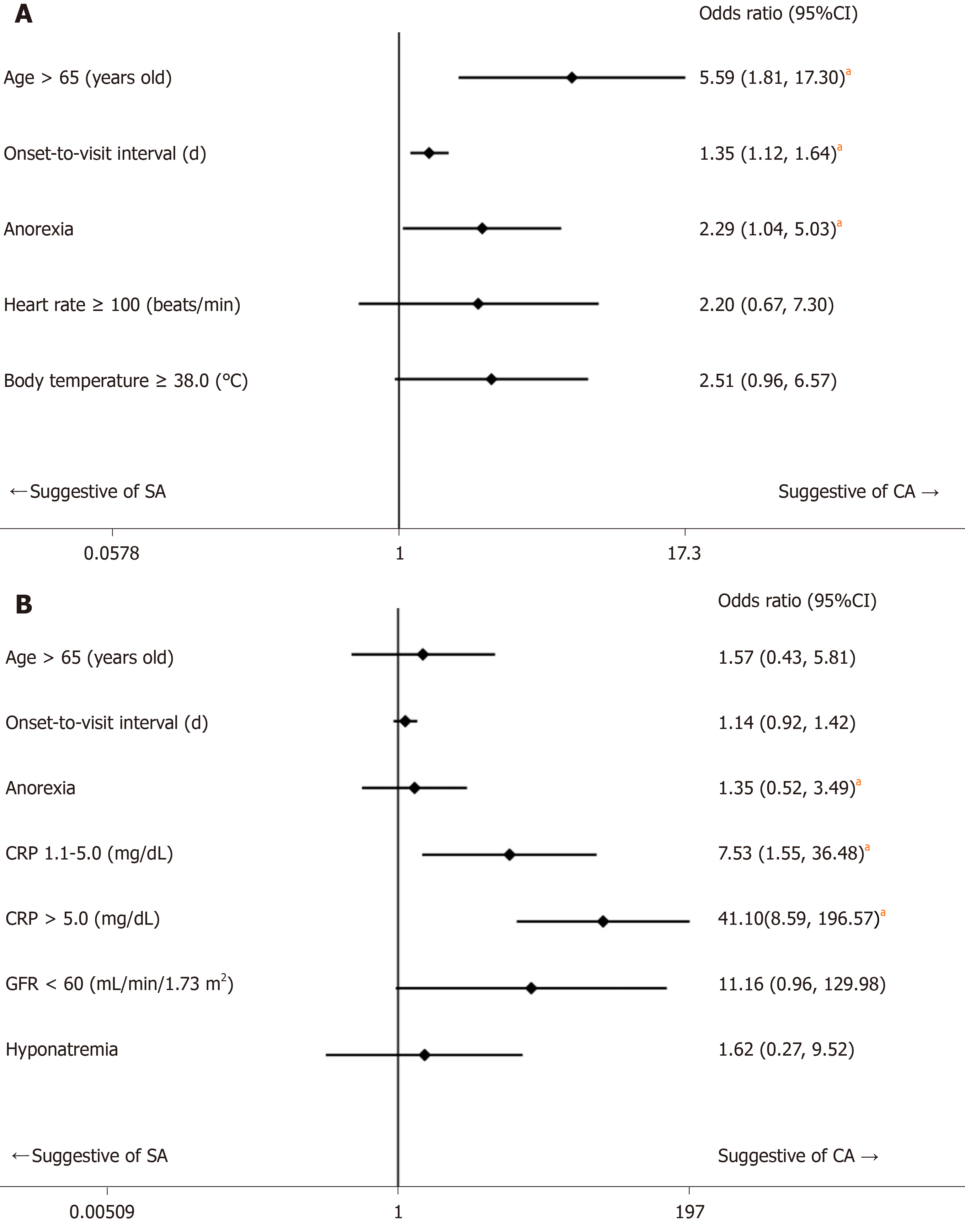
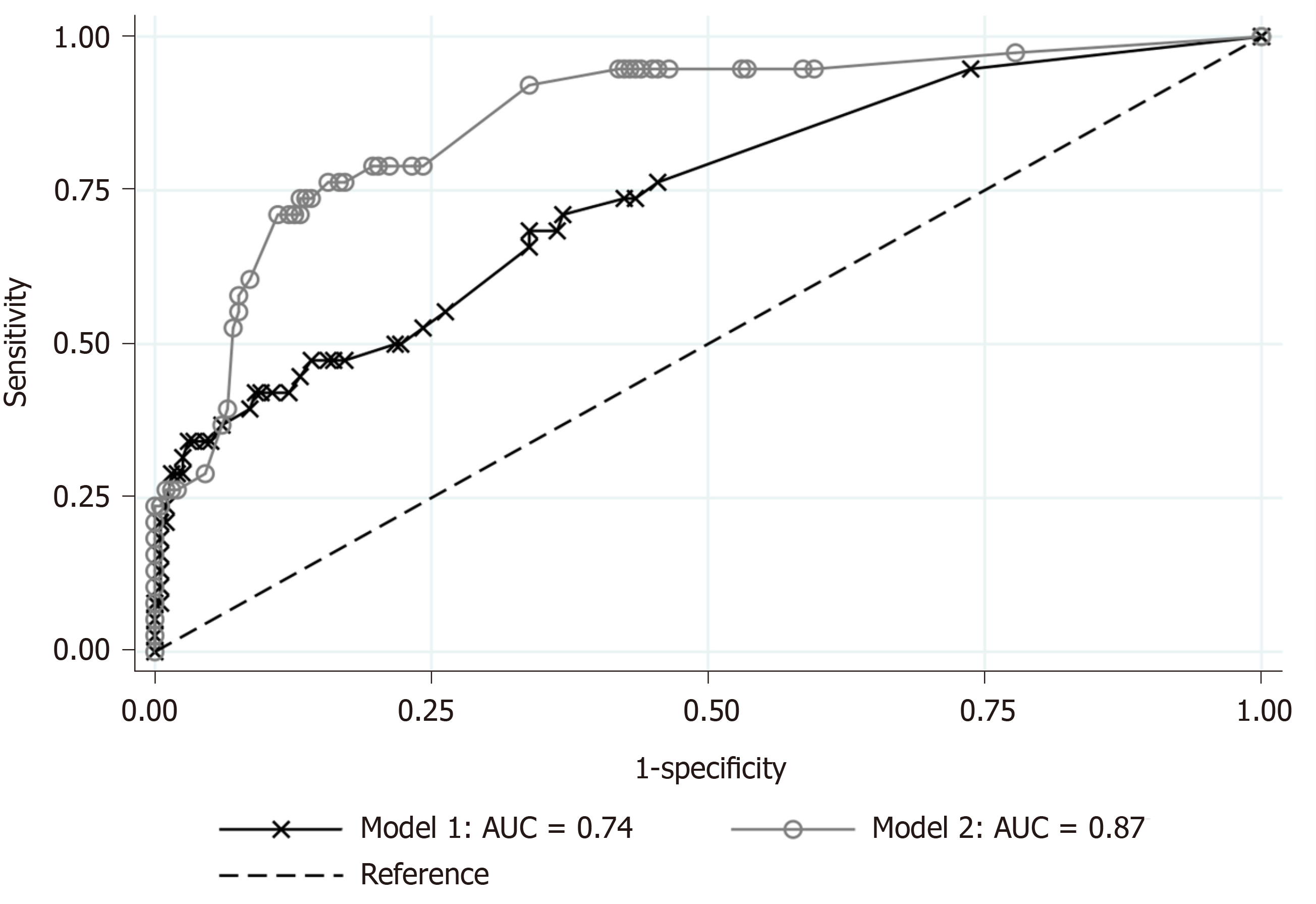
The 236 eligible patients consisted of 198 patients (83.9%) with SA and 38 patients (16.1%) with CA. The median ages were 34 years old [interquartile ranges (IR), 24-45 years] in the SA group and 49 years old (IR, 35-63 years) in the CA group (P < 0.001). The median onset-to-visit interval was 1 d (IR, 0-1) and 1 d (IR, 1-2) in the SA and CA groups, respectively (P < 0.001). Heart rate, body temperature, and serum CRP level in the CA group were significantly higher than in the SA group; glomerular filtration rate and serum sodium were significantly lower in the CA group. Anorexia was significantly more prevalent in the CA group. The regression model including age, onset-to-visit interval, anorexia, tachycardia, and fever as non-laboratory predictive factors of CA (Model 1) showed that age ≥ 65 years old, longer onset-to-visit interval, and anorexia had significantly high odds ratios. The logistic regression for prediction of CA including age, onset-to-visit interval, anorexia, serum CRP level, hyponatremia (serum sodium < 135 mEq/L), and glomerular filtration rate < 60 mL/min/1.73 m2 (Model 2) showed that only elevated CRP levels had significantly high odds ratios. Under the curve values of receiver operating characteristics curves of each regression model were 0.74 for Model 1 and 0.87 for Model 2.
Our logistic regression analysis on differentiating factors of CA from SA showed that high CRP level was a strong dose-dependent predictor of CA.
Core tip: Given few multivariate analyses on C-reactive protein (CRP) as a predictor of complicated appendicitis (CA), we performed a logistic regression analysis of 198 patients with simple appendicitis and 38 patients with CA. The regression model including non-laboratory factors of CA showed that age ≥ 65 years old, longer onset-to-visit interval, and anorexia had significantly high odds ratios. The logistic regression that additionally included laboratory data showed that only elevated CRP levels had significantly high odds ratios, which suggested that high CRP level can be a dose-dependent predictor of CA.
- Citation: Sasaki Y, Komatsu F, Kashima N, Suzuki T, Takemoto I, Kijima S, Maeda T, Miyazaki T, Honda Y, Zai H, Shimada N, Funahashi K, Urita Y. Clinical prediction of complicated appendicitis: A case-control study utilizing logistic regression. World J Clin Cases 2020; 8(11): 2127-2136
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2127.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2127
Emergent appendectomy has been the only standard form of management for acute appendicitis (AA). However, several well-organized, multi-center, randomized trials comparing conservative management with antibiotics to appendectomy have shown favorable or comparable results[1-4]. Based on these findings, a recent meta-analysis reported that conservative treatment can be considered an alternative to appendectomy[5]. Because conservative management of AA will become more popular thanks to this recent evidence, differentiation of high-risk patients with complicated appendicitis (CA) such as gangrenous appendicitis, perforated appendicitis, or cases complicated with intra-abdominal abscess from simple appendicitis (SA) has become increasingly important. Many studies on predictive factors of CA in both pediatric and adult patients have revealed that male gender, advanced age, comorbid conditions, prehospital delay, fever, anorexia, leukocytosis, elevated serum C-reactive protein (CRP) level, and hyponatremia are risk factors for perforated appendicitis[6-11]. However, confounding between various factors is problematic because most previous studies were limited to univariate analysis[12-16]. Thus, the aim of the present study was to evaluate clinical predictive factors of CA including non-laboratory and laboratory data using logistic regression analyses.
In this single-center, retrospective case-control study, we evaluated medical records from patients of the Toho University Medical Center Omori Hospital, which has 948 beds and is located in Tokyo, Japan. The center’s ethics committee approved the study’s protocol (M19023). We initially considered all patients who were ≥ 16 years old and hospitalized for AA between January 2012 and December 2016 from the discharge summary database of the hospital (Patients younger than 16 years old are treated by pediatricians in the hospital). Subsequently, the authors (Sasaki Y, Komatsu F, and Kashima N) reviewed the medical records of all the potential patients and manually collected pertinent data. We ultimately included only patients who were diagnosed as AA by computed tomography (CT) scans instead of surgical or pathological findings because of the low proportion of the patients treated with appendectomy according to the management policy of surgeons at our hospital as an advanced medical center (We will discuss the reason for the low proportion of operated patients in the Discussion section). We divided the enrolled patients into SA and CA groups based on the findings of the CT scan and ultrasound as follows: Patients were diagnosed with SA if they were clinically diagnosed with AA and had radiological/sonographical findings compatible with appendicitis catarrhalis or appendicitis phlegmonosa such as swelling of appendicitis and inflammatory findings of adjacent adipose tissue without any of the following findings of CA; patients were diagnosed with CA if they had gangrenous appendicitis, perforated appendicitis, or appendicitis complicated with an intra-abdominal abscess. All CT findings were reviewed by several different radiologists and surgeons. Gastroenterologists and surgeons reviewed all sonographic findings. These reviewing processes were timely or retrospectively performed within 48 h after the imaging studies.
The patients’ records were searched to collect data from their first visit, such as age, sex, time interval from the onset of symptoms until the time of the visit (onset-to-visit interval), epigastric/periumbilical pain, right lower quadrant pain, nausea/vomiting, diarrhea, anorexia, past medical history (of previous AA treated without appendectomy, diabetes, hypertension, hyperlipidemia, liver cirrhosis, hemodialysis, chronic lung diseases, malignant tumors, immunosuppressant use, and antiplatelet use), body temperature, blood pressure, heart rate, right lower quadrant tenderness, peritoneal signs, leukocyte count, glomerular filtration rate (GFR), serum CRP level, and serum alanine aminotransferase level, along with findings of CT scan and ultrasound at admission. We regarded clinical symptoms including pain, nausea/vomiting, diarrhea, and anorexia as positive if the patients complained of these symptoms at initial history taking.
All continuous variables except for onset-to-visit interval were categorized for statistical analyses as follows: We defined patient as old if his/her age was ≥ 65 years according to previous researches[17]. Fever was defined as an axillary measured body temperature of ≥ 38.0°C based on a previous study on the diagnosis of acute appendicitis[18]. Shock was defined as systolic blood pressure < 12.0 kPa (< 90 mmHg)[19]. Tachycardia was defined as a heart rate ≥ 100 beats/minute. Leukocytosis was defined as a leukocyte count > 10000/mm3 based on a previous study on the diagnosis of appendicitis[20]. Elevated liver enzyme was defined as alanine aminotransferase > 29 IU/L[21]. Renal dysfunction was defined as a GFR < 60 mL/min/1.73 m2 based on the guidelines of chronic kidney diseases[22]. CRP level was categorized into the following three groups: 0.0 to 1.0 mg/dL, 1.1 to 5.0 mg/dL, and over 5 mg/dL, based on the distribution of CRP level of the participants and previous studies on the cut-off value of CRP[8,12,14,15,23,24]. Hyponatremia was defined as serum sodium < 135 mEq/L[25].
Univariate comparisons: We compared all evaluated patient characteristics with SA and CA to select candidates for independent variables of logistic regressions. The χ2 test was used for all dichotomous/categorical variables, while the Wilcoxon rank-sum test was used for continuous variables because of their skewed distributions.
Logistic regression model: Logistic regression analysis was subsequently performed based on the results of the univariate analyses. As mentioned above, we converted all continuous variables, except for onset-to-visit interval, into categorized variables for logistic regression. To evaluate predictive factors of CA that were available prior to obtain laboratory results, we initially performed logistic regression using non-laboratory data including patient profiles, symptoms, and physical findings as explanatory variables (Model 1). Subsequently, we performed logistic regression using both laboratory factors and non-laboratory factors that were significant in Model 1 as explanatory variables (Model 2). We examined the variance inflation factors (VIF) to evaluate the multicollinearity of the regression models.
Discrimination, calibration, and internal validation of the regression model: We performed discrimination of the regression models by creating a receiver operating characteristic (ROC) curve. The differentiation abilities of the two regression models were compared using the methods described by DeLong et al[26]. We also calibrated the models using the Hosmer–Lemeshow (HL) goodness-of-fit test. Finally, we performed internal validation using bootstrap methods involving 100 samples tested five times.
All statistical analyses were performed using Stata/IC software (version 15.1; Stata Corp, United States). A P value < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Takuhiro Moromizato from the Internal Medicine Department, Renal and Rheumatology Division at the Okinawa Nanbu Medical Center and Children's Medical Center.
The 236 eligible patients consisted of 198 patients (83.9%) with SA and 38 patients (16.1%) with CA. Patient characteristics and the results of the univariate analyses are shown in Table 1. There were no patients with liver cirrhosis, receiving hemodialysis, and taking antiplatelets in either of the groups. Patients ≥ 65 years old were significantly prevalent in the CA group; the median ages were 34 years old [interquartile ranges (IR), 24-45 years] in the SA group and 49 years old (IR, 35-63 years) in the CA group (P < 0.001). The median onset-to-visit interval was 1 d (IR, 0-1) and 1 d (IR, 1-2) in the SA and CA groups, respectively (P < 0.001). Because even most cases with CA were conservatively treated with antibiotics in our hospital, appendectomy was performed in only 22/198 patients (11.1%) and 12/38 patients (31.6%) in the SA and CA groups, respectively (P = 0.001). Judgment on indication, reason, and selection of operated patients were based on the expert opinion of the surgeons on each individual case. In 34 operated patients, complications such as abscess formation, perforation, and wall necrosis (gangrenous appendicitis) were pathologically verified in 15/22 cases (68.2%) and 11/12 cases (91.7%) in the SA and CA groups, respectively. According to these results, sensitivity and specificity for differentiation of SA and CA based on the CT findings were 91.7% and 31.8%, respectively.
| Characteristics | All (n = 236) | SA (n = 198) | CA (n = 38) | P value |
| Age ≥ 65 (yr) | 17 (7.2) | 10 (5.1) | 7 (18.4) | 0.001a |
| Age (yr) | 35.5 (25-50.5) | 34 (24-45) | 49 (35-63) | < 0.00a |
| Male | 129 (54.7) | 105 (53.0) | 24 (63.2) | 0.251 |
| Onset-to-visit interval (d) | 1 (0-1) | 1 (0-1) | 1 (1-2) | < 0.001a |
| Epigastric/periumbilical pain | 119 (50.4) | 104 (52.5) | 15 (39.5) | 0.141 |
| RLQ pain | 171 (72.5) | 141 (71.2) | 30 (79.0) | 0.328 |
| Nausea/vomiting | 123 (52.1) | 106 (53.5) | 17 (44.7) | 0.32 |
| Diarrhea | 46 (19.5) | 38 (19.2) | 8 (21.1) | 0.791 |
| Anorexia | 64 (27.1) | 48 (24.2) | 16 (42.1) | 0.023a |
| Previous appendicitis | 32 (13.6) | 30 (15.2) | 2 (5.3) | 0.103 |
| Diabetes mellitus | 11 (4.7) | 7 (3.5) | 4 (10.5) | 0.061 |
| Hypertension | 24 (10.2) | 20 (10.1) | 4 (10.5) | 0.937 |
| Dyslipidemia | 21 (8.9) | 16 (8.1) | 5 (13.2) | 0.314 |
| Chronic lung diseases | 2 (0.9) | 1 (0.5) | 1 (2.6) | 0.19 |
| Malignancy | 1 (0.4) | 1 (0.5) | 0 | 0.661 |
| Immunosuppressant use | 1 (0.4) | 1 (0.5) | 0 | 0.661 |
| Fever (≥ 38.0 °C) | 34 (14.4) | 23 (11.6) | 11 (29.0) | 0.005a |
| Body temperature (°C) | 37.0 (36.7-37.5) | 37.0 (36.6-37.4) | 37.5 (37.1-37.9) | < 0.001a |
| Tachycardia | 18 (7.6) | 11 (5.6) | 7 (18.4) | 0.006a |
| Heart rate (beats/min) | 78 (68-88) | 76 (66-85) | 85 (76-96) | < 0.001a |
| Shock | 6 (2.5) | 5 (2.5) | 1 (2.6) | 0.97 |
| RLQ tenderness | 230 (97.5) | 192 (97.0) | 38 (100) | 0.277 |
| Peritoneal signs | 137 (58.1) | 113 (57.1) | 24 (63.2) | 0.486 |
| Leukocytosis | 177 (75.0) | 146 (73.7) | 31 (81.6) | 0.307 |
| Leukocyte count (103/mm3) | 12.6 (10.1-15.2) | 12.6 (10.0-15.2) | 13.2 (10.8-15.4) | 0.497 |
| CRP (mg/dL) | 1.1 (0.2-4.1) | 0.7 (0.2-2.7) | 8.8 (3.8-19.0) | < 0.001a |
| GFR < 60 mL/min/1.73 m2 | 6 (2.5) | 1 (0.5) | 3 (13.2) | < 0.001a |
| GFR (mL/min/1.73 m2) | 120.7 (101.6-140.4) | 123.8 (103.7-142.9) | 105.1 (82.83-122.1) | 0.007a |
| Serum sodium (mEq/L) | 139 (137-140) | 139 (138-140) | 137.5 (136-139) | 0.003a |
| Hyponatremia | 9 (3.8) | 5 (2.5) | 4 (10.5) | 0.019a |
| ALT > 29 IU/L | 38 (16.1) | 30 (15.2) | 8 (21.1) | 0.365 |
| Appendectomy | 34 (14.4) | 22 (11.1) | 12 (31.6) | 0.001a |
As shown in Table 1, heart rate, body temperature, and serum CRP level in the CA group were significantly higher than in the SA group (Table 1). On the other hand, GFR and serum sodium level were significantly lower in the CA group (Table 1). The prevalence of anorexia was significantly higher in the CA group compared to the SA group (Table 1).
Based on the results of univariate comparisons, we selected age, onset-to-visit interval, anorexia, tachycardia, and fever as non-laboratory predictive factors of CA and performed a logistic regression (Model 1). The regression model showed that advanced age (≥ 65 years old), longer onset-to-visit interval, and anorexia had significantly high ORs (Figure 1A). The logistic regression for prediction of CA including age, onset-to-visit interval, anorexia, serum CRP level, renal dysfunction (defined as GFR < 60 mL/min/1.73 m2), and hyponatremia (defined as serum sodium < 135 mEq/L) showed that only elevated CRP levels had significantly high ORs (Figure 1B). The methods of DeLong et al[26] revealed that the area under the curve (AUC) values were 0.74 for Model 1 and 0.87 for Model 2 (Figure 2). Although the discrimination ability of Model 2 was better than that of Model 1, both models were considered moderately accurate because the AUC values were > 0.7.
The Model 1 regression showed good calibration (HL χ2: 5.88, P = 0.437), and there was no multicollinearity because the VIF of all explanatory variables was 1.2 or less and the mean VIF was 1.07. Optimism, as calculated by the bootstrap method, was 2.0 × 10-6. Model 2 also showed good calibration (HL χ2: 7.08, P value = 0.420) and there was no multicollinearity (all VIFs < 1.3, mean VIF = 1.12). Optimism was 0.0001.
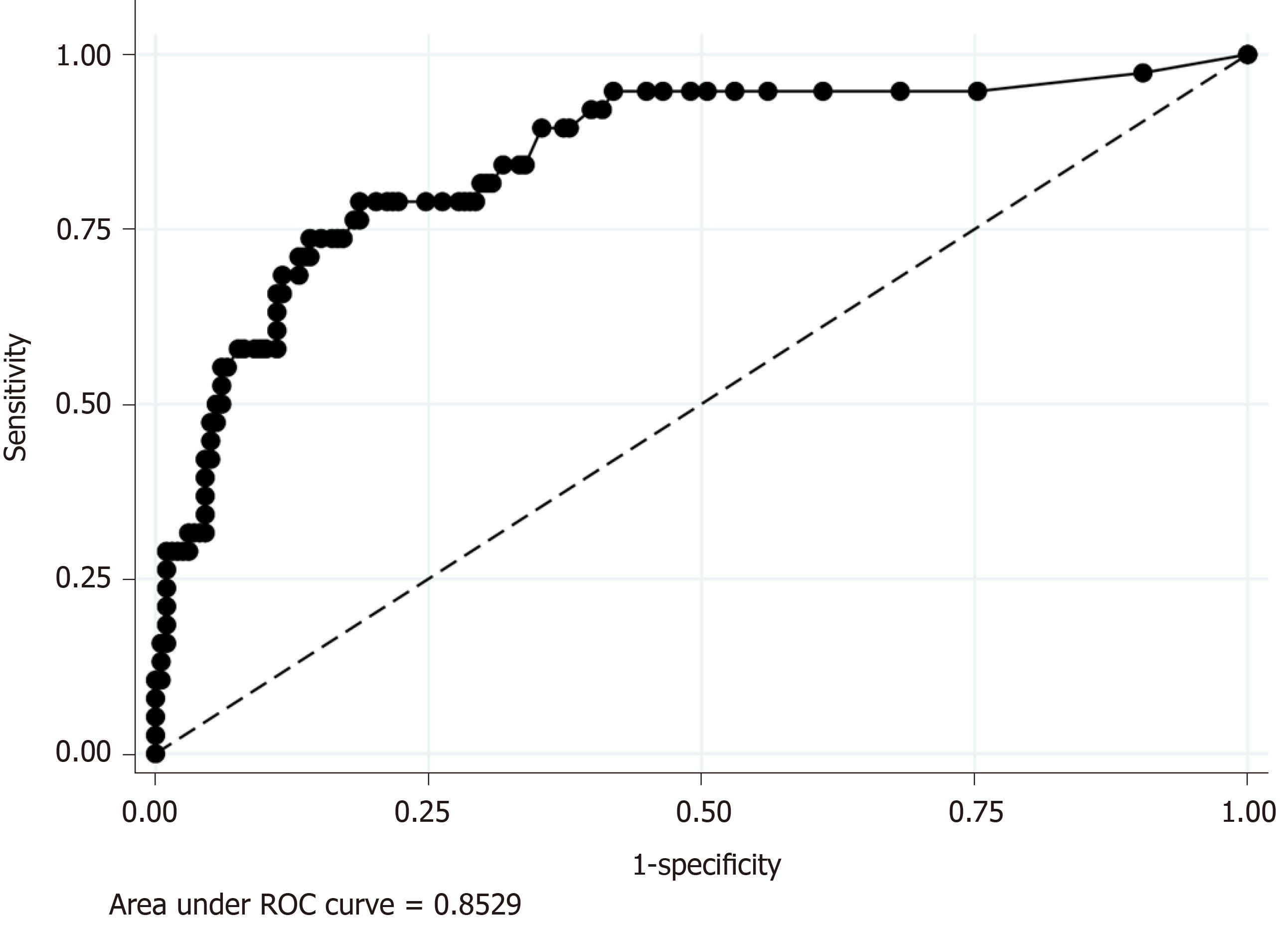
Because CRP was the only significant predictive factor of CA in our regression model (Model 2), we additionally performed ROC analyses to evaluate the discrimination ability of serum CRP level for the prediction of CA, which revealed the AUC value was 0.85. The sensitivity and specificity of each serum CRP value were as follows: Sensitivity and specificity of 94.7% and 55.1 % when was CRP ≥ 1.0 mg/dL and sensitivity and specificity of 68.4% and 86.9% when was CRP ≥ 4.9 (Figure 3).
We performed a single-center, retrospective cohort study to evaluate clinical predictive factors of CA using univariate comparison and logistic regression. Univariate comparisons showed that advanced age, longer onset-to-visit interval, anorexia, tachycardia, fever, elevated CRP level, renal dysfunction, and hyponatremia were significantly more prevalent in the CA group than in the SA group. Logistic regression on non-laboratory factors of CA showed that advanced age (≥ 65 years old), longer onset-to-visit interval, and anorexia had significantly high ORs (Model 1). Logistic regression including laboratory factors showed that only elevated CRP level had a significantly high OR (Model 2). Furthermore, the ORs increased in proportion to a rise in CRP level (Model 2).
In terms of the univariate analysis, our study showed that advanced age was a significant predictor of CA, which is compatible with the findings of previous studies[6]. On the other hand, male gender was not a significant predictor of CA in our study; nonetheless, male gender has been reported as a risk factor of CA[6,7]. The results of the significance of the preoperative interval as a predictor of CA are inconsistent. Some studies showed that longer durations before operation or admission are risk factors for perforation[7,8]. However, another high-quality study using logistic regression showed that the preoperative interval was insignificant[6]. In our study, the onset-to-visit interval was a statistically significant predictor of CA. However, because we could not evaluate the difference by the order of hours in this retrospective study and the medians were the same (1 d) in both the SA and CA groups, the clinical significance was limited. Although only a few studies have been conducted, hyponatremia has been reported as a predictor of CA[9,11]. We believe that our study adds new evidence in support of these previous studies.
To the best of our knowledge, significant differences in anorexia, tachycardia, and renal dysfunction in our study are previously unreported findings because previous analyses on risk factors of CA were limited to patient profiles, underlying conditions, and inflammatory biomarkers[6-8].
Our study showed that CRP was the single significant predictor of CA in proportion to the value of serum CRP level. Furthermore, ROC analysis showed that serum CRP level had a high discrimination ability for the prediction of CA (AUC = 0.85). Our results are compatible with those of previous studies, which have shown that CRP is a useful predictor of CA[14-16,23,24,27]. Previously, the proposed cut-off values of CRP ranged among studies from 0.3 mg/dL to 5.1 mg/dL; one previous study revealed CRP > 0.3 mg/dL was associated with CA[15]. Other studies proposed a cut-off value of around 1.0 mg/dL[12,16]. Using logistic regression, another study showed that CRP > 5.1 mg/dL had an OR as high as 3.076[23]. In addition to these previous studies, our study revealed a dose-dependent increase of the OR predicting CA was associated with the CRP value. Our study also indicated that CRP > 1.0 mg/dL, a relatively low cut-off value, was useful for predicting CA. We believe that our study provides important knowledge on the usefulness of CRP for the clinical prediction of CA because a previous study using multivariate analysis that included both non-laboratory and laboratory factors was limited[23]. A previous multivariate analysis using logistic regression showed that male gender, age, comorbid conditions, and lack of insurance were significant predictors of perforated appendicitis. However, our logistic regression showed CRP elevation was a single significant predictor of CA. Given that previous studies did not include CRP as an explanatory variable, other risk factors are adjusted by CRP, a strong laboratory predictor, in the present study.
Our study has three major limitations. First, because the present study was a retrospective, case-controlled study using medical records, we could not collect some previously reported important information, such as migration of abdominal pain or other components of the Alvarado score[20]. As mentioned above, we also could not evaluate the precise onset-to-visit interval by the order of hours.
Second, despite the fact that most of the previous studies distinguished between CA and SA based on surgical or pathological findings, we differentiated CA from SA based on the findings of CT scan instead of pathological or surgical findings in the present study because our surgeons aggressively selected a combination of conservative therapy with antibiotics and subsequent elective appendectomy (interval appendectomy)[28] as a form of advanced medical care, and we could therefore not evaluate surgical and pathological findings of the resected appendix in most cases due to the low proportion of appendectomies, which were performed in only 11.1% and 31.6% of patients in the SA and CA groups, respectively. The discrepancy between radiological findings and surgical/pathological findings diminished the reliability of the study although the sensitivity was as high as 91.7%. We should note that our study was conducted under somewhat unique circumstances: Conservative management for AA that we routinely performed in our hospital is not the global standard management of AA (i.e., despite the fact that non-operative management of AA is increasingly accepted as an option of management of AA in some patents, such as those who prefer non-operative management or high-risk patients for operation[29], emergent or urgent appendectomy is still the global standard management in most cases)[5]. However, because the evidence on conservative management and interval appendectomy is increasing, as mentioned above, our differentiation of SA and CA based on radiological findings as a surrogate for surgical or pathological findings may become more clinically significant.
The third limitation is the small sample size, especially for cases with CA. This may decrease the statistical reliability of logistic regression models that include five explanatory variables.
In conclusion, our study, which utilized logistic regression analysis on differentiating factors of CA from SA, showed that high CRP level was a strong dose-dependent predictor of CA. Because of the scarcity of the studies using multivariate analysis on the prediction of CA, further study is needed to confirm our findings.
Because evidence on conservative treatment of acute appendicitis has increased, differentiation of patients with complicated appendicitis (CA) from those with simple appendicitis (SA) has become increasingly important. Previous studies have revealed that male gender, advanced age, comorbid conditions, prehospital delay, fever, and anorexia are risk factors of perforated appendicitis. Elevated serum C-reactive protein (CRP) level and hyponatremia have also been reported as predictive biomarkers of CA.
Confounding between various factors is problematic because most previous studies were univariate analyses. Thus, we performed a study using logistic regression analyses.
The objective of the study was to evaluate non-laboratory and laboratory predictive factors of CA using logistic regression analyses using logistic regression analyses.
We performed a single-center, retrospective case-control study that evaluated 198 patients (83.9%) with SA and 38 patients (16.1%) with CA. Diagnoses were confirmed by computed tomography images for all cases. We compared age, sex, clinical symptoms, past medical history, vital signs, physical findings, and laboratory data. Based on the comparisons, we performed logistic regression for clinical differentiation between CA and SA using only non-laboratory factors and also including both non-laboratory and laboratory factors.
The 236 eligible patients consisted of 198 patients (83.9%) with SA and 38 patients (16.1%) with CA. The median ages were 34 years old in the SA group and 49 years old in the CA group. The median onset-to-visit interval was 1 d and 1 d in the SA and CA groups, respectively. Heart rate, body temperature, and serum CRP level in the CA group were significantly higher than in the SA group; glomerular filtration rate (GFR) and serum sodium were significantly lower in the CA group. Anorexia was significantly more prevalent in the CA group. The regression model including age, onset-to-visit interval, anorexia, tachycardia, and fever as non-laboratory predictive factors of CA (Model 1) showed that age ≥ 65 years old, longer onset-to-visit interval, and anorexia had significantly high odds ratios. The logistic regression for prediction of CA including age, onset-to-visit interval, anorexia, serum CRP level, hyponatremia, and glomerular filtration rate < 60 mL/min/1.73 m2 (Model 2) showed that only elevated CRP levels had significantly high odds ratios.
Our logistic regression analysis on differentiating factors of CA from SA showed that high CRP level was a strong dose-dependent predictor of CA.
We would like to acknowledge the excellent assistance of the staff of the Harvard Medical School Introduction to Clinical Research Training Japan and that of the Okinawa Asia Clinical Investigation Synergy (OACIS). Moreover, we would like to thank Dr. Takuhiro Moromizato (Internal Medicine Department, Renal and Rheumatology Division at the Okinawa Nanbu Medical Center and Children's Medical Center) for his assistance and review of the statistical analysis.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kristek J, Shiryajev Y S-Editor: Wang JL L-Editor: A E-Editor: Liu MY
| 1. | Eriksson S, Granström L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg. 1995;82:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 284] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Styrud J, Eriksson S, Nilsson I, Ahlberg G, Haapaniemi S, Neovius G, Rex L, Badume I, Granström L. Appendectomy versus antibiotic treatment in acute appendicitis. a prospective multicenter randomized controlled trial. World J Surg. 2006;30:1033-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 3. | Turhan AN, Kapan S, Kütükçü E, Yiğitbaş H, Hatipoğlu S, Aygün E. Comparison of operative and non operative management of acute appendicitis. Ulus Travma Acil Cerrahi Derg. 2009;15:459-462. [PubMed] |
| 4. | Hansson J, Körner U, Khorram-Manesh A, Solberg A, Lundholm K. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg. 2009;96:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 355] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 5. | Wilms IM, de Hoog DE, de Visser DC, Janzing HM. Appendectomy versus antibiotic treatment for acute appendicitis. Cochrane Database Syst Rev. 2011;CD008359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Drake FT, Mottey NE, Farrokhi ET, Florence MG, Johnson MG, Mock C, Steele SR, Thirlby RC, Flum DR. Time to appendectomy and risk of perforation in acute appendicitis. JAMA Surg. 2014;149:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Sirikurnpiboon S, Amornpornchareon S. Factors Associated with Perforated Appendicitis in Elderly Patients in a Tertiary Care Hospital. Surg Res Pract. 2015;2015:847681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Omari AH, Khammash MR, Qasaimeh GR, Shammari AK, Yaseen MK, Hammori SK. Acute appendicitis in the elderly: risk factors for perforation. World J Emerg Surg. 2014;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Kim DY, Nassiri N, de Virgilio C, Ferebee MP, Kaji AH, Hamilton CE, Saltzman DJ. Association Between Hyponatremia and Complicated Appendicitis. JAMA Surg. 2015;150:911-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Rentea RM, Peter SDS, Snyder CL. Pediatric appendicitis: state of the art review. Pediatr Surg Int. 2017;33:269-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Pham XD, Sullins VF, Kim DY, Range B, Kaji AH, de Virgilio CM, Lee SL. Factors predictive of complicated appendicitis in children. J Surg Res. 2016;206:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Buyukbese Sarsu S, Sarac F. Diagnostic Value of White Blood Cell and C-Reactive Protein in Pediatric Appendicitis. Biomed Res Int. 2016;2016:6508619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Ozan E, Ataç GK, Alişar K, Alhan A. Role of inflammatory markers in decreasing negative appendectomy rate: A study based on computed tomography findings. Ulus Travma Acil Cerrahi Derg. 2017;23:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Dal F, Cicek Y, Pekmezci S, Kocazeybek B, Tokman HB, Konukoglu D, Şimşek O, Taner Z, Sirekbasan S, Uludağ SS. Role of Alvarado score and biological indicators of C-reactive protein, procalicitonin and neopterin in diagnosis of acute appendicitis. Ulus Travma Acil Cerrahi Derg. 2019;25:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Kim M, Kim SJ, Cho HJ. International normalized ratio and serum C-reactive protein are feasible markers to predict complicated appendicitis. World J Emerg Surg. 2016;11:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Shogilev DJ, Duus N, Odom SR, Shapiro NI. Diagnosing appendicitis: evidence-based review of the diagnostic approach in 2014. West J Emerg Med. 2014;15:859-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Orimo H, Ito H, Suzuki T, Araki A, Hosoi T, Sawabe M. Reviewing the definition of “elderly”. Geriatr Gerontol Int. 2006;6:149-158. [RCA] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 18. | Chen SC, Chang KJ, Wei TC, Yu SC, Wang SM. Can cecal diverticulitis be differentiated from acute appendicitis? J Formos Med Assoc. 1994;93:263-265. [PubMed] |
| 19. | Edelman DA, White MT, Tyburski JG, Wilson RF. Post-traumatic hypotension: should systolic blood pressure of 90-109 mmHg be included? Shock. 2007;27:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 814] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 21. | Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112:18-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 713] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 22. | Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013;3:19-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 766] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 23. | Yazar CKD, Urfalioglu A, Bakacak M, Boran ÖF, Bülbüloğlu E. Efficacy of the Evaluation of Inflammatory Markers for the Reduction of Negative Appendectomy Rates. Indian J Surg. 2018;80:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Ahmed N. C-Reactive Protein: An Aid For Diagnosis Of Acute Appendicitis. J Ayub Med Coll Abbottabad. 2017;29:250-253. [PubMed] |
| 25. | Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1106] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 26. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] |
| 27. | March B, Leigh L, Brussius-Coelho M, Holmes M, Pockney P, Gani J. Can CRP velocity in right iliac fossa pain identify patients for intervention? A prospective observational cohort study. Surgeon. 2019;17:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Perez KS, Allen SR. Complicated appendicitis and considerations for interval appendectomy. JAAPA. 2018;31:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Flum DR. Clinical practice. Acute appendicitis--appendectomy or the "antibiotics first" strategy. N Engl J Med. 2015;372:1937-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |