Published online Nov 26, 2018. doi: 10.12998/wjcc.v6.i14.800
Peer-review started: August 7, 2018
First decision: October 3, 2018
Revised: October 16, 2018
Accepted: October 23, 2018
Article in press: October 23, 2018
Published online: November 26, 2018
Processing time: 111 Days and 18.1 Hours
Carney complex (CNC) is an extremely rare genetic syndrome of pigmented skin lesions, endocrine hyperfunction and myxoma. Given its diverse clinical manifestations, CNC is often misdiagnosed. Recognition of some special clinical manifestations and imaging features may help with the diagnosis. Early diagnosis of CNC would alert ongoing surveillance of tumors and complications; the prognosis of CNC may thus be improved by early treatment. Herein, we report two cases of CNC with bone lesions.
Core tip: Carney complex (CNC) is a very rare disease which is often misdiagnosed because of its diverse clinical characteristics. The imaging features of bone lesions in two cases have been summarized in this paper. Recognition of some special clinical manifestations and imaging features may help with the diagnosis; the prognosis of CNC may thus be improved by early treatment.
- Citation: Li S, Duan L, Wang FD, Lu L, Jin ZY. Carney complex: Two case reports and review of literature. World J Clin Cases 2018; 6(14): 800-806
- URL: https://www.wjgnet.com/2307-8960/full/v6/i14/800.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i14.800
Carney complex (CNC) was first described in 1985 by J Aidan Carney, and its main clinical features are spotty pigmentation, endocrine overactivity, and myxoma[1].
The mutation of the PRKAR1A gene on chromosome 17q22-24 and another gene called CNC2 on chromosome 2p16 is considered to be associated with the cause of the disease[2,3]. The prevalence of CNC is unknown, and only about 750 patients have been reported worldwide with CNC by January 2008, based on reports from the National Institutes of Health and the Mayo Clinic (the United States), and the Hospital Cochin (France)[4,5]. Moreover, the diagnosis is often delayed owing to its rarity and complexity. Although some non-endocrine tumors are rare, they are highly characteristic, such as cardiac myxomas and osteochondromyxomas. Herein, we report two cases of CNC with bone lesions and review the relevant literature.
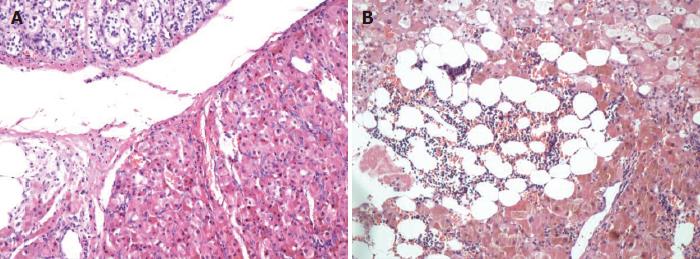
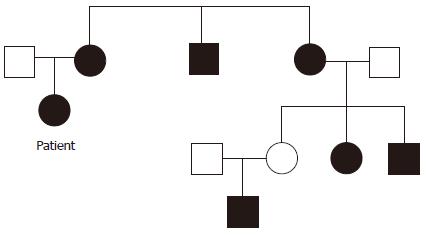
A 27-year-old female who had suffered from recurrent fractures for 17 years, visual decline for 12 years, and a gradually rounded face for 7 years was admitted to our hospital. For 17 years, she has often suffered from various fractures (involving almost every part of the body) after mild activity. Twelve years ago, she suffered ocular proptosis, widened eye distance, and decreased binocular vision. In recent years, she has slowly developed a rounded face and her weight has increased significantly. Three years ago, she developed amenorrhea, and both adrenals were found by abdominal computed tomography (CT) to have multiple nodules. Therefore, the patient underwent bilateral adrenalectomy, and primary pigmented nodular adrenocortical disease (PPNAD) was confirmed (Figure 1A). After the surgery, she regained her normal menstrual cycles. Physical examination revealed an overweight female patient with a full moon face, thin skin, multiple purple striae on both sides of the abdomen, and numerous punctate areas of black or brown pigmentation on her lips and buccal mucosa. Detailed enquiry found a family history of “facial asymmetry”, as demonstrated in Figure 2.
Laboratory tests revealed an abnormal rhythm of cortisol secretion and increased urine free cortisol. Both the low-dose and high-dose dexamethasone suppression tests were not inhibited, indicating primary hypercortisolism. Serum adrenocorticotropic hormone (ACTH) level was less than 5.00 pg/mL (normal range: 5-50 pg/mL).
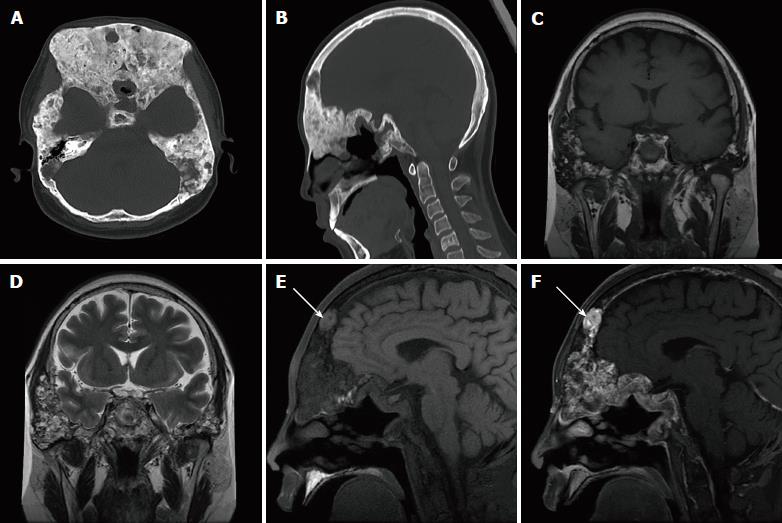
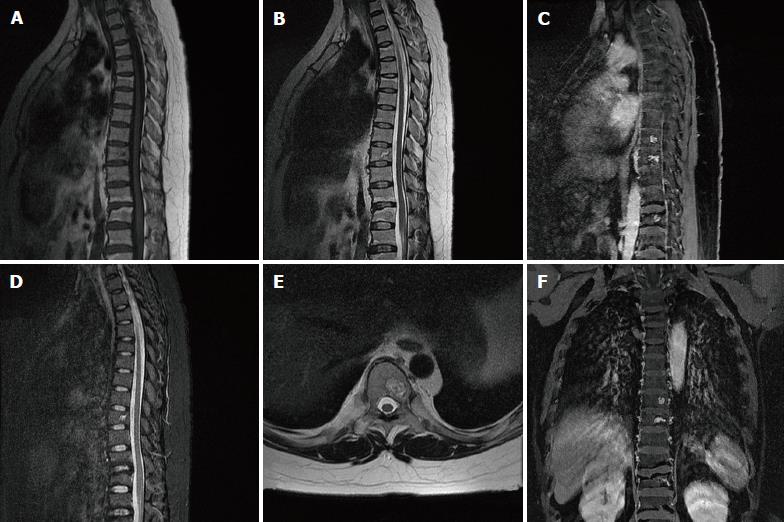
Skull CT examination showed that the skull and maxillofacial bones were remarkably enlarged, with both sclerotic and lytic lesions (Figure 3A and B). Head magnetic resonance imaging (MRI) revealed diffused bone lesions in the frontal, occipital, and sphenoid bones, with low to intermediate and high signal intensity on both T1-weighted (WI) and T2WI images. Some of these retained high signal intensity on fat saturated T1WI and were markedly enhanced after the injection of gadolinium (Figure 3C-F). Spine MRI demonstrated multiple flatted vertebrae, with patchy bone lesions that were of low signal intensity on T1WI, mixed signal intensity on T2WI, and high signal intensity on fat saturated T2WI, with enhancement on gadolinium enhanced T1WI (Figure 4).
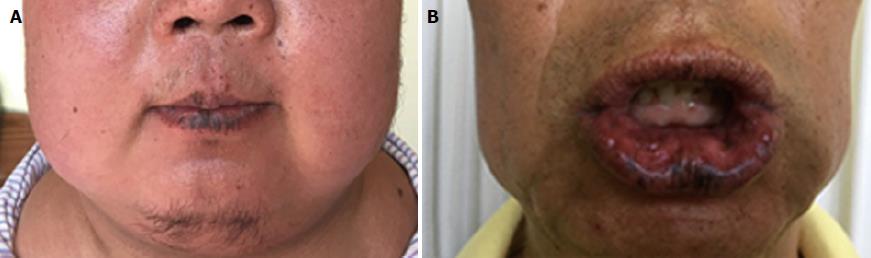
A 26-year-old male with a gradually rounded face and increased abdominal circumference for 2 years was admitted to our hospital. For 2 years, he had noticed his face becoming rounder and redder, the abdominal circumference had increased slowly, and more and more purple lines had developed on both sides of the abdominal skin. Right adrenalectomy was performed because of bilateral adrenal nodular hyperplasia and the postoperative pathology proved to be PPNAD (Figure 1B). On physical examination, the patient had a full, sanguineous moon face and scattered spots of pigmentation on his lips and buccal mucosa, which existed at birth and were similar to his father’s (Figure 5).
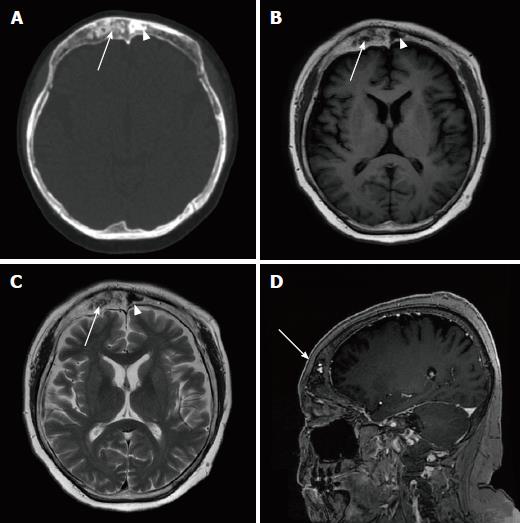
Laboratory tests confirmed hypercortisolism because both the low-dose and high-dose dexamethasone suppression tests were not inhibited. Serum ACTH level was less than 5.00 pg/mL (normal range: 5-50 pg/mL). An enlarged frontal bone of inhomogeneous density with scattered small lytic lesions was found on skull CT (Figure 6A). The sclerotic lesion on the left part of the frontal bone was of high density on CT and low intensity on T1WI and T2WI MRI. In contrast, the lytic lesion was of low density on CT, low intensity on T1WI, and high intensity on T2WI, and was enhanced on gadolinium enhanced T1WI (Figure 6B-D).
Genetic testing was undertaken by both patients to detect the mutation of the PRKAR1A gene, which was negative in the first case and positive in the second case. According to the patients’ clinical findings, imaging manifestations, and gene mutation, the diagnosis of CNC was made. Both patients were regularly followed after discharge, which was uneventful.
CNC is an autosomal dominant disorder, characterized by multiple endocrine tumors and skin and heart involvement. The diagnostic criteria for CNC are: (1) at least two manifestations out of spotty skin pigmentation with a typical distribution (lips, conjunctiva and inner or outer canthi, vaginal and penile mucosa), myxoma (cutaneous and mucosal), cardiac myxoma, breast myxomatosis, PPNAD, acromegaly due to growth hormone-producing adenoma, large-cell calcifying Sertoli cell tumor, thyroid carcinoma, psammomatous melanotic schwannoma, blue nevus, breast ductal adenoma, and osteochondromyxoma; or (2) one of these manifestations plus one of the supplemental criteria (an affected first-degree relative or an inactivating mutation of the PRKAR1A gene)[6]. Both of the cases reported here had a positive family history, typical skin changes, and endocrine abnormalities, and one of them had PRKAR1A gene mutation. Thus, the diagnosis of CNC was made.
These two cases are interesting because both of them had multiple bone lesions. The most common bone lesions in CNC patients are osteochondromyxoma. Osteochondromyxoma is an extremely rare kind of bone tumor, presenting within the context of 1% of CNC cases[7], and is specific for the diagnosis. “Osteochondromyxoma” was used as a key word to search the PubMed database; only nine cases were identified after exclusion of repeated cases. All of them were CNC combined with osteochondromyxoma, three of which were in Japanese, Russian, and Italian, respectively[8-10]; only six cases are reported in English literature[11-13]. According to these limited reports, osteochondromyxoma usually presents as painless masses, which are noticed for their compression effects, such as proptosis and nasal obstruction.
Our first case was a young woman whose bone lesions involved the skull and maxillofacial bones; the second case was a young man with only the frontal bone involved. Both of our cases had a mixed pattern of osteosclerotic bone lesions combined with osteolytic lesions, with Patient 1 affected more markedly. Specifically, the cranial bone lesion in the first patient showed regions with high intensity on fat saturated T1WI and T2WI, which may be the “myxoid matrix”. Furthermore, the enhanced MRI scan revealed marked enhancement of osteolytic regions, for the tumor may contain rich sinusoidal blood vessels. The first patient also has spinal lesions. Golden et al[14] found spinal osteochondromyxoma, presenting with increased T2WI signal intensity and enhancement on post-contrast studies[15], which is similar to our patient. But Patient 1 also had flat vertebral bodies, presumably due to osteoporosis. It was proposed by Golden et al[14] that although little was known about the appearance of osteochondromyxoma, it can be distinguished based on its unique site, symptoms, and radiographic appearance which are different from other bone lesions. Therefore, even though there was no bone biopsy in our two cases, osteochondromyxoma was highly suggested on the basis of the imaging features analysed above.
CNC has been previously called NAME (Nevi, Atrial Myxoma, Ephelides) and LAMB (Lentigines, Atrial myXoma, Blue nevi) syndrome. However, it is different from Carney triad, which presents with gastrointestinal stromal tumours, lung chondromas, and paragangliomas. The clinical features of CNC and some similar genetic syndromes associated with lentigines are summarized in Table 1[16-22]. Molecular genetic studies of CNC have shown that it is linked to the regulatory subunit type I alpha of protein kinase A (PRKAR1A) gene located on 17q22–24, referred to as CNC1. CNC1 encodes PRKAR, which plays an important role in the cAMP signaling pathway[2]. In addition, the CNC2 gene located on 2p16 was also detected in CNC, but its role needs to be further studied[3]. The main causes of CNC death are heart related diseases (57%), mainly cardiac myxomas and complications of operation.
| Clinical features | Estimated incidence[7-13] | |
| Carney complex | Lentigines | 70%-80% |
| Blue nevi | 40% | |
| Cutaneous myxomas | Less than 50% | |
| Primary pigmented nodular adrenocortical disease | 25%-45% | |
| Asymptomatic growth hormone hypersecretion | 66% | |
| Large cell calcifying Sertoli cell tumors | 75% of male patients | |
| Thyroid nodules | 75% | |
| Cardiac myxomas | 30%-72% | |
| Psammomatous melanotic schwannomas | 18% | |
| Benign breast tumor | 14% of female patients | |
| Osteochondromyxomas | < 1% | |
| Peutz-Jeghers syndrome | Mucocutaneous pigmentation | More than 95% |
| Hamartomatous polyps | Most of the patients | |
| McCune-Albright syndrome | Peripheral precocious puberty | 50% in females at 4 yr in de Sanctis C’s research |
| Irregular café-au-lait skin pigmentation | Almost all the patients in de Sanctis C’s research | |
| Fibrous dysplasia of bone | 50% at 8 yr of age in de Sanctis C’s research |
CNC with osteochondromyxoma should be differentiated from McCune–Albright syndrome (MAS) clinically and radiologically. MAS is a syndrome characterized by skin pigmentation, precocious puberty, and fibrous dysplasia (FD)[23,24]. It is caused by a mutation in the guanine nucleotide-binding protein, alpha-stimulating activity polypeptide (GNAS) gene, which lies on chromosome 20q13 and whose mutation leads to adenylyl cyclase over-activity and abnormally increased cyclic adenosine monophosphate (cAMP) levels. Both of these diseases involve the dysregulation of the cAMP signaling pathway, which may explain their clinical and radiological similarity. However, the location of disease may help to make the differential diagnosis between FD in MAS and osteochondromyxoma in CNC from imaging findings: FD in MAS is the polyostotic FD type, which may involve almost the whole skeleton, but rarely the spine. In contrast, the spine is a common target for osteochondromyxoma. In addition, the majority of FD is surrounded by a sclerotic border that is of low signal intensity on T1WI and T2WI. However, osteochondromyxoma has no capsule and it may even show an invasive character, thus its boundary is not very clear. Peutz–Jeghers syndrome also has hyperpigmented macules on the lips and oral mucosa, but it at the same time exhibits benign hamartomatous polyps in the gastrointestinal tract which CNC does not have. Multiple endocrine neoplasia syndromes are inherited as autosomal dominant disorders encompassing several distinct syndromes featuring tumors of endocrine glands, each with its own characteristic pattern. CNC also has a variety of endocrine tumors such as thyroid carcinoma and PPNAD, but there are other symptoms at the same time, for example, spotty skin pigmentation and cardiac myxoma.
If osteochondromyxoma can be removed completely, it is considered to be curable. Although there is a possibility of recurrence at the resection site, no distant metastasis has been reported so far. MRI, the highest resolution imaging modality for soft tissues, can identify the osteolytic lesions and indicate the pathological components through detailed analysis of the signal characteristics in various sequences. Therefore, early identification and preoperative assessment by MRI may help patients to achieve a better prognosis.
Admittedly, there is an obvious limitation of these two cases that there was no bone biopsy since both of them concerned about the invasiveness of the procedure. Nevertheless, the diagnosis of CNC is solid based on the diagnostic criteria.
In conclusion, two cases of CNC with bone lesions have been reported. Detailed analysis of the imaging manifestations of bone lesions may help in the recognition of this rare and complicated syndrome.
Carney complex (CNC) is a very rare disease with diverse clinical characteristics. Osteochondromyxoma is an extremely rare kind of bone tumor, presenting within the context of 1% of CNC cases, and is specific for the diagnosis.
CNC.
Peutz-Jeghers syndrome and McCune-Albright syndrome.
Hypercortisolism.
Osteochondromyxoma.
Primary pigmented nodular adrenocortical disease.
Surgery.
Nine cases were CNC combined with osteochondromyxoma, three of which were in Japanese, Russian and Italian, and only 6 cases are reported in English literature.
CNC.
This case will contribute to improvements in our understanding of the clinical and imaging features, especially osteochondromyxoma, of CNC. In clinical practice, osteochondromyxoma should be taken into account in patients with CNC complicated with skeletal lesions. Early diagnosis will help to improve the prognosis of patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Higa K, Carney JA S- Editor: Dou Y L- Editor: Wang TQ E- Editor: Song H
| 1. | Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore). 1985;64:270-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 654] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 2. | Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 735] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 3. | Matyakhina L, Pack S, Kirschner LS, Pak E, Mannan P, Jaikumar J, Taymans SE, Sandrini F, Carney JA, Stratakis CA. Chromosome 2 (2p16) abnormalities in Carney complex tumours. J Med Genet. 2003;40:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Espiard S, Bertherat J. Carney complex. Front Horm Res. 2013;41:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I. Mutations in regulatory subunit type 1A of cyclic adenosine 5’-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041-4046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 399] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 7. | Kitsoulis P, Galani V, Stefanaki K, Paraskevas G, Karatzias G, Agnantis NJ, Bai M. Osteochondromas: review of the clinical, radiological and pathological features. In Vivo. 2008;22:633-646. [PubMed] |
| 8. | Katsube Y, Fukuchi T, Ito E. Report of a case of osteochondromyxoma of the chest wall. Gan No Rinsho. 1965;11:706-709. [PubMed] |
| 9. | Sokolov AV, Gretskaia EV, Mazanov GS. Case of a large osteochondromyxoma of the rib. Vestn Rentgenol Radiol. 1972;47:93-94. [PubMed] |
| 10. | POLI A. Giant sacroiliac osteochondromyxoma in dyschondroplasia. Arch Ortop. 1955;68:539-549. [PubMed] |
| 11. | Carney JA, Boccon-Gibod L, Jarka DE, Tanaka Y, Swee RG, Unni KK, Stratakis CA. Osteochondromyxoma of bone: a congenital tumor associated with lentigines and other unusual disorders. Am J Surg Pathol. 2001;25:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Mountricha A, Zotalis N, Giannakou N, Xenelis J, Maroudias N. Osteochondromyxoma of Maxilla: Case Report. Skull Base Surg. 2009;19:A101. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Waissbluth S, Winter M, Dreyse X, Solar A, Castro M, Rosenblut A. Osteochondromyxoma of the nasal cavity: Case report and review of the literature. EJMCR. 2018;2:12-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Golden T, Siordia JA. Osteochondromyxoma: Review of a rare carney complex criterion. J Bone Oncol. 2016;5:194-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Courcoutsakis NA, Tatsi C, Patronas NJ, Lee CC, Prassopoulos PK, Stratakis CA. The complex of myxomas, spotty skin pigmentation and endocrine overactivity (Carney complex): imaging findings with clinical and pathological correlation. Insights Imaging. 2013;4:119-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Correa R, Salpea P, Stratakis CA. Carney complex: an update. Eur J Endocrinol. 2015;173:M85-M97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 17. | Carney JA, Stratakis CA. Ductal adenoma of the breast and the Carney complex. Am J Surg Pathol. 1996;20:1154-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Irani AD, Estrera AL, Buja LM, Safi HJ. Biatrial myxoma: a case report and review of the literature. J Card Surg. 2008;23:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Jelsig AM, Qvist N, Brusgaard K, Nielsen CB, Hansen TP, Ousager LB. Hamartomatous polyposis syndromes: a review. Orphanet J Rare Dis. 2014;9:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Mateus C, Palangié A, Franck N, Groussin L, Bertagna X, Avril MF, Bertherat J, Dupin N. Heterogeneity of skin manifestations in patients with Carney complex. J Am Acad Dermatol. 2008;59:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Maleszewski JJ, Larsen BT, Kip NS, Castonguay MC, Edwards WD, Carney JA, Kipp BR. PRKAR1A in the development of cardiac myxoma: a study of 110 cases including isolated and syndromic tumors. Am J Surg Pathol. 2014;38:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | de Sanctis C, Lala R, Matarazzo P, Balsamo A, Bergamaschi R, Cappa M, Cisternino M, de Sanctis V, Lucci M, Franzese A. McCune-Albright syndrome: a longitudinal clinical study of 32 patients. J Pediatr Endocrinol Metab. 1999;12:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1002] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 24. | Martin-Carreras T, Sanchez E, Bancroft LW. Polyostotic fibrous dysplasia. Orthopedics. 2014;37:722-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |