Published online Feb 16, 2024. doi: 10.12998/wjcc.v12.i5.931
Peer-review started: November 9, 2023
First decision: December 21, 2023
Revised: January 4, 2024
Accepted: January 22, 2024
Article in press: January 22, 2024
Published online: February 16, 2024
Processing time: 82 Days and 22.4 Hours
There are limited data on the use of glucose transport protein 1 (GLUT-1) expre
To evaluate GLUT-1, GLUT-3, HK-II, and HIF-1 expressions as biomarkers for detecting primary tumors and lymph node metastasis with 18F-FDG-PET/CT.
This retrospective study included 169 patients with colorectal cancer who underwent colectomy and preoperative 18F-FDG-PET/CT at Chungbuk National University Hospital between January 2009 and May 2012. Two tissue cores from the central and peripheral areas of the tumors were obtained and were examined by a dedicated pathologist, and the expressions of GLUT-1, GLUT-3, HK-II, and HIF-1 were determined using immunohistochemical staining. We analyzed the correlations among their expressions, various clinicopathological factors, and the maximum standardized uptake value (SUVmax) of PET/CT.
GLUT-1 was found at the center or periphery of the tumors in 109 (64.5%) of the 169 patients. GLUT-1 positivity was significantly correlated with the SUVmax of the primary tumor and lymph nodes, regardless of the biopsy site (tumor center, P < 0.001 and P = 0.012; tumor periphery, P = 0.030 and P = 0.010, respectively). GLUT-1 positivity and negativity were associated with higher and lower sensitivities of PET/CT, respectively, for the detection of lymph node metastasis, regardless of the biopsy site. GLUT3, HK-II, and HIF-1 expressions were not significantly correlated with the SUVmax of the primary tumor and lymph nodes.
GLUT-1 expression was significantly correlated with the SUVmax of 18F-FDG-PET/CT for primary tumors and lymph nodes. Clinicians should consider GLUT-1 expression in preoperative endoscopic biopsy in interpreting PET/CT findings.
Core Tip: Glucose transport protein 1 (GLUT-1) expression is a significant predictor of lymph node metastasis in patients with colorectal cancer. Positron emission tomography/computed tomography showed a higher sensitivity for detecting lymph node metastasis for GLUT-1-positive tumors, suggesting that GLUT-1 expression can be used to improve the accuracy of preoperative staging and guide treatment planning in patients with colorectal cancer.
- Citation: Kim H, Choi SY, Heo TY, Kim KR, Lee J, Yoo MY, Lee TG, Han JH. Value of glucose transport protein 1 expression in detecting lymph node metastasis in patients with colorectal cancer. World J Clin Cases 2024; 12(5): 931-941
- URL: https://www.wjgnet.com/2307-8960/full/v12/i5/931.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i5.931
Colorectal cancer is the third most common malignant tumor globally, with a high incidence in developed countries[1]. Preoperative staging is important for predicting prognosis and determining appropriate treatment modalities. Although various imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)/CT are used, the diagnostic accuracy for lymph node metastasis varies from 54% to 80%[2,3]. Several studies have suggested the importance of PET/CT in patients with colorectal cancer; however, the extent of its contribution has not been well-established[4,5]. Its sensitivity for diagnosing the primary site is as high as 95%-100%, while its sensitivity for diagnosing lymph node metastasis remains low (29%-37%), although a high specificity of 83%-96% has been reported[6-9].
18F-fluorodeoxyglucose (18F-FDG)-PET/CT is widely used to detect primary tumors and metastasis, monitor recurrence, and evaluate therapeutic response[10]. It is based on the enhanced glucose metabolism of malignant tumors. Malignant tumors increase glucose utilization via increasing the expression of glucose transporter (GLUT) and upregulating intracellular enzymes such as hexokinase (HK) that promote glycolysis and result in the accumulation of FDG[11,12]. Further, malignant tumors grow abnormally and become hypoxic, which induces the expression of hypoxia induced factor-1 (HIF-1). HIF-1 is a transcription factor that promotes the expression of several genes involved in glucose utilization, including glucose transporters and glycolytic enzymes such as GLUT and HK-II[13]. Some studies have evaluated the relationship between the expression of several proteins, including GLUT, HK-II, and HIF-1, and FDG uptake on PET/CT to increase the accuracy of PET/CT in detecting various malignancies[14-16]. However, the correlation between protein expression and FDG uptake in various malignancies remains controversial[11]. While FDG uptake in colorectal cancer has been reported, its correlation with protein expression has not been established[17,18].
Previous studies have shown that low GLUT expression at the tumor center (tissue obtained by surgery) is correlated with a false-negative diagnosis based on PET/CT[19,20]. Therefore, further studies investigating whether PET/CT findings in combination with GLUT expression in preoperative endoscopic biopsy of colorectal cancer can help provide a more accurate assessment of lymph node staging are in demand. Studies assessing GLUT expressions in relation to biopsy sites (tumor center with the tissue being obtained by surgery vs tumor periphery with the sample being obtained by endoscopy) are also needed.
In this study, we used immunohistochemistry (IHC) to determine the correlation between GLUT-1, GLUT-3, HK-II, and HIF-1 expressions in the central and peripheral areas of a primary tumor and investigated their values as biomarkers for detecting primary colorectal cancer and lymph node metastasis in combination with FDG uptake on PET/CT.
We retrospectively analyzed 240 patients with colorectal cancer who had undergone colectomy and were diagnosed with adenocarcinoma at Chungbuk National University Hospital between January 2009 and May 2012. The histologic findings were interpreted based on the World Health Organization classification. The colon cancer stage evaluation was based on the American Joint Committee on Cancer 7th Edition Cancer Staging Manual. A total of 71 patients were excluded from this study: 66 patients were not examined with 18F-FDG-PET/CT, and 5 patients were not followed up. None of the patients had received neoadjuvant chemotherapy or radiotherapy.
Two tissue cores from the central and peripheral areas of the tumor were obtained from the 169 included patients for pathological examination, and GLUT-1 expression was evaluated using IHC staining. The samples were examined by a dedicated pathologist (SYC). All clinical, laboratory, and radiological data were collected from the electronic medical records. The Institutional Review Board (IRB No. 2013-03-003) of Chungbuk National University Hospital approved this study, and the requirement for informed consent was waived due to the retrospective nature of the study.
All cases were reviewed, and 3 mm-sized tissue microarrays (TMA) were reconstructed. Two cores (tumor center and periphery) were obtained for each case. The core obtained from the center of the tumor close to the mucosa was indicated as “tumor center,” and the core obtained from the deep invasive front was designated as “periphery.” Sections of 4-µm thickness were cut and placed on SuperfrostPlus microscope slides (Fisher Scientific). IHC staining was performed using the BenchMark XT automated immunohistochemistry stainer (Ventana Medical Systems, Inc., United States) and signal was detected using a Ventana Ultraview DAB Kit (Ventana Medical Systems). Briefly, sections were deparaffinized using the EZ Prep solution. Antigen retrieval was performed using the CC1 standard automated process (pH 8.4 buffer contained Tris/Borate/EDTA). A DAB inhibitor (3% H2O2 endogenous peroxidase) was blocked for 4 min at 37 °C temperature. The slides were incubated with primary antibodies specific to GLUT-1 (1:100, Thermo Fisher Scientific, USA), GLUT-3 (1:50, Thermo Fisher Scientific, USA), HK-II (1:100, Cell Signaling Technology, United States), and HIF-1 (1:50, Thermo Fisher Scientific) for 30 min at 37 °C followed by incubation with a secondary antibody (Universal HRP Multimer) for 8 min at 37 °C. The slides were incubated with DAB (chromogen) + H2O2 (substrate) for 8 min, followed by counterstaining using hematoxylin and DAPI at 37 °C. Tris buffer (pH 7.6) was used as the washing solution.
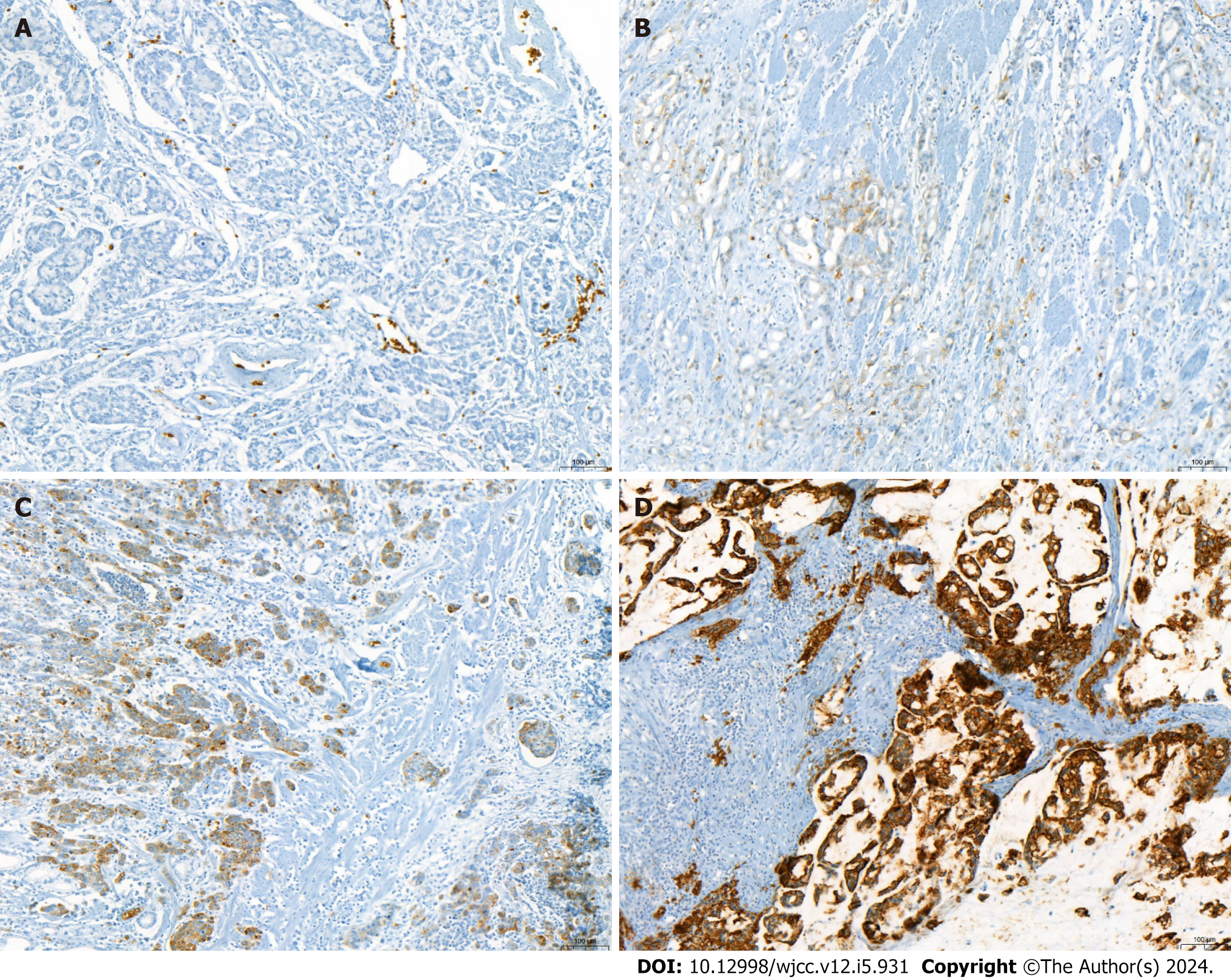
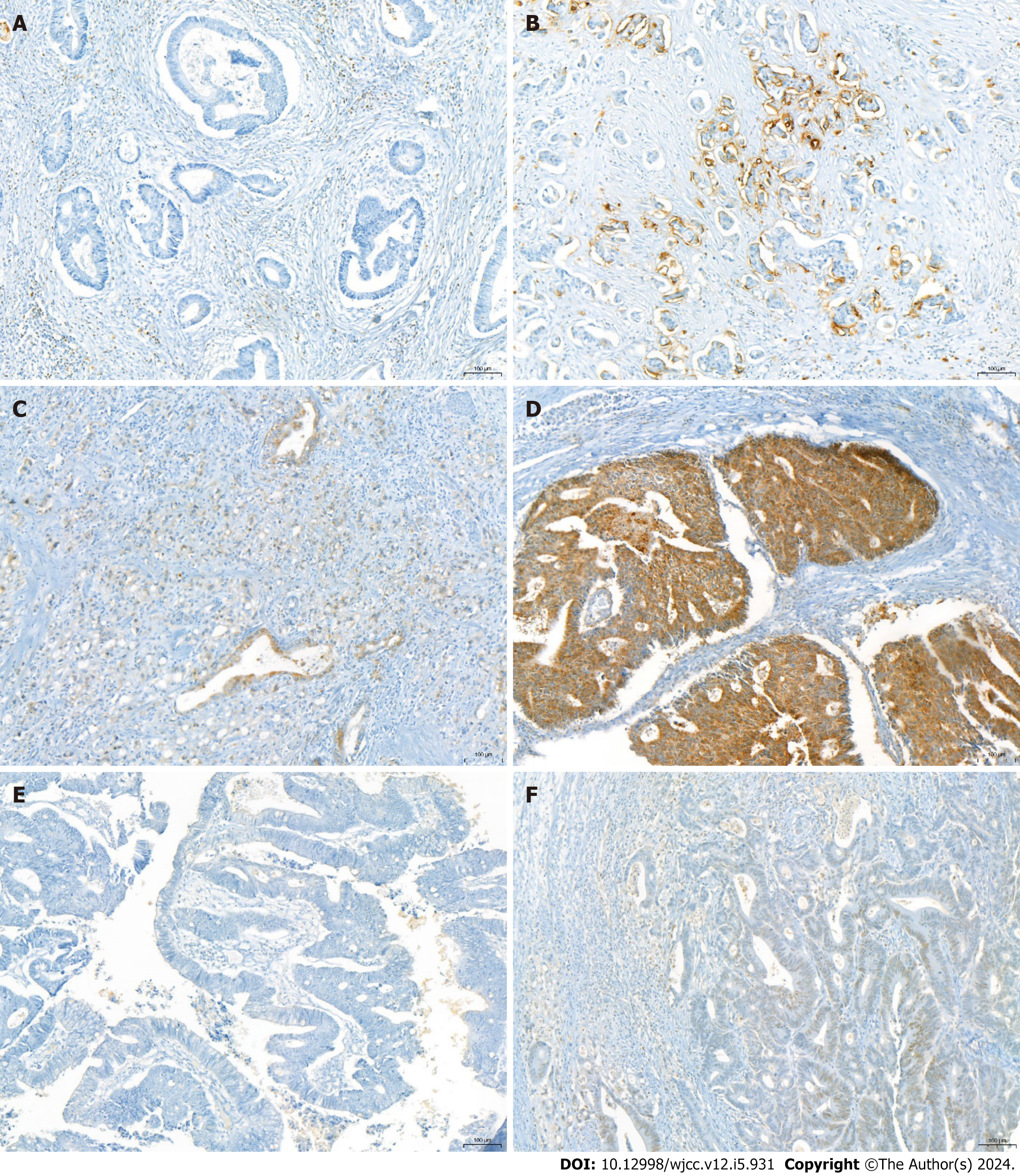
GLUT-1, GLUT-3, HK-II, and HIF-1 expressions were considered positive when > 5% of tumor cells demonstrated cytoplasmic or membranous staining. The immunoreactive score was rated as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong) based on the average staining intensity. A score of 2 or higher indicated positivity. HIF-1 expression was considered positive when > 5% of tumor cells demonstrated nuclear staining (Figures 1 and 2).
Descriptive statistics were used to summarize the patient and tumor characteristics, and the data are reported as proportions and medians for continuous variables. The categorical data are presented as numbers (%). The continuous variables were compared using the Student’s t-test, and the categorical variables were compared using the chi-squared or Fisher’s exact test. Statistical significance was set at P < 0.05. Statistical analyses were performed using IBM SPSS Statistics version 25 (IBM, Armonk, NY, United States).
Table 1 presents the relationship between GLUT-1 expression and clinicopathological parameters. Among the 169 patients, 86 (50.9%) and 91 (53.8%) patients showed GLUT-1 positivity in the tumor center and periphery, respectively. GLUT-1 positivity was significantly correlated with the SUVmax of the primary tumor and lymph nodes, regardless of the biopsy site (tumor center, P < 0.001 and P = 0.012; tumor periphery P = 0.030 and P = 0.010, respectively).
| GLUT-1 (center) | P value | GLUT-1 (periphery) | P value | |||
| (-) (n = 83) | (+) (n = 86) | (-) (n = 78) | (+) (n = 91) | |||
| Age (yr ± SD) | 64.0 ± 11.4 | 64.7 ± 12.6 | 0.740 | 64.0 ± 11.6 | 64.7 ± 12.4 | 0.718 |
| Sex | ||||||
| Male | 52 | 54 | 0.985 | 51 | 55 | 0.507 |
| Female | 31 | 32 | 27 | 36 | ||
| Tumor size (mean ± SD) | 4.9 ± 1.8 | 5.2 ± 2.0 | 0.277 | 5.1 ± 1.7 | 5.1 ± 2.1 | 0.932 |
| T stage | ||||||
| T1, 2 | 16 | 7 | 0.035 | 11 | 12 | 0.836 |
| T3, 4 | 67 | 79 | 67 | 79 | ||
| N stage | ||||||
| N0 | 51 | 48 | 0.457 | 48 | 51 | 0.47 |
| N1-2 | 32 | 38 | 30 | 40 | ||
| AJCC stage | ||||||
| I, II | 49 | 48 | 0.672 | 47 | 50 | 0.486 |
| III, IV | 34 | 38 | 31 | 41 | ||
| Lymphatic invasion | ||||||
| Negative | 70 | 72 | 0.913 | 64 | 78 | 0.517 |
| Positive | 13 | 14 | 14 | 13 | ||
| Perineural invasion | ||||||
| Negative | 66 | 73 | 0.361 | 68 | 71 | 0.12 |
| Positive | 17 | 13 | 10 | 20 | ||
| Blood glucose level (mean ± SD) | 110.5 ± 36.2 | 111.6 ± 25.8 | 0.818 | 112.4 ± 38.8 | 109.9 ± 23.2 | 0.612 |
| SUVmax of primary tumor (mean ± SD) | 12.3 ± 5.9 | 16.1 ± 7.4 | <0.001 | 13.0 ± 6.4 | 15.3 ± 7.3 | 0.030 |
| SUVmax of lymph node (mean ± SD) | 1.04 ± 2.6 | 2.15 ± 3.1 | 0.012 | 0.99 ± 2.6 | 2.13 ± 3.0 | 0.010 |
We analyzed the predictive values of PET/CT, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for lymph node metastasis (Table 2).
| Pathology | PET/CT diagnosis | Sensitivity | Specificity | PPV (%) | NPV (%) | |||
| PET LN (+) | PET LN (-) | (%) | (%) | |||||
| All patients (n = 169) | LN (+) | 35 | 35 | 50 | 81.8 | 66.03 | 69.82 | |
| LN (-) | 18 | 81 | ||||||
| Tumor center | GLUT-1 negative (n = 83) | LN (+) | 12 | 20 | 37.5 | 88.2 | 66.6 | 69.2 |
| LN (-) | 6 | 45 | ||||||
| GLUT-1 positive (n = 86) | LN (+) | 23 | 15 | 60.5 | 75 | 65.7 | 70.6 | |
| LN (-) | 12 | 36 | ||||||
| Tumor periphery | GLUT-1 negative (n = 78) | LN (+) | 9 | 21 | 29 | 87.5 | 60 | 66.6 |
| LN (-) | 6 | 42 | ||||||
| GLUT-1 positive (n = 91) | LN (+) | 26 | 14 | 65 | 76.5 | 68.4 | 73.6 | |
| LN (-) | 12 | 39 | ||||||
Among the 169 patients, 53 were classified as positive for lymph node metastasis on PET/CT, and 35 were histologically confirmed positive, with a sensitivity of 50.0% and a PPV of 66.0%. A total of 116 patients were classified as negative for lymph node metastasis on PET/CT, and 81 patients were histologically confirmed as negative with a specificity of 81.8% and an NPV of 69.8%.
We investigated whether GLUT-1 expression in peripheral and central sites of the primary tumor could facilitate a more accurate assessment of lymph node staging based on FDG uptake on PET/CT (Table 2). Patients with GLUT-1 positivity demonstrated higher sensitivity of lymph node metastasis prediction by PET/CT than all patients considered together (overall patients, 50.0%; tumor center, 60.5%; tumor periphery, 65.0%). In contrast, patients with GLUT-1 negativity demonstrated overall lower sensitivity for the prediction of lymph node metastasis by PET/CT than all patients considered together (overall patients, 50.0%; tumor center, 37.5%; tumor periphery, 29.0%).
We investigated the expressions of other proteins associated with glucose metabolism that could be associated with false-negative prediction of lymph node metastasis by PET/CT. Due to the staining of TMA slides, a few tissue cores were lost; therefore, different cases were analyzed using each antibody. The GLUT3, HK-II, and HIF-1 expressions did not significantly correlate with the SUVmax of the primary tumor and lymph nodes, other than for GLUT-3 in the tumor periphery (P = 0.013) (Tables 3-5).
| GLUT-3 (center) | P value | GLUT-3 (periphery) | P value | |||
| (-) (n = 153) | (+) (n = 12) | (-) (n = 78) | (+) (n = 91) | |||
| Age (yr), mean ± SD | 64.5 ± 11.8 | 63.0 ± 14.6 | 0.729 | 64.1 ± 11.9 | 67.8 ± 13.0 | 0.194 |
| Sex | ||||||
| Male | 98 | 5 | 0.135 | 93 | 7 | 0.013 |
| Female | 55 | 7 | 49 | 13 | ||
| Tumor size (mean ± SD) | 5.07 ± 2.0 | 5.02 ± 2.0 | 0.931 | 5.05 ± 2.0 | 5.20 ± 1.6 | 0.743 |
| T stage | ||||||
| T1, 2 | 20 | 2 | 0.663 | 20 | 1 | 0.475 |
| T3, 4 | 133 | 10 | 122 | 19 | ||
| N stage | ||||||
| N0 | 93 | 4 | 0.074 | 89 | 6 | 0.007 |
| N1-2 | 60 | 8 | 53 | 14 | ||
| AJCC stage | ||||||
| I, II | 91 | 4 | 0.127 | 87 | 6 | 0.014 |
| III, IV | 62 | 8 | 53 | 14 | ||
| Lymphatic invasion | ||||||
| Negative | 128 | 11 | 0.693 | 120 | 17 | 1.000 |
| Positive | 25 | 1 | 22 | 33 | ||
| Perineural invasion | ||||||
| Negative | 127 | 8 | 0.234 | 117 | 17 | 1.000 |
| Positive | 26 | 4 | 25 | 3 | ||
| Blood sugar level (mean ± SD) | 111.3 ± 32.0 | 109.2 ± 26.9 | 0.803 | 112.7 ± 33.0 | 101.8 ± 20.3 | 0.150 |
| SUV max of primary tumor (mean ± SD) | 14.1 ± 7.5 | 13.5 ± 4.7 | 0.678 | 14.3 ± 7.6 | 12.9 ± 4.8 | 0.433 |
| SUV max of lymph node (mean ± SD) | 1.6 ± 2.8 | 2.8 ± 4.0 | 0.328 | 1.4 ± 2.7 | 3.8 ± 4.0 | 0.013 |
| Hexokinase-II (center) | P value | Hexokinase-II (periphery) | P value | |||
| (-) (n = 94) | (+) (n = 70) | (-) (n = 114) | (+) (n = 48) | |||
| Age (yr ± SD) | 65.1 ± 12.3 | 63.5 ± 11.6 | 0.403 | 64.3 ± 12.3 | 64.8 ± 11.5 | 0.806 |
| Sex | ||||||
| Male | 61 | 41 | 0.409 | 73 | 27 | 0.352 |
| Female | 33 | 29 | 41 | 21 | ||
| Tumor size (mean ± SD) | 5.1 ± 1.8 | 5.1 ± 2.2 | 0.976 | 5.1 ± 2.0 | 5.1 ± 1.9 | 0.977 |
| T stage | ||||||
| T1, 2 | 15 | 7 | 0.268 | 16 | 5 | 0.531 |
| T3, 4 | 79 | 63 | 98 | 43 | ||
| N stage | ||||||
| N0 | 54 | 42 | 0.743 | 68 | 28 | 0.876 |
| N1-2 | 40 | 28 | 46 | 20 | ||
| AJCC stage | ||||||
| I, II | 53 | 41 | 0.779 | 67 | 27 | 0.766 |
| III, IV | 41 | 29 | 47 | 21 | ||
| Lymphatic invasion | ||||||
| Negative | 77 | 61 | 0.365 | 97 | 40 | 0.778 |
| Positive | 17 | 9 | 17 | 8 | ||
| Perineural invasion | ||||||
| Negative | 75 | 60 | 0.325 | 95 | 38 | 0.528 |
| Positive | 19 | 10 | 19 | 10 | ||
| Blood glucose level (mean ± SD) | 105.2 ± 22.8 | 116.8 ± 35.1 | 0.017 | 110.2 ± 32.0 | 113.9 ± 31.3 | 0.493 |
| SUVmax of primary tumor (mean ± SD) | 14.0 ± 7.1 | 14.2 ± 7.7 | 0.874 | 14.3 ± 7.2 | 13.9 ± 7.7 | 0.786 |
| SUVmax of lymph node (mean ± SD) | 1.6 ± 2.8 | 1.8 ± 3.2 | 0.712 | 1.5 ± 2.6 | 2.0 ± 3.7 | 0.450 |
| HIF-1 (center) | P value | HIF-1 (periphery) | P value | |||
| (-) (n = 94) | (+) (n = 72) | (-) (n = 89) | (+) (n = 75) | |||
| Age (yr), mean ± SD | 64.0 ± 12.4 | 65.1 ± 11.5 | 0.576 | 63.5 ± 12.6 | 65.6 ± 11.3 | 0.246 |
| Sex | ||||||
| Male | 59 | 45 | 0.972 | 58 | 45 | 0.495 |
| Female | 35 | 27 | 31 | 30 | ||
| Tumor size (mean ± SD) | 4.7 ± 1.7 | 5.5 ± 2.2 | 0.014 | 4.8 ± 1.7 | 5.3 ± 2.2 | 0.082 |
| T stage | ||||||
| T1, 2 | 17 | 6 | 0.071 | 16 | 7 | 0.112 |
| T3, 4 | 77 | 66 | 73 | 68 | ||
| N stage | ||||||
| N0 | 59 | 38 | 0.196 | 57 | 40 | 0.164 |
| N1-2 | 35 | 34 | 32 | 35 | ||
| AJCC stage | ||||||
| I, II | 57 | 38 | 0.310 | 55 | 40 | 0.274 |
| III, IV | 37 | 34 | 34 | 35 | ||
| Lymphatic invasion | ||||||
| Negative | 80 | 60 | 0.755 | 76 | 62 | 0.634 |
| Positive | 14 | 12 | 13 | 13 | ||
| Perineural invasion | ||||||
| Negative | 77 | 59 | 0.996 | 73 | 61 | 0.909 |
| Positive | 17 | 13 | 16 | 14 | ||
| Blood glucose level (mean ± SD) | 109.91 ± 30.8 | 112.29 ± 32.6 | 0.632 | 108.97 ± 32.2 | 113.69 ± 31.1 | 0.342 |
| SUVmax of primary tumor (mean ± SD) | 14.0 ± 7.1 | 14.0 ± 7.6 | 0.979 | 14.2 ± 7.0 | 13.8 ± 7.9 | 0.754 |
| SUVmax of lymph node (mean ± SD) | 1.4 ± 2.9 | 2.0 ± 2.9 | 0.229 | 1.6 ± 2.9 | 1.8 ± 2.9 | 0.713 |
In this study, we analyzed the role of GLUT-1 expression as a biomarker for lymph node metastasis detected by FDG uptake on PET/CT and assessed whether GLUT-1 expression differed in the peripheral and central areas of the tumor. We discovered that 109/169 patients (64.5%) exhibited GLUT-1 positivity in the center or periphery of the primary tumor. The expression of GLUT-1 was significantly correlated with the SUVmax of the primary tumor and lymph node, regardless of the biopsy site. In addition, patients with GLUT-1 positivity demonstrated overall higher sensitivity for the prediction of lymph node metastasis by PET/CT. These findings suggest that GLUT-1 expression is a useful biomarker for predicting lymph node metastasis when combined with PET/CT. In this study, the tumor periphery was regarded as the edge of the tumor close to the mucosa; this is the part of the tumor that can be reached during preoperative endoscopic biopsy, which suggests that the measurement of GLUT-1 expression in endoscopic tumor biopsy specimens can predict lymph node metastasis. Therefore, we recommend that clinicians evaluate GLUT-1 expression in preoperative endoscopic biopsy specimens and consider interpreting PET/CT results according to GLUT-1 expression. Since lymph node metastasis is underestimated in patients with colorectal cancer with GLUT-1 negativity, clinicians should be cautious when interpreting PET/CT results in preoperative patients, because of the possibility of false negative findings.
Previous studies have reported a correlation between GLUT-1 expression and SUVmax in various carcinomas; however, discordant findings were reported[21-23]. Meyer et al[11] conducted a meta-analysis and reported that GLUT-1 and SUVmax were highly correlated in patients with pancreatic cancer and cervical cancer but not in those with colorectal cancer and endometrial cancer. However, Zhang et al[23] and Gu et al[24] reported a significant correlation between GLUT-1 expression and SUVmax in patients with colorectal cancer, which is consistent with our results. However, both studies included a small number of patients (n < 40), and the correlation between GLUT-1 expression and the SUVmax of the primary tumor remains unclear. In addition, few studies have reported an association between GLUT-1 expression and the prediction of lymph node metastasis by PET/CT in patients with non-small cell lung cancer. Taira et al[22] demonstrated that the predictive accuracy of lymph node metastasis by PET/CT differed from that obtained when GLUT-1 expression in patient samples was incorporated in the assessment, which is consistent with our results. However, to date, the exact reasons underlying these controversial results remains unclear. Probably, the complex glucose meta
We analyzed the expression of other proteins (including GLUT3, HK-II, and HIF-1) associated with glucose meta
To the best of our knowledge, this is the first study to evaluate the relationship between GLUT-1 expression and the SUVmax of primary tumors and lymph node metastasis in patients with colorectal cancer, as well as its role in detecting lymph node metastasis using 18F-FDG-PET/CT according to biopsy sites. We discovered that GLUT-1 expression was significantly correlated with the SUVmax of primary tumors and facilitated the prediction of lymph node metastasis using 18F-FDG-PET/CT. Moreover, GLUT-1 expression did not differ based on whether it was measured in either central or peripheral primary tumor biopsy sites in patients with colorectal cancer.
Our study has several limitations. First, it was retrospective, and our results should be confirmed in a prospective study. Second, only Asian patients with colorectal cancer were analyzed in this study, limiting the generalizability of our results as molecular profiles and clinical features of Western and Eastern populations differ significantly. Third, IHC cannot differentiate membranous from cytoplasmic GLUT-1 expressions. Cytoplasmic GLUT-1 is known to be inactive. Thus, to determine the clinical effects of GLUT-1, it is necessary to measure the expression of membranous GLUT-1. However, this distinction is presently challenging and limits the application of our findings[21].
GLUT-3, HK-II, and HIF-1 expressions were not significantly correlated with the SUVmax of the primary tumor and lymph nodes. GLUT-1 expression, on the other hand, demonstrated a significant correlation and may be a useful biomarker to be used in conjunction with PET/CT to diagnose colorectal cancers. Clinicians should consider GLUT-1 expression during preoperative endoscopic biopsy in interpreting PET/CT findings for the diagnosis of colorectal cancers and lymph node metastases.
Further studies are needed to evaluate clinical benefit of glucose transport protein 1 (GLUT-1) expression as biomarkers for detecting primary tumors and lymph node metastasis via fluorodeoxyglucose (FDG)-positron emission tomography/computed tomography (PET/CT).
GLUT-1 expression was significantly correlated with the maximum standardized uptake value (SUVmax) of 18F-FDG-PET/CT for primary tumors and lymph nodes. Clinicians should consider GLUT-1 expression in preoperative endoscopic biopsy when interpreting PET/CT findings.
GLUT-1 positivity was significantly correlated with the SUVmax of the primary tumor and lymph nodes, regardless of biopsy site (tumor center, P < 0.001 and P = 0.012; tumor periphery, P = 0.030 and P = 0.010, respectively). GLUT-1 positivity and negativity were associated with higher and lower sensitivities of PET/CT, respectively, for the detection of lymph node metastasis, regardless of biopsy site. GLUT3, HK-II, and HIF-1 expressions were not significantly associated with the SUVmax of the primary tumor and lymph nodes.
Two tissue cores from the central and peripheral areas of the tumors were examined and the expressions of GLUT-1, GLUT-3, HK-II, and HIF-1 were determined via immunohistochemical staining. We analyzed the correlations among their expressions, various clinicopathological factors, and the SUVmax of PET/CT.
The research objective was to investigate the role of GLUT-1 expression as a biomarker for lymph node metastasis detected by FDG uptake on PET/CT; in addition, we aimed to assess whether GLUT-1 expression differed in the peripheral and central areas of the tumor.
The research motivation was to evaluate GLUT-1, GLUT-3, HK-II, and HIF-1 expressions as biomarkers for detecting primary tumors and lymph node metastasis with 18F-FDG-PET/CT.
There are limited data on the use of GLUT-1 and GLUT-3, HK-II, and HIF-1 expressions as biomarkers for predicting lymph node metastasis when combined with FDG uptake on PET/CT in patients with colorectal cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shah SIA, Pakistan; Yan B, China S-Editor: Liu JH L-Editor: A P-Editor: Zheng XM
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64624] [Article Influence: 16156.0] [Reference Citation Analysis (176)] |
| 2. | Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, Brown G, McLeod R, Kennedy E. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 396] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 3. | Dighe S, Purkayastha S, Swift I, Tekkis PP, Darzi A, A'Hern R, Brown G. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol. 2010;65:708-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Nasseri Y, Langenfeld SJ. Imaging for Colorectal Cancer. Surg Clin North Am. 2017;97:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Park IJ, Kim HC, Yu CS, Ryu MH, Chang HM, Kim JH, Ryu JS, Yeo JS, Kim JC. Efficacy of PET/CT in the accurate evaluation of primary colorectal carcinoma. Eur J Surg Oncol. 2006;32:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Abdel-Nabi H, Doerr RJ, Lamonica DM, Cronin VR, Galantowicz PJ, Carbone GM, Spaulding MB. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology. 1998;206:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 262] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Kijima S, Sasaki T, Nagata K, Utano K, Lefor AT, Sugimoto H. Preoperative evaluation of colorectal cancer using CT colonography, MRI, and PET/CT. World J Gastroenterol. 2014;20:16964-16975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Kantorová I, Lipská L, Bêlohlávek O, Visokai V, Trubaĉ M, Schneiderová M. Routine (18)F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med. 2003;44:1784-1788. [PubMed] |
| 9. | Mukai M, Sadahiro S, Yasuda S, Ishida H, Tokunaga N, Tajima T, Makuuchi H. Preoperative evaluation by whole-body 18F-fluorodeoxyglucose positron emission tomography in patients with primary colorectal cancer. Oncol Rep. 2000;7:85-87. [PubMed] |
| 10. | Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJ; European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2053] [Cited by in RCA: 2291] [Article Influence: 229.1] [Reference Citation Analysis (0)] |
| 11. | Meyer HJ, Wienke A, Surov A. Associations between GLUT expression and SUV values derived from FDG-PET in different tumors-A systematic review and meta analysis. PLoS One. 2019;14:e0217781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | van Berkel A, Rao JU, Kusters B, Demir T, Visser E, Mensenkamp AR, van der Laak JA, Oosterwijk E, Lenders JW, Sweep FC, Wevers RA, Hermus AR, Langenhuijsen JF, Kunst DP, Pacak K, Gotthardt M, Timmers HJ. Correlation between in vivo 18F-FDG PET and immunohistochemical markers of glucose uptake and metabolism in pheochromocytoma and paraganglioma. J Nucl Med. 2014;55:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Busk M, Horsman MR, Jakobsen S, Bussink J, van der Kogel A, Overgaard J. Cellular uptake of PET tracers of glucose metabolism and hypoxia and their linkage. Eur J Nucl Med Mol Imaging. 2008;35:2294-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Yang HJ, Xu WJ, Guan YH, Zhang HW, Ding WQ, Rong L, Qiu ZB, Zhong L. Expression of Glut-1 and HK-II in Pancreatic Cancer and Their Impact on Prognosis and FDG Accumulation. Transl Oncol. 2016;9:583-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, Brys M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol Oncol Res. 2012;18:721-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138-1143. [PubMed] |
| 17. | Saigusa S, Toiyama Y, Tanaka K, Okugawa Y, Fujikawa H, Matsushita K, Uchida K, Inoue Y, Kusunoki M. Prognostic significance of glucose transporter-1 (GLUT1) gene expression in rectal cancer after preoperative chemoradiotherapy. Surg Today. 2012;42:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Yang J, Wen J, Tian T, Lu Z, Wang Y, Wang Z, Wang X, Yang Y. GLUT-1 overexpression as an unfavorable prognostic biomarker in patients with colorectal cancer. Oncotarget. 2017;8:11788-11796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Carvalho KC, Cunha IW, Rocha RM, Ayala FR, Cajaíba MM, Begnami MD, Vilela RS, Paiva GR, Andrade RG, Soares FA. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics (Sao Paulo). 2011;66:965-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Hong R, Lim SC. ¹⁸F-fluoro-2-deoxyglucose uptake on PET CT and glucose transporter 1 expression in colorectal adenocarcinoma. World J Gastroenterol. 2012;18:168-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Choi WH, Yoo IeR, O JH, Kim TJ, Lee KY, Kim YK. Is the Glut expression related to FDG uptake in PET/CT of non-small cell lung cancer patients? Technol Health Care. 2015;23 Suppl 2:S311-S318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Taira N, Atsumi E, Nakachi S, Takamatsu R, Yohena T, Kawasaki H, Kawabata T, Yoshimi N. Comparison of GLUT-1, SGLT-1, and SGLT-2 expression in false-negative and true-positive lymph nodes during the (18)F-FDG PET/CT mediastinal nodal staging of non-small cell lung cancer. Lung Cancer. 2018;123:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Zhang M, Yang J, Jiang H, Wang Z. Correlation between glucose metabolism parameters derived from FDG and tumor TNM stages and metastasis-associated proteins in colorectal carcinoma patients. BMC Cancer. 2021;21:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Gu J, Yamamoto H, Fukunaga H, Danno K, Takemasa I, Ikeda M, Tatsumi M, Sekimoto M, Hatazawa J, Nishimura T, Monden M. Correlation of GLUT-1 overexpression, tumor size, and depth of invasion with 18F-2-fluoro-2-deoxy-D-glucose uptake by positron emission tomography in colorectal cancer. Dig Dis Sci. 2006;51:2198-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Baek HJ, Chung JH, Park JH, Zo JI, Cheon GJ, Choi CW, Lim SM, Choi SY, Hong JM, Hong JS. FDG-PET in Mediastinal Nodal Staging of Non-small Cell Lung Cancer: Correlation of False Results with Histopathologic Finding. Cancer Res Treat. 2003;35:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |