Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1416
Peer-review started: November 12, 2023
First decision: December 28, 2023
Revised: January 9, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 16, 2024
Processing time: 120 Days and 18.5 Hours
Epidural analgesia is the most effective analgesic method during labor. Butor
To assess butorphanol's safety and efficacy for epidural labor analgesia.
The PubMed, Cochrane Library, EMBASE, Web of Science, China National Know
This study provides reliable information regarding the safety and efficacy of using butorphanol as an epidural analgesic during labor.
To support clinical practice and development, this study provides evidence-based findings regarding the safety and efficacy of using butorphanol as an epidural analgesic during labor.
Core Tip: Because κ-receptors appear to be involved in visceral pain modulation, butorphanol, which has a strong visceral component, has been recommended as an effective treatment for labor-related pain. Nevertheless, no study has comprehensively examined the effectiveness and safety of using butorphanol as an epidural analgesic during labor. The safety and effectiveness of butorphanol for epidural analgesia during labor will be thoroughly and systematically investigated in this study. Future research and clinical practice will benefit from the conclusions of the present study.
- Citation: Tang GC, He M, Huang ZZ, Cheng Y. Safety and effectiveness of butorphanol in epidural labor analgesia: A protocol for a systematic review and meta-analysis. World J Clin Cases 2024; 12(8): 1416-1421
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1416.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1416
The pain that a woman feels during delivery is regarded as one of the most excruciating sensations she can experience, and it can have a severe influence on both maternal and fetal physiology[1]. The use of neuraxial analgesic treatments during labor increases patient satisfaction and decreases pain levels without affecting maternal cardiovascular or lung function or fetal physiology[2]. The most effective analgesic technique during childbirth is epidural analgesia[3]. Previous research has demonstrated that combining epidural narcotics with local anesthetics results in a quicker onset and longer duration of analgesia[4]. Butorphanol is a lipid-soluble narcotic that has modest agonistic and antagonistic effects as well as significant κ-receptor agonism. Butorphanol, which has a strong visceral component, has been proposed to be effective at reducing pain during labor because κ-receptors appear to be involved in visceral pain regulation[5-10]. However, no study has comprehensively examined the safety and efficacy of using butorphanol as an epidural analgesic during labor.
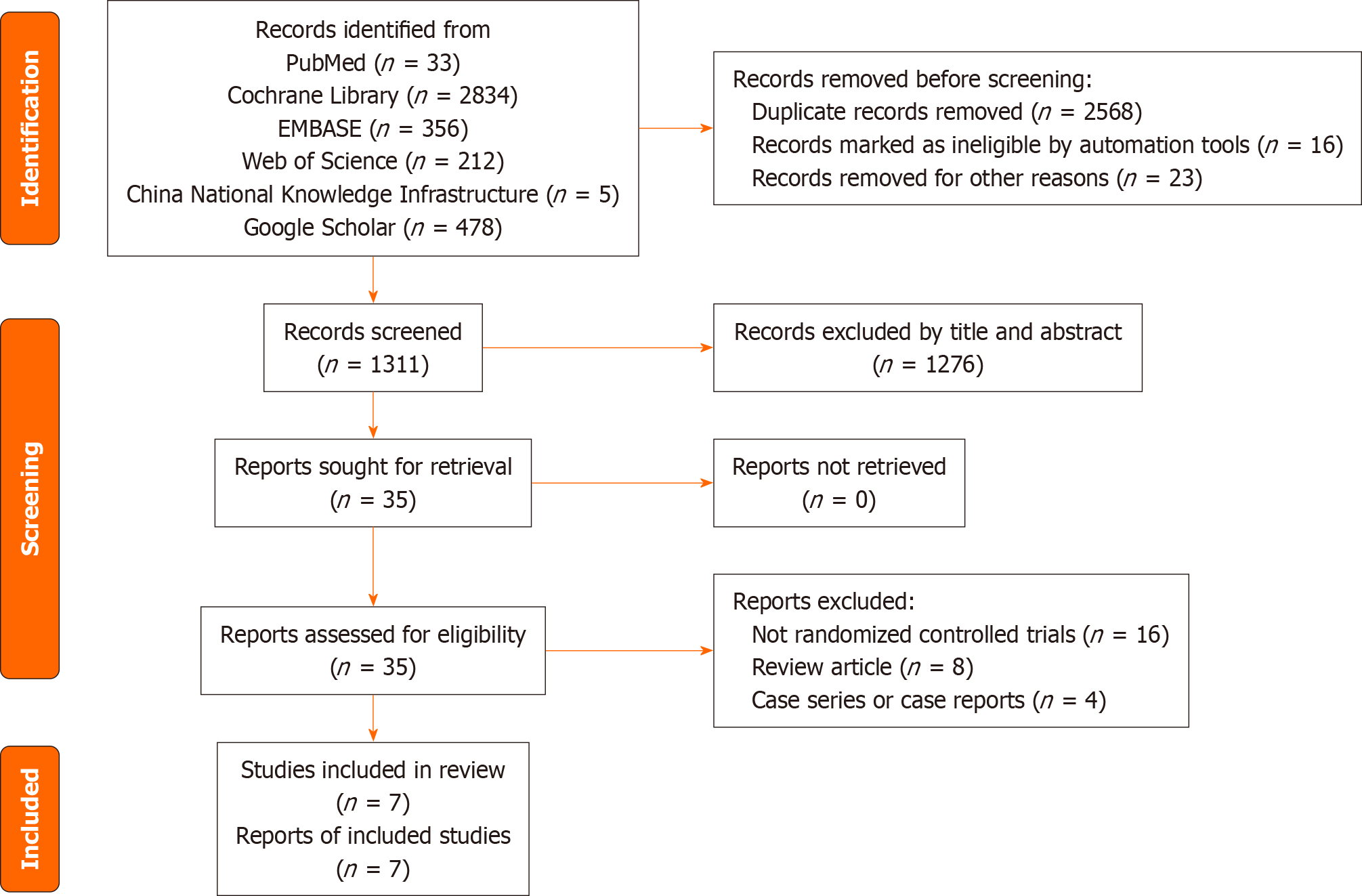
This systematic review will be conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines[11]. We will construct a PRISMA flow chart to illustrate the study selection process (Figure 1). This protocol was developed in accordance with the PRISMA Protocol guidelines. The PRISMA checklist was used to ensure the quality of the protocol, as shown in the PRISMA 2009 checklist statement[12]. Our protocol has been registered in the international prospective register of a systematic review with registration number PROSPERO CRD42022383830. Any amendments to the currently registered protocol will be submitted to the PROSPERO database along with the reasons for such changes. The amended version of the protocol will then be made public through the database.
Types of studies: This systematic review will include randomized controlled trials (RCTs) examining the use of butor
Types of participants: We will include parturients aged 18 years and older from any country who have received an epi
Types of interventions: All subjects in the experimental group will have received butorphanol in combination with local anesthetics such as bupivacaine or ropivacaine.
All participants in the control group will have received epidural analgesia during labor with or without opioids mixed with local anesthetics.
Types of outcome measures: Primary outcomes: (1) Visual analog scale score for the first stage of labor; and (2) Fetal effects and Apgar scores.
Secondary outcomes: Duration of the first stage of labor, duration of the second stage of labor, incidence of side effects, vaginal delivery rate, and degree of motor block.
Search strategy: The PubMed, Cochrane Library, EMBASE, Web of Science, China National Knowledge Infrastructure, and Google Scholar electronic databases will be searched from inception. The databases will be searched without language or publication status restrictions. All database searches will be tailored to the specific database using a combination of Medical Subject Headings and free terms. The following terms will be used: butorphanol, parturient, epidural anesthetic, epidural labor analgesia, and RCT. Table 1 presents the search strategy for PubMed. Similar search algorithms will be developed for additional datasets. To prevent missing any potentially eligible research, we will search gray literature such as conference abstracts and evaluated references.
| Search number | Query | Results |
| 1 | "Butorphanol"[MeSH Terms] OR (("17"[All Fields] AND "Cyclobutylmethyl"[All Fields]) AND "morphinan 3 14 diol"[Title/Abstract]) OR "BC-2627"[Title/Abstract] OR "BC-2627"[Title/Abstract] OR "Beforal"[Title/Abstract] OR "butorphanol tartrate"[Title/Abstract] OR "Moradol"[Title/Abstract] OR "Stadol"[Title/Abstract] OR "stadol ns"[Title/Abstract] OR "Torbugesic"[Title/Abstract] | 1268 |
| 2 | "anesthesia, epidural"[MeSH Terms] OR "anesthesia peridural"[Title/Abstract] OR (("anaesthesia"[All Fields] OR "Anesthesia"[MeSH Terms] OR "Anesthesia"[All Fields] OR "anaesthesias"[All Fields] OR "Anesthesias"[All Fields]) AND "Peridural"[Title/Abstract]) OR "peridural anesthesia"[Title/Abstract] OR "peridural anesthesias"[Title/Abstract] OR "anesthesia extradural"[Title/Abstract] OR (("anaesthesia"[All Fields] OR "Anesthesia"[MeSH Terms] OR "Anesthesia"[All Fields] OR "anaesthesias"[All Fields] OR "Anesthesias"[All Fields]) AND "Extradural"[Title/Abstract]) OR "extradural anesthesia"[Title/Abstract] OR (("Extradural"[All Fields] OR "extradurally"[All Fields]) AND "Anesthesias"[Title/Abstract]) OR "epidural anesthesia"[Title/Abstract] OR (("anaesthesia"[All Fields] OR "Anesthesia"[MeSH Terms] OR "Anesthesia"[All Fields] OR "anaesthesias"[All Fields] OR "Anesthesias"[All Fields]) AND "Epidural"[Title/Abstract]) OR "epidural anesthesias"[Title/Abstract] OR "intraspinal labor analgesia"[Title/Abstract] OR "epidural analgesia in labor"[Title/Abstract] OR "labour epidural analgesia"[Title/Abstract] OR (("labor s"[All Fields] OR "labored"[All Fields] OR "laborer"[All Fields] OR "laborer s"[All Fields] OR "laborers"[All Fields] OR "laboring"[All Fields] OR "labors"[All Fields] OR "labour"[All Fields] OR "work"[MeSH Terms] OR "work"[All Fields] OR "labor"[All Fields] OR "labor, obstetric"[MeSH Terms] OR ("labor"[All Fields] AND "obstetric"[All Fields]) OR "obstetric labor"[All Fields] OR "laboured"[All Fields] OR "labourer"[All Fields] OR "labourers"[All Fields] OR "labouring"[All Fields] OR "labours"[All Fields]) AND "with epidural analgesia"[Title/Abstract]) | 26241 |
| 3 | ("randomized controlled trial"[Publication Type] OR "controlled clinical trial"[Publication Type] OR "randomized"[Title/Abstract] OR "placebo"[Title/Abstract] OR "drug therapy"[MeSH Subheading] OR "randomly"[Title/Abstract] OR "trial"[Title/Abstract] OR "groups"[Title/Abstract]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]) | 5022394 |
| 4 | 1 AND 2 AND 3 | 33 |
Options for examination: Two authors will separately and sequentially screen the titles and abstracts of all identified entries. All irrelevant records will be removed. After that, we will get the full texts of all the other articles that fit the requirements and evaluate them all to see if they should be included. A third author will settle any disputes that arise throughout the verification process. A flowchart illustrating the complete study selection procedure is provided, and any studies that are missed will be noted.
To decrease the potential of bias, two writers will independently extract data from the included studies using a standardized data extraction form. Any differences between the two writers will be settled by discussion with a third author. The following information will be extracted: first author, year of publication, inclusion and exclusion criteria, race, age, sample size, study methodology, treatment specifics, outcome measures, safety, and any other pertinent information. We will contact the original writers to get or clarify any missing or confusing information.
Using the Cochrane risk of bias methodology for RCTs, we will evaluate the included studies' risk of bias. Seven domains will be used to assess the bias risk. Every study will be assigned a risk of bias classification: low, unsure, or high. Before a decision is reached, a third author will debate any discrepancies between the two writers who will independently examine the probability of bias.
We will perform a statistical analysis using RevMan 5.4 software. For dichotomous data, odds ratios and 95%CI will be utilized, but for continuous data, mean differences or normalized mean differences and 95%CI will be employed. As shown below, we will utilize I2 statistics to evaluate any possible heterogeneity among the included research. When there is moderate heterogeneity, as indicated by an I2 of 50%, a fixed effects model will be applied. When there is substantial heterogeneity, as indicated by an I2 > 50%, a random effects model will be applied. The same treatments, controls, and outcomes will be used in a meta-analysis if there is little variation among the qualifying trials. In order to identify the source of any evident heterogeneity, a subgroup analysis will be carried out.
Based on the study and patient characteristics, study quality, treatments, controls, and outcomes, subgroup analyses will be carried out.
By excluding subpar research, a sensitivity analysis will be carried out to evaluate the consistency of the results.
Egger's or Begg's tests will be used to measure the funnel plot's asymmetry in order to determine the likelihood of publication bias. Since the test power is sometimes insufficient to distinguish between chance and true asymmetry in such circumstances, the test for funnel plot asymmetry will not be used when the meta-analysis includes less than ten primary studies. The trim and fill method will be used to address any potential publishing bias if there is a significant amount of it. In addition, the degree to which the funnel plot's significant asymmetry is susceptible to additional biases that could account for it will be assessed.
During labor, lipid-soluble opioids are often used in conjunction with local anesthetics to improve epidural analgesia. This allows for a lower dose of local anesthetic with a decreased risk of motor block[12-14]. Fentanyl and sufentanil are widely used as epidural analgesics but carry the risks of delayed respiratory depression, pruritus, and vomiting[15]. These adverse reactions have prompted a search for alternative drugs or methods to provide labor pain relief[16]. Epidural butorphanol has been used effectively for analgesia during labor and after cesarean section[5,17-19], but no neurotoxic effects have been reported in humans. However, no comprehensive study has been published on the safety and efficacy of using butorphanol as an epidural analgesic during labor. As a result, this study systematically and completely explored the safety and efficacy of using butorphanol as an epidural analgesic during labor. The findings of this study will be valuable for clinical practice as well as for future research.
To support clinical practice and development, this study provides evidence-based findings regarding the safety and efficacy of using butorphanol as an epidural analgesic during labor.
Butorphanol has been used successfully as an epidural analgesic during labor. However, no study has comprehensively examined the safety and effectiveness of using butorphanol as an epidural analgesic during labor.
To assess butorphanol's safety and efficacy for epidural labor analgesia.
To provide a safe and reliable theoretical basis for the use of butorphanol in epidural labor analgesia.
Six databases will be searched to identify find relevant randomized controlled trials. The visual analog scale score during the first stage of labor, fetal effects, and Apgar score will be the primary outcomes.
This study will provide trustworthy data on the safety and efficacy of using butorphanol as an epidural analgesic during labor.
This study provides evidence-based verification of the safety and efficacy of using butorphanol as an epidural analgesic during labor, thus providing guidance for clinical practice.
Butorphanol dose in combination with opioids for epidural labor analgesia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kang JH, South Korea S-Editor: Li L L-Editor: A P-Editor: Zheng XM
| 1. | Li Y, Hu C, Fan Y, Wang H, Xu H. Epidural analgesia with amide local anesthetics, bupivacaine, and ropivacaine in combination with fentanyl for labor pain relief: a meta-analysis. Med Sci Monit. 2015;21:921-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Cambic CR, Wong CA. Labour analgesia and obstetric outcomes. Br J Anaesth. 2010;105:i50-i60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 177: Obstetric Analgesia and Anesthesia. Obstet Gynecol. 2017;129:e73-e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Halliday L, Nelson SM, Kearns RJ. Epidural analgesia in labor: A narrative review. Int J Gynaecol Obstet. 2022;159:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 5. | Hunt CO, Naulty JS, Malinow AM, Datta S, Ostheimer GW. Epidural butorphanol-bupivacaine for analgesia during labor and delivery. Anesth Analg. 1989;68:323-327. [PubMed] |
| 6. | Lawhorn CD, McNitt JD, Fibuch EE, Joyce JT, Leadley RJ Jr. Epidural morphine with butorphanol for postoperative analgesia after cesarean delivery. Anesth Analg. 1991;72:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Bharti N, Chari P. Epidural butorphanol-bupivacaine analgesia for postoperative pain relief after abdominal hysterectomy. J Clin Anesth. 2009;21:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Camann WR, Loferski BL, Fanciullo GJ, Stone ML, Datta S. Does epidural administration of butorphanol offer any clinical advantage over the intravenous route? A double-blind, placebo-controlled trial. Anesthesiology. 1992;76:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Shrestha CK, Sharma KR, Shrestha RR. Comparative study of epidural administration of 10 mL of 0.1% bupivacaine with 2 mg butorphanol and 10 mL of 0.25% plain bupivacaine for analgesia during labor. JNMA J Nepal Med Assoc. 2007;46:1-6. [PubMed] |
| 10. | Shankar K A, Puri R, Goel JK. Butorphanol-bupivacaine vs Fentanyl-bupivacaine for Extradural Analgesia during Labour. Med J Armed Forces India. 2006;62:224-227. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40536] [Article Influence: 10134.0] [Reference Citation Analysis (2)] |
| 12. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15886] [Article Influence: 1588.6] [Reference Citation Analysis (1)] |
| 13. | Chestnut DH, Owen CL, Bates JN, Ostman LG, Choi WW, Geiger MW. Continuous infusion epidural analgesia during labor: a randomized, double-blind comparison of 0.0625% bupivacaine/0.0002% fentanyl vs 0.125% bupivacaine. Anesthesiology. 1988;68:754-759. [PubMed] |
| 14. | Li DF, Rees GA, Rosen M. Continuous extradural infusion of 0.0625% or 0.125% bupivacaine for pain relief in primigravid labour. Br J Anaesth. 1985;57:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Stoddart AP, Nicholson KE, Popham PA. Low dose bupivacaine/fentanyl epidural infusions in labour and mode of delivery. Anaesthesia. 1994;49:1087-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Chassard D, Mathon L, Dailler F, Golfier F, Tournadre JP, Boulétreau P. Extradural clonidine combined with sufentanil and 0.0625% bupivacaine for analgesia in labour. Br J Anaesth. 1996;77:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Leighton BL, DeSimone CA, Norris MC, Ben-David B. Intrathecal narcotics for labor revisited: the combination of fentanyl and morphine intrathecally provides rapid onset of profound, prolonged analgesia. Anesth Analg. 1989;69:122-125. [PubMed] |
| 18. | Palacios QT, Jones MM, Hawkins JL, Adenwala JN, Longmire S, Hess KR, Skjonsby BS, Morrow DH, Joyce TH 3rd. Post-caesarean section analgesia: a comparison of epidural butorphanol and morphine. Can J Anaesth. 1991;38:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Abboud TK, Moore M, Zhu J, Murakawa K, Minehart M, Longhitano M, Terrasi J, Klepper ID, Choi Y, Kimball S. Epidural butorphanol or morphine for the relief of post-cesarean section pain: ventilatory responses to carbon dioxide. Anesth Analg. 1987;66:887-893. [PubMed] |