Published online Apr 6, 2019. doi: 10.12998/wjcc.v7.i7.891
Peer-review started: December 6, 2018
First decision: December 15, 2018
Revised: January 2, 2019
Accepted: January 26, 2019
Article in press: January 26, 2019
Published online: April 6, 2019
Processing time: 123 Days and 19.6 Hours
Villous adenomas of the urinary tract are uncommon. They are morphologically similar to and difficult to differentiate from their counterpart in the colon. The histogenesis and malignant potential are uncertain.
A 63-year-old woman was admitted to our hospital with a mass in the urethral orifice. Gross and microscopic pathological examination was suggestive of urethral villous adenoma with focal well-differentiated adenocarcinoma. The whole urethra and part of the bladder were excised. No further treatment was offered. Carcinoembryonic antigen, cytokeratin 7, cytokeratin 20, epithelial membrane antigen, and p53 protein were positive, and the ratio of Ki-67 was 60%. After follow-up at 11 mo, the patient was cured and had no recurrence.
Immunohistochemistry is important for differential diagnosis of villous adenoma of the urinary system. Complete surgical resection of the urinary tract is curative.
Core tip: Villous adenoma coexisting with adenocarcinoma of the urinary system is uncommon. Differential diagnosis from villous adenoma of the digestive tract is difficult. We present a case of villous adenoma coexisting with adenocarcinoma of the female urethral orifice and provide an accurate method to differentiate villous adenomas of the urinary and digestive tracts.
- Citation: Qin LF, Liang Y, Xing XM, Wu H, Yang XC, Niu HT. Villous adenoma coexistent with focal well-differentiated adenocarcinoma of female urethral orifice: A case report and review of literature. World J Clin Cases 2019; 7(7): 891-897
- URL: https://www.wjgnet.com/2307-8960/full/v7/i7/891.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i7.891
Villous adenomas are frequent benign tumors of the digestive tract. However, they are uncommon in the urinary system. To date, only two case series[1,2] and several sporadic cases have been reported in the English language literature. In the urinary tract, villous adenoma has a predilection for the bladder and urachus, followed by the urethra and prostate[1-5]. As far as we know, this is the first case of villous adenoma coexistent with focal well-differentiated adenocarcinoma of the female urethral orifice. We also include a review of the relevant literature.
A 63-year-old woman was admitted to our hospital in December 2017 with a mass in the urethral orifice. She had noticed the mass 2 yr prior without any diagnosis and treatment, and it had slowly increased in size in the past few months. She had no other symptoms. Physical examination found a fleshy, hemorrhaged, uneven polypoidal mass 3 cm × 4 cm in diameter located at the bottom right of the urethral orifice. Radiographic examination of the chest, abdomen, and pelvis was unremarkable. Urine analysis showed that urinary occult blood test was positive and 65.34 red blood cells were observed per high-power field. There was no significant past medical history except for hypertension, and other laboratory tests were normal. Cystourethroscopy demonstrated a villous mass with exophytic growth in the distal urethra and smooth mucosa of the bladder. Biopsy of the urethral lesion showed villous adenoma with well-differentiated adenocarcinoma. Complete gastrointestinal evaluation failed to find any similar lesions. The whole urethra and part of the bladder were excised and the specimen was sent to the Department of Pathology for pathological and immunohistochemical examination. No further treatment was offered. After follow-up at 11 mo, the patient had no recurrence. Publication of this case report was approved by the Ethics Committee of Affiliated Hospital of Qingdao University.
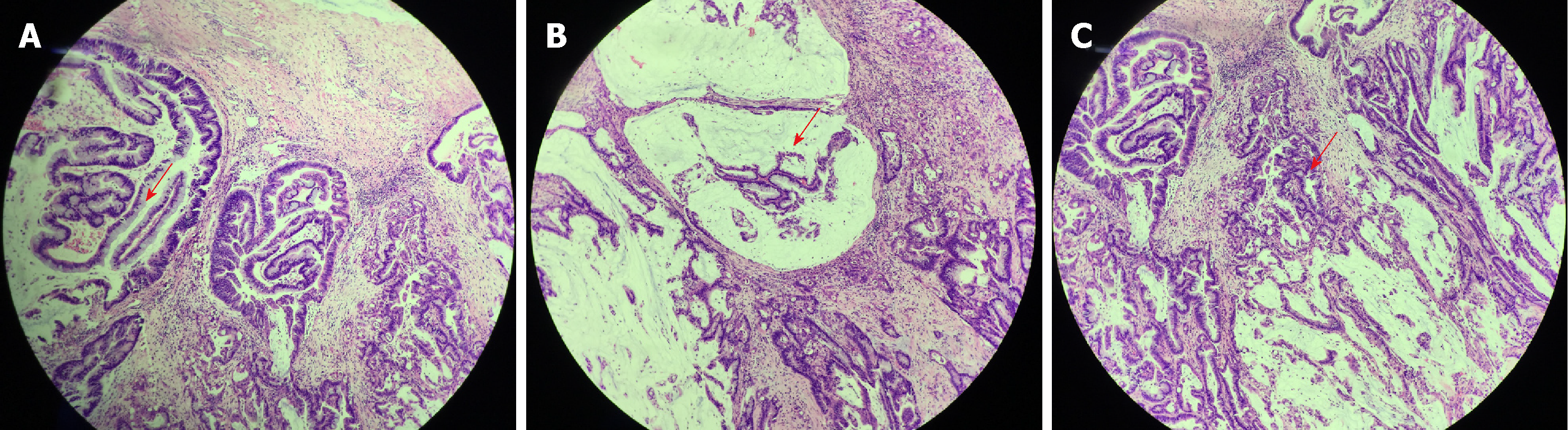
The excised lesion was a soft grayish mass measuring 1.5 cm in diameter and appeared papillary and fleshy with hemorrhage and negative surgical margins. The appearance was identical to villous adenoma of the colon. Several blunt finger-like processes lined by pseudostratified columnar cells with frequent goblet cells were observed under a light microscope (Figure 1A). The nuclei were stratified atypical and hyperchromatic. Abundant mucin was seen both intracellularly and extracellularly (Figure 1B). Carcinomatous areas consisted of dysplastic glands and some of the glands presented with high-grade intraepithelial neoplasia (Figure 1C). In focal areas, the glandular component was characterized by increased disorganization of structure. More importantly, the carcinoma invaded the muscularis layer. The gross and microscopic examination was suggestive of urethral villous adenoma with focal well-differentiated adenocarcinoma.
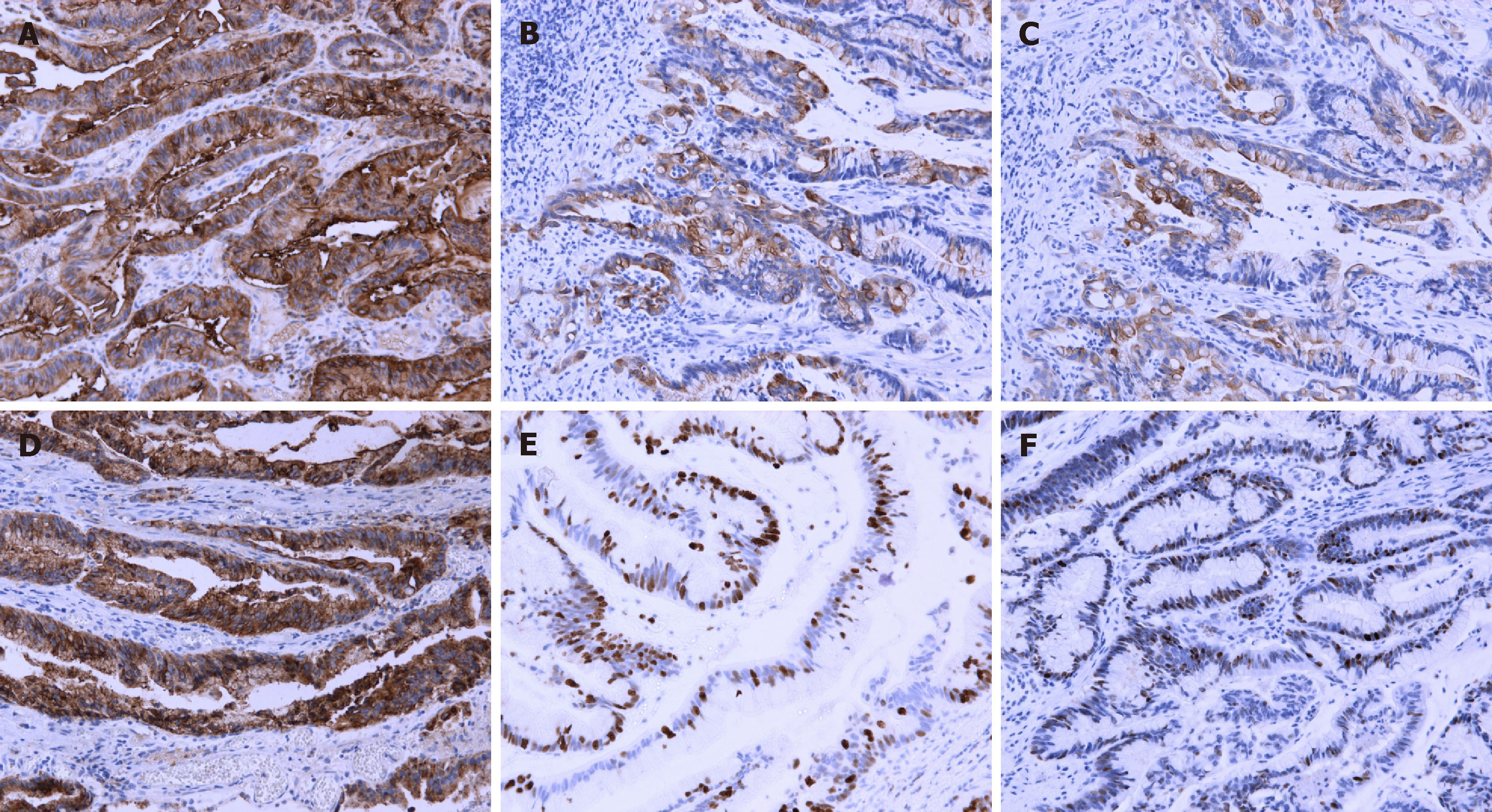
Formalin-fixed, paraffin-embedded tissue was cut into 5-µm sections for im-munohistochemical evaluation. Immunohistochemical examination was positive for carcinoembryonic antigen (CEA), cytokeratin (CK) 7, CK20, epithelial membrane antigen (EMA), and p53 protein, and the positive ratio of Ki-67 was 60% in the adenocarcinoma (Figure 2). All immunohistochemical staining was carried out by the avidin–biotin–complex method, as previously described[6,7].
We carried out a review of the literature on urethral villous adenoma. PubMed and Embase were searched using the following keywords: urethra OR urethral AND villous adenoma. We only included articles in English. A total of 11 cases with urethral villous adenoma were reported from 1981 to 2003 (Table 1).
| Ref. | Time of publication | Age in yr | Clinical symptoms | Associated adenocarcinoma | Treatment | Follow-up in mo | Outcome |
| Powel et al[8] | 1981 | 59 | Hematuria | Yes | Transurethral resection | 15 | Alive |
| Howells et al[9] | 1985 | 70 | Vaginal discharge; Dysuria | No | Surgical resection | 22 | Alive |
| Raju et al[10] | 1987 | 58 | Asymptomatic mass increasing in size | No | Surgical resection | 72 | Alive |
| Morgan et al[11] | 1998 | 87 | Asymptomatic polypoid urethral mass | No | Surgical resection | 24 | Dead |
| Cheng et al[1] | 1999 | 81 | Hematuria | No | Unknown | Unknown | Unknown |
| 1999 | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | |
| 2002 | 63 | Unknown | Unknown | Transurethral resection; Cystoprostatectomy | 84 | Alive | |
| Sung et al[12] | 2000 | 57 | Acute attack of urinary retention | No | Transurethral resection | Unknown | Alive |
| Seibel et al[2] | 2002 | 93 | Unknown | Unknown | Transurethral resection; cystoprostatectomy | Unknown | Unknown |
| 2002 | 73 | Unknown | Unknown | Transurethral resection; prostatectomy | 24 | Local recurrence; Lung metastasis | |
| Noel et al[13] | 2003 | 49 | Hematuria | Adenosquamous carcinoma | Surgical resection | 16 | Alive |
The final diagnosis of the patient was urethral villous adenoma with focal well-differentiated adenocarcinoma.
The whole urethra and part of the bladder of the patient were excised under general anesthesia.
After follow-up at 11 mo, the patient had no recurrence.
Villous adenoma of the urinary tract is uncommon and occurs principally in elderly patients, without gender predominance, and is mostly located in the urachus and bladder, followed by the urethra and ureter[1-5]. The typical clinical presentation includes hematuria and urinary tract irritation[1-5].
The gross and microscopic characteristics of urinary villous adenoma are identical to those of gastrointestinal villous adenoma. Pure villous adenomas are usually small and solitary. The tumors show papillary or finger-like processes and have a soft texture without prominent hemorrhage and necrosis. Microscopically, they all exhibit papillary architecture with central lamina propria cores, consisting of pointed or blunt finger-like processes lined by pseudostratified columnar epithelial cells with goblet-type cells. Nuclear atypia is common[1,14-16]. Adenocarcinoma presents with brittle texture, erosion, and hemorrhage. Microscopically, the pseudostratified columnar epithelial cells show stratification, crowding, hyperchromasia, and irregular nuclear mitosis[1]. The pathological diagnosis of our case was villous adenoma coexistent with focal well-differentiated adenocarcinoma. The gross and microscopic presentations were consistent with previous reports.
As for the immunophenotypic profile, we found that CEA, CK7, CK20, EMA, and p53 were positive and the positive ratio of Ki-67 was 60%. As reported previously[1,2,17], almost all the tumor lesions were stained for CEA and CK20 and approximately 50% for CK7, but generally EMA was negative. Cheng et al[1] reported that CK7 was positive in 56% of patients with urinary tract adenoma and 22% were positive for EMA. The immunohistochemical staining patterns were also identical with those of intestinal villous adenoma. Our results were consistent with prior studies.
The etiopathogenesis of urinary tract villous adenoma is not entirely understood. Atik et al[18] hypothesized that embryologically the urinary tract and distal colorectum both arise from partitioning of the cloaca. The cloaca is divided into two parts by the urorectal septum. The dorsal side of the cloaca turns into the rectum and the ventral side of cloaca into the urogenital sinus. The former splits into three sections: presumptive bladder, pelvic urethra, and presumptive definitive urogenital sinus, which becomes the vaginal vestibule, and the glandular remnant in the bladder or urethra may give rise to these villous tumors. Based on this pathogenic hypothesis, villous adenomas in the bladder, urethra, and vaginal vestibule have been reported[9]. There is another assumption that these lesions originate from injured urothelial stem cells due to long-term irritation, such as chronic infection and chemical injury, which result in glandular metaplasia[1,2,19,20]. In our case, we found that CK7, CK20 and EMA were positive, which was similar to the immunophenotype in primary villous adenoma of the intestinal tract. Based on this result, we assume that the pluripotent stem cells with glandular differentiation capability might cause intestinal metaplasia and be the origin of adenocarcinoma.
The current studies have suggested that villous adenoma is a well-recognized premalignant lesion of the colon. Villous adenoma of the urinary tract shares the same origins as colon villous adenoma and can be considered as a potential precursor of adenocarcinoma of the urinary tract[21]. Villous adenoma of the urinary tract coexistent with in situ or invasive adenocarcinoma shows marked dysplastic pseudostratified epithelium with enlarged, crowded, and hyperchromatic nuclei[1,21-23]. Adegboyega et al[4] found that villous adenoma of the bladder was heteroploid with high expression of p53, which suggests the potential carcinogenesis of the benign tumor. In addition, both villous adenoma and adenocarcinoma of the bladder are commonly located in the dome of the bladder and urachus. They have similar immunohistochemical features and diffuse expression of CEA, demonstrating that adenocarcinoma originates from villous adenoma[1]. Our case presented with villous adenoma with focal well-differentiated adenocarcinoma, suggesting that villous adenoma in the urinary tract has similar malignant transformation from adenoma to adenocarcinoma to that in the gastrointestinal tract. Therefore, villous adenoma of the urinary tract should be removed completely and sampled adequately to avoid leaving out any malignant lesions.
As the villous adenoma can transform from benign tumor to malignant adenocarcinoma, the latter two share identical morphological and immuno-histochemical features. It is important to make a differential diagnosis between villous adenoma of the urinary tract and secondary involvement from adjacent anatomical sites and particularly metastases, such as from the colon, female genital tract, and prostate gland[1,2,4]. For most patients with prostatic adenocarcinoma, morphological features can be easily identified by histology alone with positive prostatic adenocarcinomas[24]. For female patients with adenocarcinoma of the genital system, carbohydrate antigen 125, estrogen receptor, and progesterone receptor can be specific diagnostic tumor markers. Unfortunately, histological examination is impossible to differentiate urinary tract villous adenoma from metastatic adenocarcinoma of the gastrointestinal tract and there is no specific tumor marker. Wang et al[25] have found that a panel consisting of CK7 and CK20 has some value in differentiating primary bladder adenocarcinoma from metastatic colorectal adenocarcinoma. Nuclear staining for CK20 favors colorectal origin, while primary bladder adenocarcinoma is usually positive for CK7[25,26]. However, clinical history and colonoscopic findings are essential to reach the correct diagnosis in most cases. Differentiation of villous adenoma with coexistent adenocarcinoma in the urinary tract from secondary involvement by colonic cancer may be impossible on morphological grounds alone, although clinical presentation, endoscopic examination, radiographic studies, and histological findings suggest a primary bladder neoplasm[27]. In our case, there were no positive radiographic examinations and complete gastrointestinal evaluation. Immunohistochemical examination was helpful in drawing the conclusion that the urethral tumor in our case was a primary neoplasm.
Patients with pure villous adenoma in the urinary tract have excellent prognosis. It has been demonstrated that surgical excision of the lesion is the most effective approach, allowing for a long-term disease-free period postoperatively. There has been no recurrence or invasive adenocarcinoma in any patients with isolated villous adenoma during a mean follow-up of 9.9 yr[1]. Even patients with in situ adenocarcinoma have shown satisfactory outcome after complete surgical resection[1,28]. However, it is uncertain whether an untreated lesion might eventually develop into an adenocarcinoma[2]. In our case, the mass was found 2 years ago without any diagnosis and treatment. The pathological results of the surgical specimen showed that the glandular structure was disordered with loss of polarity and nuclear atypia. The suggested diagnosis was villous adenoma with focal well-differentiated adenocarcinoma. Therefore, strict follow-up and periodical re-examination are essential because of malignant transformation of the tumor. In our case, there has been no recurrence over the past 11 mo.
In summary, villous adenoma of the urinary tract usually occurs in elderly patients, with a predilection for the urachus, dome, and trigone of the urinary bladder, but rarely for the urethra. Immunohistochemistry is important for the differential diagnosis of villous adenoma of the urinary tract. Patients with isolated villous adenoma have excellent prognosis, and surgical excision might be a good treatment choice and offer long-term survival. Patients with coexistent adenocarcinoma may experience recurrence or distant metastasis, and more aggressive treatments may be indicated. Further study is needed to establish the precursor nature of this uncommon lesion.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nag DSS, Vaudo G S-Editor: Ji FF L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Cheng L, Montironi R, Bostwick DG. Villous adenoma of the urinary tract: a report of 23 cases, including 8 with coexistent adenocarcinoma. Am J Surg Pathol. 1999;23:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Seibel JL, Prasad S, Weiss RE, Bancila E, Epstein JI. Villous adenoma of the urinary tract: a lesion frequently associated with malignancy. Hum Pathol. 2002;33:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Sung W, Park BD, Lee S, Chang SG. Villous adenoma of the urinary bladder. Int J Urol. 2008;15:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Adegboyega PA, Adesokan A. Tubulovillous adenoma of the urinary bladder. Mod Pathol. 1999;12:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Eble JN, Hull MT, Rowland RG, Hostetter M. Villous adenoma of the urachus with mucusuria: a light and electron microscopic study. J Urol. 1986;135:1240-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10193] [Cited by in RCA: 10432] [Article Influence: 237.1] [Reference Citation Analysis (0)] |
| 7. | Lopez-Beltran A, Pacelli A, Rothenberg HJ, Wollan PC, Zincke H, Blute ML, Bostwick DG. Carcinosarcoma and sarcomatoid carcinoma of the bladder: clinicopathological study of 41 cases. J Urol. 1998;159:1497-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 163] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Powell I, Cartwright H, Jano F. Villous adenoma and adenocarcinoma of female urethra. Urology. 1981;18:612-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Howells MR, Baylis MS. Benign urethral villous adenoma. Case report. Br J Obstet Gynaecol. 1985;92:1070-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Raju GC, Roopnarinesingh A, Woo J. Villous adenoma of female urethra. Urology. 1987;29:446-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Morgan DR, Dixon MF, Harnden P. Villous adenoma of urethra associated with tubulovillous adenoma and adenocarcinoma of rectum. Histopathology. 1998;32:87-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Sung MT, Lin JW, Chen WJ. Villous adenomas of the urinary tract: report of two cases. Chang Gung Med J. 2000;23:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Noel JC, Fayt I, Aguilar SF. Adenosquamous carcinoma arising in villous adenoma from female vulvar urethra. Acta Obstet Gynecol Scand. 2006;85:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Tran KP, Epstein JI. Mucinous adenocarcinoma of urinary bladder type arising from the prostatic urethra. Distinction from mucinous adenocarcinoma of the prostate. Am J Surg Pathol. 1996;20:1346-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Channer JL, Williams JL, Henry L. Villous adenoma of the bladder. J Clin Pathol. 1993;46:450-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Trotter SE, Philp B, Luck R, Ali M, Fisher C. Villous adenoma of the bladder. Histopathology. 1994;24:491-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Mazzucchelli R, Scarpelli M, Montironi R. Mucinous adenocarcinoma with superficial stromal invasion and villous adenoma of urachal remnants: a case report. J Clin Pathol. 2003;56:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Atik E, Akansu B, Davarci M, Inci M, Yalcinkaya F, Rifaioglu M. Villous adenoma of the urinary bladder: rare location. Contemp Oncol (Pozn). 2012;16:276-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Chaudhuri A, Sandhu DP, Xuereb J. Villous adenoma of the urinary bladder. BJU Int. 1999;84:177-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Corica FA, Husmann DA, Churchill BM, Young RH, Pacelli A, Lopez-Beltran A, Bostwick DG. Intestinal metaplasia is not a strong risk factor for bladder cancer: study of 53 cases with long-term follow-up. Urology. 1997;50:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Joniau S, Lerut E, Van Poppel H. A Giant Mucinous Adenocarcinoma Arising within a Villous Adenoma of the Urachus: Case Report and Review of the Literature. Case Rep Med. 2009;2009:818646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Yip SK, Wong MP, Cheung MC, Li JH. Mucinous adenocarcinoma of renal pelvis and villous adenoma of bladder after a caecal augmentation of bladder. Aust N Z J Surg. 1999;69:247-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | O'Brien AM, Urbanski SJ. Papillary adenocarcinoma in situ of bladder. J Urol. 1985;134:544-546. [PubMed] |
| 24. | Ford TF, Butcher DN, Masters JR, Parkinson MC. Immunocytochemical localisation of prostate-specific antigen: specificity and application to clinical practice. Br J Urol. 1985;57:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Wang HL, Lu DW, Yerian LM, Alsikafi N, Steinberg G, Hart J, Yang XJ. Immunohistochemical distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Am J Surg Pathol. 2001;25:1380-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Rao Q, Williamson SR, Lopez-Beltran A, Montironi R, Huang W, Eble JN, Grignon DJ, Koch MO, Idrees MT, Emerson RE, Zhou XJ, Zhang S, Baldridge LA, Cheng L. Distinguishing primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma: extended immunohistochemical profiles emphasizing novel markers. Mod Pathol. 2013;26:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Silver SA, Epstein JI. Adenocarcinoma of the colon simulating primary urinary bladder neoplasia. A report of nine cases. Am J Surg Pathol. 1993;17:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Kato Y, Konari S, Obara W, Sugai T, Fujioka T. Concurrence of villous adenoma and non-muscle invasive bladder cancer arising in the bladder: a case report and review of the literature. BMC Urol. 2013;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |