Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1442
Peer-review started: November 13, 2023
First decision: January 9, 2024
Revised: January 15, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 16, 2024
Processing time: 119 Days and 21.7 Hours
Immature ovarian teratoma is a rare and aggressive neoplasm that affects young women. This report is the first to describe the development of immature teratoma after ovarian cystectomy for mature teratoma of the ovary in an adolescent female with a family history of ovarian teratoma.
A 16-year-old girl who had undergone bilateral ovarian cystectomy for mature teratomas 3 years ago showed bilateral adnexal tumors during her regular ultrasonography follow-up every 6 months. She received laparoscopic bilateral ovarian cystectomy, and final histopathology showed grade-1 immature teratoma of the left ovary and mature teratoma of the right ovary. Laparoscopic left salpingo-oophorectomy and staging procedures were performed again. Her mother, maternal aunt, and maternal grandmother had also received surgeries for mature ovarian teratomas.
It is important to have guidance on management of patient and family members with familial ovarian teratomas.
Core Tip: This report describes a unique case of an adolescent woman with familial ovarian teratoma that was initially diagnosed as mature ovarian teratoma and later recurred and identified as immature ovarian teratoma during follow-up. Our case highlights the importance of genetic counseling, screening, and close surveillance in families with a predisposition to ovarian teratomas. Collaborative efforts between oncologists, geneticists, and researchers are necessary to determine the underlying mechanisms and develop targeted interventions for individuals at high risk of immature teratomas.
- Citation: Ju UC, Kang WD, Kim SM. Development of immature ovarian teratoma after mature teratoma in a girl with familial ovarian teratoma: A case report. World J Clin Cases 2024; 12(8): 1442-1447
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1442.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1442
The teratomas of ovary derive from the primordial ovarian germ cells and are categorized into three groups: Benign mature teratoma, non-malignant monodermal teratoma and malignant immature teratoma. Mature cystic or solid ovarian teratomas account for 90% of ovarian tumors in premenarchal girls and 60% in women under 20-years-old[1]. Immature ovarian teratoma is a malignant germ cell tumor that originates from pluripotent cells in the ovary. It predominantly affects pediatric and adolescent females, and accounts for approximately 10%-20% of all ovarian teratomas[2]. Although ovarian teratomas are common, the incidence of familial ovarian teratomas is very rare, with only a handful of published case reports[1-3]. Moreover, we could not find reports of immature teratoma developing after ovarian cystectomy for mature teratoma of the ovary in an adolescent woman with a family history of ovarian teratomas in the English literature.
This report describes a unique case of an adolescent woman with familial ovarian teratoma that was initially diagnosed as mature ovarian teratoma and later recurred and identified as immature ovarian teratoma during follow-up.
Bilateral adnexal tumors detected during her regular ultrasonography (US) examination.
A 16-year-old girl (G0P0) presented to our gynecology department at Chonnam National University Hospital with bilateral adnexal tumors detected during her regular US follow-up.
She had undergone bilateral ovarian cystectomy for mature teratomas at our hospital 3 years ago and was under regular 6-month follow-up at a local gynecology center.
Her mother, maternal aunt, and maternal grandmother had undergone surgeries for mature ovarian teratomas.
She was systemically healthy. Her vital sign was stable. Non-specific sign was showed on physical examination.
Her laboratory findings were normal. The tumor markers, α-fetoprotein and β-human chorionic gonadotropin were within normal range.
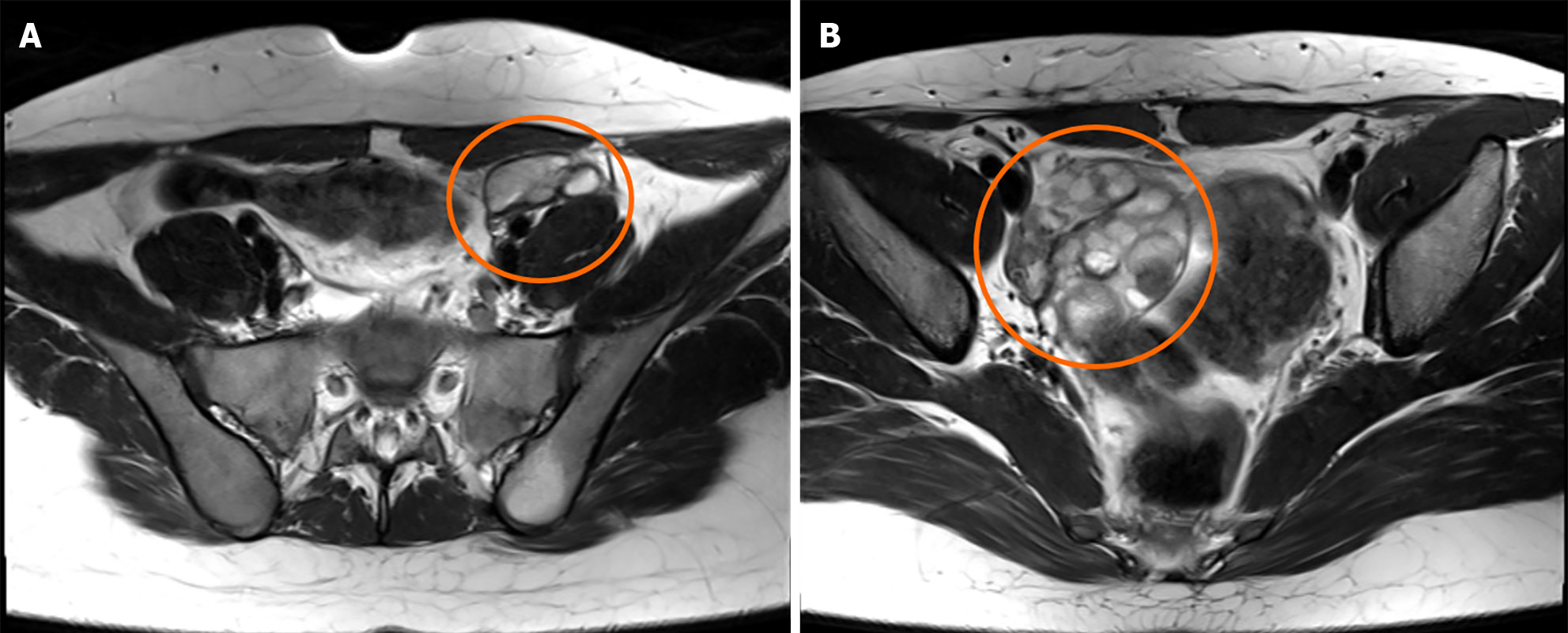
Subsequent pelvic magnetic resonance imaging (MRI) showed multiple cystic masses in the left ovary and multiple cystic masses in the right ovary (Figure 1), which strongly indicated recurrent mature cystic teratomas.
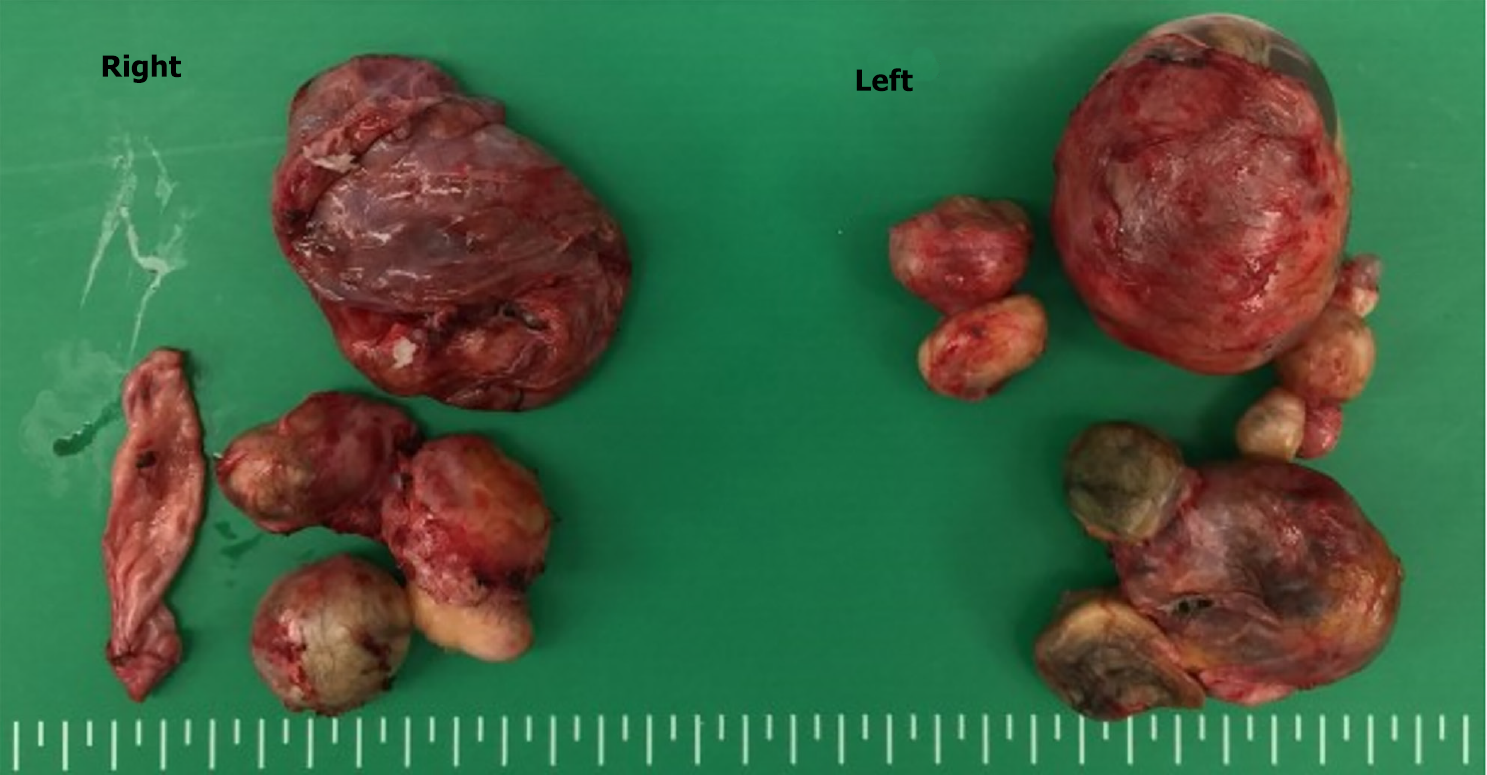
We performed laparoscopic bilateral ovarian cystectomy based on the findings (Figure 2). She recovered well and was discharged to home on the third postoperative day. The final histopathology confirmed grade-1 immature teratoma of the left ovary and mature cystic teratoma of the right ovary.
She then underwent laparoscopic left salpingo-oohorectomy and staging procedures again. Subsequent examination showed no remnant tumor and no metastasis. We recommended frequent follow-up to the patient and her parents.
After 3 years of follow-up, she experienced a relapse of 3 cm sized mature cystic tumor at the remnant right ovary, as observed on pelvic MRI.
Familial ovarian teratomas are extremely rare, and their exact incidence has not been reported. Although several studies reported a common genetic basis, none provide definitive evidence. These tumors could carry significant consequences for every female member within the family. The risk factors for a secondary tumor and the potential complications that may follow, such as malignant transformation, rupture, or torsion, are yet to be ascertained. The scarcity of data regarding this medical condition presents a substantial hurdle for pediatric surgeons when it comes to patient care, guidance, and addressing the concerns of family members.
We conducted a literature search in the Medline and PubMed databases, encompassing studies available until 2022, with the aim of collecting data that can assist in shaping guiding principles. Our literature review showed that only few case reports describe familial ovarian teratomas occurring across several generations. Moreover, there is limited evidence available in the literature regarding the management of isolated, nonfamilial ovarian teratomas.
Up to now, there have been only 7 instances resembling this in the published literature (as indicated in the Table 1). Of these 7 cases, only 2 involve an ovarian teratoma occurring in 3 consecutive generations. Moreover, only one of these reports described an immature teratoma. We reported a case of an immature ovarian teratoma recurring after treatment for a mature ovarian teratoma, which has not been described in previous reports. In addition, previous reports have predominantly centered on the theories explaining the origin of ovarian teratomas rather than addressing the management and counseling of patients and their families, respectively.
| Ref. | Year of issue | Relation | Pathology | Age |
| Plattner and Oxorn[1] | 1973 | Mother | Mature | 22 |
| Daughter 1 | Mature | 23 | ||
| Daughter 2 | Mature | 23 | ||
| Hecht et al[8] | 1976 | Grandmother | Mature | N/A |
| Granddaughter | Mature | 21 | ||
| Brenner and Wallach[9] | 1983 | Three successive generation | Mature | 7, 34, 45 |
| Gustavson and Rune[10] | 1988 | Mother | Mature | 26 |
| Daughter 1 | Mature | 24 | ||
| Daughter 2 | Mature | 22 | ||
| Kim and Böhm-Vélez[11] | 1994 | Mother | Mature | 42 |
| Daughter 1 | Mature | 26 | ||
| Daughter 2 | Mature | 20 | ||
| Nezhat et al[2] | 2010 | Mother | Mature | 26 |
| Daughter 1 | Mature | 32 | ||
| Daughter 2 | Mature | 25 | ||
| Daughter 3 | Mature | 22 | ||
| Braungart and McCullagh[3] | 2016 | Grandmother | Mature | 25 |
| Mother | Mature | 21 | ||
| Daughter | Immature | 8 | ||
| This report | Grandmother | Mature | 47 | |
| Mother | Mature | 33 | ||
| Aunt | Mature | 29 | ||
| Daughter | Mature immature | 13 |
A Canadian research project conducted a retrospective analysis of the postoperative care for children with nonfamilial ovarian teratomas at their medical facility[4]. The findings of the study indicated a recurrence rate of as high as 15%. It remains uncertain whether these recurrences represented authentic metachronous tumors or a resurgence of inadequately removed tumors. Regardless, the recurrence rate in this study is significantly higher than that previously published for adult populations (0%-4%). The subsequent assessment after the initial treatment exhibited discrepancies across the studies, encompassing scenarios ranging from a sole follow-up appointment devoid of any imaging to yearly follow-ups with imaging for a minimum of 5 years following the surgery. US was the prevailing imaging method employed for follow-up across all the studies. The literature review unmistakably highlights the absence of concrete guidance for patient care and post-treatment monitoring.
US is the primary diagnostic imaging tool for patients with potential ovarian cysts. US serves as an efficient imaging technique for detecting ovarian abnormalities and is frequently employed to verify the existence of a tumor[5]. It is useful in determining morphologic characteristics such as multilocularity, thickened walls, projections, or irregular cyst contents. It is relatively inexpensive and easy to perform. A 98% positive predictive value with 85%-94% sensitivity has been reported for the diagnosis and identification of mature ovarian teratomas using US[6,7]. Transvaginal US helps determine the cyst structure, whereas 3D US enables visualizing blood flow within and around the cyst. However, the appearance of mature ovarian teratomas on US is frequently nonspecific. US is a valuable imaging technique for the initial screening of ovarian abnormalities. However, in some cases, it can be challenging to differentiate between certain pathologies, like hemorrhagic cysts with blood clots, which may resemble mature ovarian teratomas. Additionally, distinguishing between a mature teratoma and an immature teratoma using US can be difficult because US often struggles to detect the solid components typical of an immature ovarian teratoma. As a result, further imaging, such as MRI, is frequently recommended to characterize the nature of an ovarian tumor.
Guidance on the appropriate follow-up imaging interval is lacking. Therefore, with regard to managing familial ovarian teratoma, evidence-based guidance on the most suitable imaging modality and the appropriate imaging follow-up interval, the appropriate follow-up duration, and the need for screening other family members cannot be established. Our patient was prescribed US imaging follow-up every 6 months after the first surgical treatment and received pelvic MRI after recurrence. After surgical treatment for the immature teratoma, she was prescribed US imaging follow-up for every 3 months and computed tomography imaging follow-up for every 6 months. We also recommended regular US examination to her maternal female cousins.
Our case highlights the importance of genetic counseling, screening, and close surveillance in families with a predisposition to ovarian teratomas. Genetic counseling should be considered for families with a history of teratoma to facilitate early detection and management of potential cases among descendants. Collaborative efforts between oncologists, geneticists, and researchers are necessary to determine the underlying mechanisms and develop targeted interventions for individuals at high risk of immature teratomas. Further studies are warranted to identify the genetic factors contributing to the development of familial ovarian teratomas and to optimize management strategies for affected individuals.
I would like to express my gratitude to all the peer reviewers and editors for their valuable feedback and recommendations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brind'Amour A, Canada S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Plattner G, Oxorn H. Familial incidence of ovarian dermoid cysts. Can Med Assoc J. 1973;108:892-893. [PubMed] |
| 2. | Nezhat C, Kotikela S, Mann A, Hajhosseini B, Veeraswamy A, Lewis M. Familial cystic teratomas: four case reports and review of the literature. J Minim Invasive Gynecol. 2010;17:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Braungart S, McCullagh M. Management of Familial Ovarian Teratoma: The Need for Guidance. European J Pediatr Surg Rep. 2016;4:31-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Rogers EM, Allen L, Kives S. The recurrence rate of ovarian dermoid cysts in pediatric and adolescent girls. J Pediatr Adolesc Gynecol. 2014;27:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Saba L, Guerriero S, Sulcis R, Virgilio B, Melis G, Mallarini G. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. Eur J Radiol. 2009;72:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Pothula V, Matseoane S, Godfrey H. Gonadotropin-producing benign cystic teratoma simulating a ruptured ectopic pregnancy. J Natl Med Assoc. 1994;86:221-222. [PubMed] |
| 7. | Mahdavi A, Berker B, Nezhat C, Nezhat F. Laparoscopic management of ovarian cysts. Obstet Gynecol Clin North Am. 2004;31:581-592, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Hecht F, McCaw BK, Patil S. Ovarian teratomas and genetics of germ-cell formation. Lancet. 1976;2:1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Brenner SH, Wallach RC. Familial benign cystic teratomata. Int J Gynaecol Obstet. 1983;21:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Gustavson KH, Rune C. Familial ovarian dermoid cysts. Ups J Med Sci. 1988;93:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Kim R, Böhm-Vélez M. Familial ovarian dermoids. J Ultrasound Med. 1994;13:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |