Published online Jun 6, 2019. doi: 10.12998/wjcc.v7.i11.1330
Peer-review started: January 22, 2019
First decision: March 9, 2019
Revised: April 2, 2019
Accepted: April 18, 2019
Article in press: April 19, 2019
Published online: June 6, 2019
Processing time: 137 Days and 4 Hours
Sarcomatoid hepatocellular carcinoma (SHC) combined with paraneoplastic leukemoid reaction (PLR), which is associated with a poor prognosis, is rarely seen in the clinic. Here, we report the case of a patient in the above situation.
A 75-year-old female patient with a past medical history of hypertension and cerebral infarction paid a hospital visit as a result of right upper quadrant abdominal pain and anorexia for two months. Laboratory examination revealed a white blood cell (WBC) count of 43790/μL, which was then increased up to 77050/μL. In addition, the results of bone marrow examination suggested a leukemoid reaction. Computed tomography (CT) revealed a focal hepatic mass, which was confirmed through pathological examination to be an SHC postoperatively. In addition, the WBC count had fallen to a normal level before she left the hospital. However, the patient died two and a half months after the second hospital admission.
This is a rare case of SHC combined with PLR, both of which have an extremely poor prognosis.
Core tip: Sarcomatoid hepatocellular carcinoma (SHC) is a rare histological subtype of hepatocellular carcinoma (HCC), with largely incompletely described clinical manifestations and outcomes. SHC combined with paraneoplastic leukemoid reaction (PLR), which is defined as reactive leukocytosis exceeding 50000/μL, is associated with a poor prognosis and is rarely seen in the clinic. A surgery or surgery-centered multidisciplinary team may benefit patients in this situation.
- Citation: Hu B, Sang XT, Yang XB. Paraneoplastic leukemoid reaction in a patient with sarcomatoid hepatocellular carcinoma: A case report. World J Clin Cases 2019; 7(11): 1330-1336
- URL: https://www.wjgnet.com/2307-8960/full/v7/i11/1330.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i11.1330
Leukemoid reaction is referred to as the condition in which reactive leukocytosis has exceeded 50000/μL, accompanied by a significant increase in the early neutrophil precursors. Typically, leukemoid reaction can serve as a paraneoplastic manifestation of various malignant tumors, including lung, gastrointestinal, genitourinary, ovarian, and head and neck cancers, as well as hepatocellular carcinoma (HCC)[1]. Generally, the most common presentation of the paraneoplastic leukemoid reaction (PLR) is less severe and lacks an increase in the levels of inflammatory markers. It shows no response to antibiotic therapy[2-5]. Moreover, the PLR-induced complications mainly result from increased blood viscosity, and gangrene in the foot has been described in one report[6]. As a result, to manage PLR is indeed to treat the underlying tumor.
Sarcomatoid hepatocellular carcinoma (SHC) is characterized by the proliferation of spindle cells or bizarre giant cells[7] and is usually associated with a dismal prognosis and high risks of recurrence and metastasis. Typically, the hepatocellular markers were negative in the malignant spindle cell component, whereas creatine kinase (CK) and vimentin were positive in most SCC patients[8]. SHC is distinct from nonsarcomatoid liver cancer. However, it can be misdiagnosed as intrahepatic cholangiocarcinoma, making differential diagnosis necessary. PLR is even more rarely seen in SHC patients, which was fatal in our case.
A 75-year-old female patient presented to the hospital as a result of right upper quadrant abdominal pain. Meanwhile, she also complained of anorexia and progressive generalized weakness.
Patient’s symptoms started two months ago with recurrent episodes of bloating.
The patient had a past history of hypertension and cerebral infarction that caused mild instability in walking.
On the first admission, her temperature was 36.4 °C, blood pressure was 100/63 mmHg, pulse rate was 82 beats/min, and respiratory rate was 16 breaths/min. Physical examination revealed tenderness in her right upper abdomen, with no evidence of any abdominal mass.
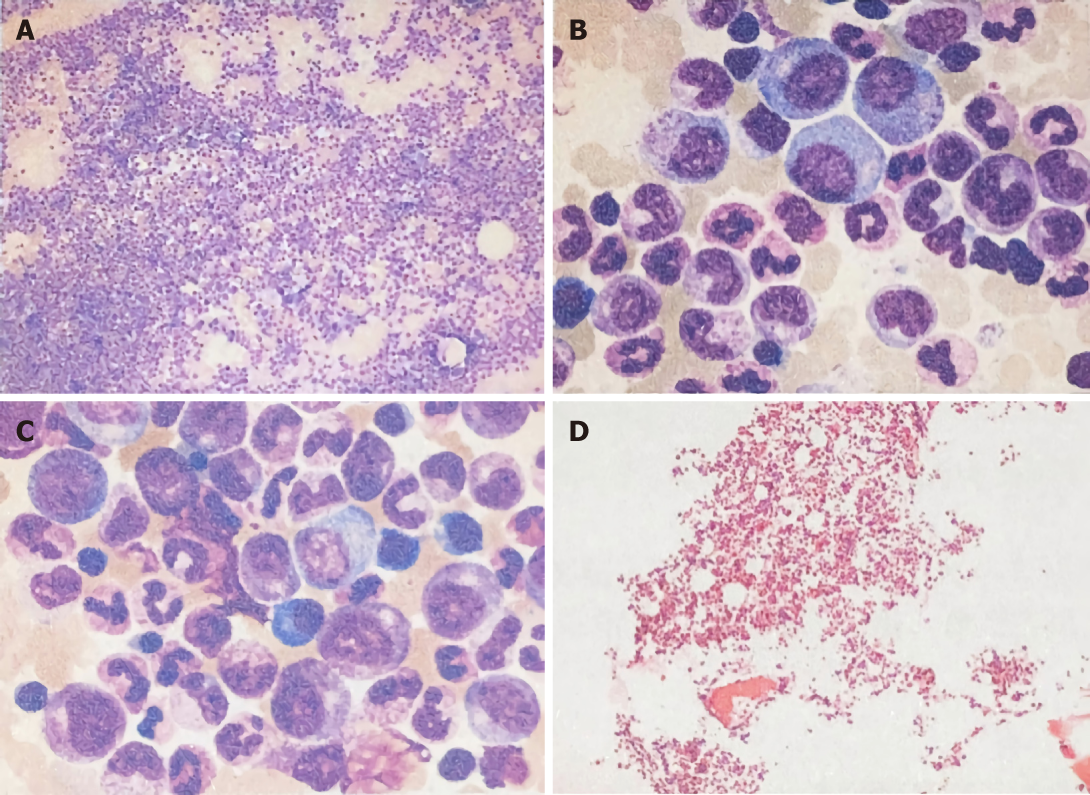
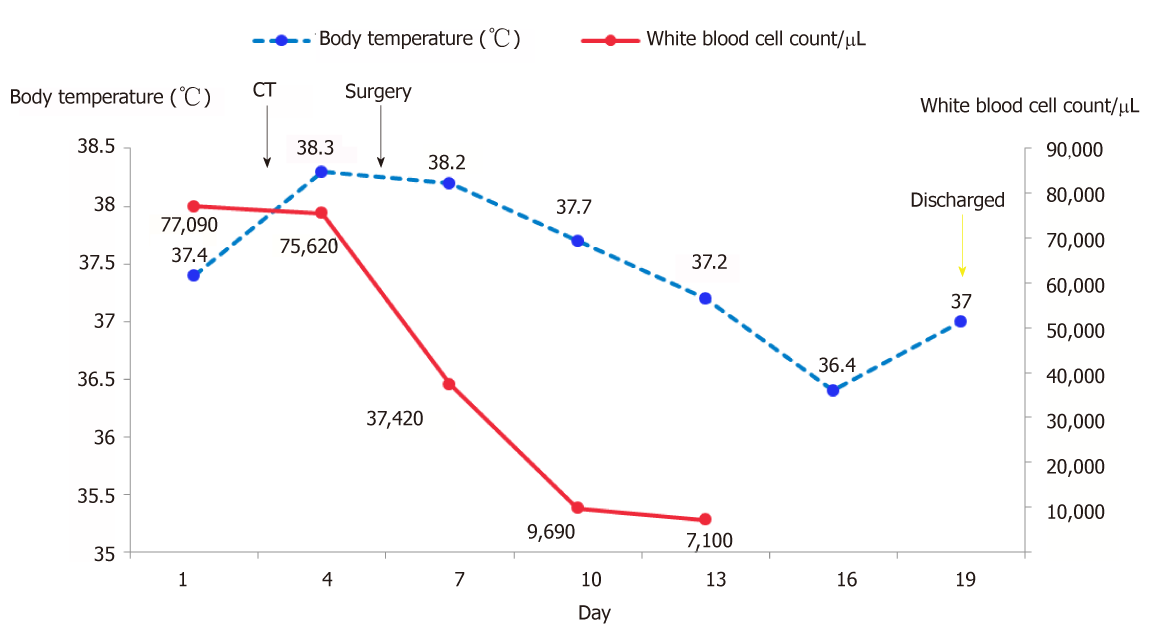
Laboratory examination showed a white blood cell (WBC) count of 43790/μL, with 87.1% of neutrophils, hemoglobin of 12.7 g/dL, platelets of 36200/mm3, total bilirubin of 0.12 mg/dL, direct bilirubin of 0.05 mg/dL, albumin of 3.7 g/dL, aspartate aminotransferase of 18 U/L, and alanine aminotransferase of 22 U/L. In addition, tests for serum tumor markers revealed no abnormalities in the levels of α-fetoprotein (AFP; 3.4 ng/mL) and carcinoembryonic antigen (1.80 ng/mL) but elevated levels of carbohydrate antigenic determinant (CA19-9; 72.6 U/mL). In addition, the coagulation profile was within normal limits, and the results of the hepatitis panel and human immunodeficiency virus antibody tests were negative. In view of the elevated WBC levels, the patient was discharged to the Hematology Clinic for bone marrow biopsy and other tests to rule out the possibility of blood diseases. Two weeks later, the patient was admitted again, and routine blood examination showed a WBC count of 77050/μL. Subsequently, the patient developed a fever of 38.6 °C, which did not subside after antibiotic infusion, and her blood WBC levels were not significantly lowered. The results of the bone marrow examination showed that the proportion of granulocyte-neutrophil nucleated cells was increased by 40%, and toxic granules could be observed (Figure 1). In addition, a peripheral smear revealed neutrophilia with band forms but no blasts. Subsequently, procalcitonin test was performed, and the result was 0.31 ng/mL, indicating a lower risk of infection. Moreover, repeat blood cultures were negative, but the WBC count was elevated continuously.
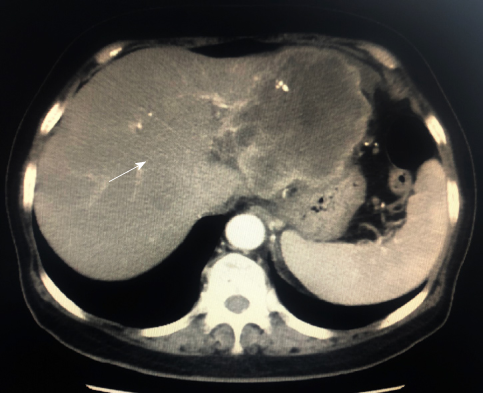
Computed tomography (CT) revealed a 9.3-cm focal hepatic mass in the left lobe of the liver, along with dilatation of the intrahepatic bile duct (Figure 2). A chest radiograph suggested no abnormality.
Hence, symptoms, signs, and laboratory studies were negative for an infectious etiology. In summary, the elevation in WBC count was believed to be caused by a PLR, which was not a surgical contraindication.
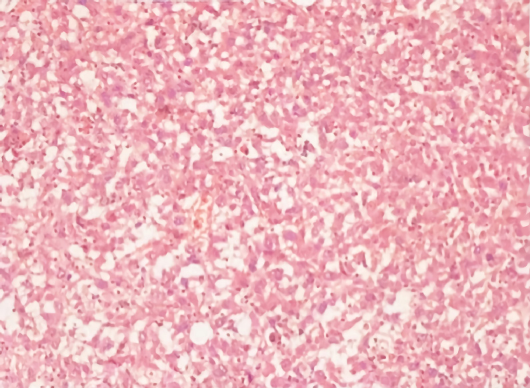
Hepatectomy for the liver mass was performed 5 d after the second hospitalization. Immediately after tumor removal, her presenting symptoms and laboratory values were improved remarkably. Typically, her WBC count had dropped to the normal level 5 d after surgery (Figure 3). Postoperative pathology examination revealed that the poorly differentiated malignant cells were positive for CD34, Vimentin, human epithelial membrane antigen, and cytokeratin 7 (CK7), while negative for CK19 (Figure 4), which was suggestive of SHC.
Unfortunately, the patient died two and a half months after the second discharge. Since the patient’s family did not cooperate with our follow-up work, we are unable to know the specific circumstances of the patient’s death.
Generally, leukemoid reaction is conventionally defined as the condition in which the peripheral WBC count has exceeded 50000/μL, with a dominance of mature neutrophils, and it is related to the reactive causes outside the bone marrow, such as severe infection, poisoning, allergic reaction, drugs, or malignant tumors[3,9,10]. After the causes of the leukemoid reaction are removed, the abnormal changes in WBC counts in blood and bone marrow can return to normal levels within a short period of time.
Specifically, a PLR is a cancer-associated leukemoid reaction. Some scholars believe that PLR is more likely to occur in malignant tumor patients at middle-age[11]. At the same time, tumor stage is also related to the occurrence of PLR, which is particularly true for stage III-IV tumors. Notably, the underlying mechanism appears to be the production of growth factors by the tumor cells, such as granulocyte macrophage colony stimulating factor (GM-CSF), granulocyte CSF (G-CSF), and interleukins (IL-3 and IL-6)[11].
PLR is rarely reported in SHC, and both have dismal prognoses. SHC is a rare histological subtype of HCC, which is discovered in 3.9%-9.4% of HCC autopsy cases and 1.8% of patients with surgically resected HCC[12-14]. However, the pathogenesis of SHC has not been fully elucidated yet, which may be associated with chronic hepatitis B, chronic hepatitis C, liver cirrhosis, preoperative radiotherapy or chemotherapy, and interventional therapy. Kim et al[15] believed that mutations in the p53 gene might be related to the occurrence of SHC. In our case, the patient showed no abovementioned predisposing factors and had not received any nonsurgical treatment. Some scholars call it “pure” SHC[16,17]. Typically, SHC is characterized by high malignancy grade, rapid progression, and poor prognosis. Compared with nonsarcomatoid HCC, SHC is characterized by lower levels of bilirubin, liver enzymes, and AFP, as well as a lower FIB-4 score. In addition, central necrosis and hemorrhage can be more frequently seen in SHC than in the ordinary type of HCC[14,18-20]. Our results are in accordance with previous studies reporting that more than 50% patients were negative for both HBsAg and hepatitis C virus antibody and had negative or low serum AFP levels. According to literature reports, some patients may have fever, but the WBC count is generally not high or is slightly elevated, suggesting the presence of noninfectious fever, which may be related to the sarcomatoid components or tumor parenchymal ischemia and necrosis. In our case, the patient developed fever after admission, with a significant increase in WBC count and neutrophil percentage, and her blood culture results were negative, which was suggestive of PLR. The WBC count in our case had dropped rapidly after surgery, which had confirmed our diagnosis.
In our case, SHC combined with a PLR was fatal, reflecting that such condition was aggressive. Shin et al reported a 71-year-old patient diagnosed with SHC, whose leukocyte count increased to 147800/μL and died on the 10th day of hospitalization, which also suggested the danger of this state. Unlike our case, the patient did not undergo surgery, and the pathological results were obtained by fine needle aspiration biopsy. We hypothesized that timely surgical treatment may be beneficial to such patient to some extent, but overall the prognosis is still poor. For malignant tumor patients with persistently unexplained elevated WBC count, PLR should be considered once infection is excluded. With regard to the relationship between PLR and sarcomatoid carcinoma, some scholars have reported cases of renal cell carcinoma and lung cancer with sarcomatous changes, accompanying PLR; however, it has not been specifically reported whether PLR is more common in sarcomatoid cancer[21,22]. It is likely that the progression and necrosis of SHC may be associated with an increase in inflammatory cytokine response, which may thereby result in leukemoid reaction. In an article examining the relationship between PLR and solid tumors, Chakraborty et al[10] reported a case of SHC with PLR who died 10 d after admission, which is earlier than most other types of tumors (such as cervical cancer, HCC, and gastric cancer). In terms of treatment, liver resection or liver transplantation is the therapeutic gold standard for such patients[23], but the effect is still unclear. On the other hand, with actively evolving tumor treatment modalities and concepts, and knowing that optimal efficacy can rarely be achieved by a single treatment regimen, surgery-centered multidisciplinary team as a collaborative health care model has been increasingly recognized[24], which may also benefit patients in this situation. In short, different factors that influence leukocyte elevation and tumor progression should be considered to understand their pathogenesis and to devise effective strategies for their clinical management.
As evidenced by our case, patients with malignant tumors should consider the possibility of PLR in the presence of persistently unexplained elevated levels of WBC. Nonetheless, the association of SHC with leukemoid reaction could not be concluded due to the limited data and care reports, but a prompt diagnostic approach to identify the underlying cause and early application of the most effective treatment might result in a better prognosis for such patients.
This is a rare case of SHC combined with PLR, both of which had carried an extremely poor prognosis and had been associated with shorter postoperative survival periods.
We would like to express our gratitude to the participants of the study, and to Dr. Hai-Tao Zhao for his contributions in preparing the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kozarek R, Labusca L, Xavier-Elsas P S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Shin HP, Jeon JW, Park JJ, Cha JM, Joo KR, Lee JI, Kim GY, Kang SY. A case of leukemoid reaction in a patient with sarcomatous hepatocellular carcinoma. Korean J Hepatol. 2011;17:226-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Schniewind B, Christgen M, Hauschild A, Kurdow R, Kalthoff H, Klomp HJ. Paraneoplastic leukemoid reaction and rapid progression in a patient with malignant melanoma: establishment of KT293, a novel G-CSF-secreting melanoma cell line. Cancer Biol Ther. 2005;4:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Sakka V, Tsiodras S, Giamarellos-Bourboulis EJ, Giamarellou H. An update on the etiology and diagnostic evaluation of a leukemoid reaction. Eur J Intern Med. 2006;17:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Wilcox RA. Cancer-associated myeloproliferation: old association, new therapeutic target. Mayo Clin Proc. 2010;85:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93:273-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Lammel V, Stoeckle C, Padberg B, Zweifel R, Kienle DL, Reinhart WH, Simon HU. Hypereosinophilia driven by GM-CSF in large-cell carcinoma of the lung. Lung Cancer. 2012;76:493-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Hung Y, Hsieh TY, Gao HW, Chang WC, Chang WK. Unusual computed tomography features of ruptured sarcomatous hepatocellular carcinoma. J Chin Med Assoc. 2014;77:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Shafizadeh N, Kakar S. Hepatocellular Carcinoma: Histologic Subtypes. Surg Pathol Clin. 2013;6:367-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Mandal SK, Ganguly J, Sil K, Mondal SS, Sardar D, Sarkar P. Renal cell carcinoma with paraneoplastic leucocytosis. J Cancer Res Ther. 2015;11:660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Chakraborty S, Keenportz B, Woodward S, Anderson J, Colan D. Paraneoplastic leukemoid reaction in solid tumors. Am J Clin Oncol. 2015;38:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | McCoach CE, Rogers JG, Dwyre DM, Jonas BA. Paraneoplastic Leukemoid Reaction as a Marker of Tumor Progression in Non-Small Cell Lung Cancer. Cancer Treat Commun. 2015;4:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Da Ines D, Bailly A, Lannareix V, Petitcolin V, Boldor L, Charpy C, Abergel A, Pezet D, Garcier JM. Hepatocellular carcinoma with sarcomatous change: prompt and fatal intraabdominal recurrence after liver transplantation. Gastroenterol Clin Biol. 2009;33:590-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Liao SH, Su TH, Jeng YM, Liang PC, Chen DS, Chen CH, Kao JH. Clinical Manifestations and Outcomes of Patients with Sarcomatoid Hepatocellular Carcinoma. Hepatology. 2019;69:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Koo HR, Park MS, Kim MJ, Lim JS, Yu JS, Jin H, Kim KW. Radiological and clinical features of sarcomatoid hepatocellular carcinoma in 11 cases. J Comput Assist Tomogr. 2008;32:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Kim DG, Park SY, Kim H, Chun YH, Moon WS, Park SH. A comprehensive karyotypic analysis on a newly established sarcomatoid hepatocellular carcinoma cell line SH-J1 by comparative genomic hybridization and chromosome painting. Cancer Genet Cytogenet. 2002;132:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Seok JY, Kim YB. [Sarcomatoid hepatocellular carcinoma]. Korean J Hepatol. 2010;16:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Wang QB, Cui BK, Weng JM, Wu QL, Qiu JL, Lin XJ. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma and carcinosarcoma of the liver. J Gastrointest Surg. 2012;16:1715-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1270] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 19. | Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, Brunello F, Pinna AD, Giorgio A, Giulini SM, De Sio I, Torzilli G, Fornari F, Capussotti L, Guglielmi A, Piscaglia F, Aldrighetti L, Caturelli E, Calise F, Nuzzo G, Rapaccini GL, Giuliante F. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 234] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 20. | Liu C, Xiao GQ, Yan LN, Li B, Jiang L, Wen TF, Wang WT, Xu MQ, Yang JY. Value of α-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J Gastroenterol. 2013;19:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Huang W, Wang F, Li Y, Duan F, Yu Z. Leukemoid reaction in sarcomatoid renal cell carcinoma: a two-case report. World J Surg Oncol. 2014;12:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Wang D, Zhang H, Yu F, Fang B. Extreme leukocytosis and leukemoid reaction associated with the lung sarcomatoid carcinoma: an unusual case report. Int J Gen Med. 2016;10:7-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Levi Sandri GB, Ettorre GM, Colasanti M, De Werra E, Mascianà G, Ferraro D, Tortorelli G, Sciuto R, Lucatelli P, Pizzi G, Visco-Comandini U, Vennarecci G. Hepatocellular carcinoma with macrovascular invasion treated with yttrium-90 radioembolization prior to transplantation. Hepatobiliary Surg Nutr. 2017;6:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Wang K, Zhang H, Xia Y, Liu J, Shen F. Surgical options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |