Published online Jul 26, 2024. doi: 10.12998/wjcc.v12.i21.4536
Revised: May 9, 2024
Accepted: June 13, 2024
Published online: July 26, 2024
Processing time: 102 Days and 0.1 Hours
Intrapancreatic fat deposition (IPFD) exerts a significant negative impact on patients with type 2 diabetes mellitus (T2DM), accelerates disease deterioration, and may lead to impaired β-cell quality and function.
To investigate the correlation between T2DM remission and IPFD.
We enrolled 80 abdominally obese patients with T2DM admitted to our institution from January 2019 to October 2023, including 40 patients with weight loss-induced T2DM remission (research group) and 40 patients with short-term intensive insulin therapy-induced T2DM remission (control group). We comparatively analyzed improvements in IPFD [differential computed tomography (CT) values of the spleen and pancreas and average CT value of the pancreas]; levels of fasting blood glucose (FBG), 2-h postprandial blood glucose (2hPBG), and insulin; and homeostasis model assessment of insulin resistance (HOMA-IR) scores. Correlation analysis was performed to explore the association between T2DM remission and IPFD.
After treatment, the differential CT values of the spleen and pancreas, FBG, 2hPBG, and HOMA-IR in the research group were significantly lower than those before treatment and in the control group, and the average CT value of the pancreas and insulin levels were significantly higher. Correlation analysis revealed that the greater the T2DM remission, the lower the amount of IPFD.
T2DM remission and IPFD are inversely correlated.
Core Tip: This study on 80 patients with type 2 diabetes mellitus (T2DM) with abdominal obesity confirmed that T2DM remission and intrapancreatic fat deposition were negatively correlated, as well as the benefits of liraglutide in glycemic control and pancreatic function repair in T2DM. Our findings are of great significance for advancing research on diabetes mellitus and fat and building appropriate treatment models for patients with T2DM. However, there are limitations such as insufficient sample size and lack of basic experiments.
- Citation: Liu J, Luo Y, Zhu YR, Wu ZT, Hou Y, Xu YJ, Li L, Ma CW. Correlation between type 2 diabetes mellitus remission and intrapancreatic fat deposition. World J Clin Cases 2024; 12(21): 4536-4542
- URL: https://www.wjgnet.com/2307-8960/full/v12/i21/4536.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i21.4536
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease with increasing prevalence and carries a high risk of nonalcoholic fatty liver disease[1,2]. T2DM risk factors include obesity, race, family history, puberty, unhealthy lifestyles, lack of sleep, and work stress[3,4]. Approximately 94% of the 34 million cases of diabetes mellitus (DM) in the United States each year are T2DM, and the cost of managing T2DM increased by 26% in 2017[5]. Intrapancreatic fat deposition (IPFD) negatively affects patients with T2DM, accelerating their deterioration[6]. This is closely related to pancreatic exocrine acinar atrophy and fibrosis associated with IPFD, which adversely affects endocrine cells and functions to varying degrees[7]. IPFD may also impair β-cell quality and function[8]. Thus, our study aimed to explore the correlation between T2DM remission and IPFD to identify effective methods for T2DM remission to prevent the deterioration of IPFD-related conditions. Our findings are of important clinical value for optimizing the management of patients with T2DM.
A systematic review and meta-analysis have shown that short-term intensive insulin therapy (IIT) significantly alleviates pathophysiological manifestations in patients with early-stage T2DM, primarily by significantly increasing β-cell functional homeostasis and decreasing insulin resistance[9]. This mechanism may be related to the elimination of glucotoxicity and lipotoxicity, which play a major role in repairing β-cell function[10]. Short-term IIT has a significant glycemic remission effect in patients with T2DM and can help maintain normal blood glucose levels for 1-2 years without antidiabetic drug intervention[11]. Although this treatment has excellent efficacy, patient response varies greatly[12]. Liraglutide is a commonly used drug for weight loss in obese or overweight patients, and its effectiveness increases when used in combination with diet control and exercise[13]. Liraglutide is a glucagon-like peptide-1 analog with 97% homology to human glucagon-like peptide-1[14]. Liraglutide treatment helps to reduce IPFD in patients with T2DM, suggesting that its use has certain advantages in slowing down IPFD[15].
Because the correlation between T2DM remission and IPFD has rarely been reported, this study aimed to fill the knowledge gap and provide relevant clinical evidence.
The study cohort comprised 80 abdominally obese patients with T2DM who visited our hospital between January 2019 and October 2023. The research group (n = 40) received weight loss therapy, and the control group (n = 40) received short-term IIT.
Patients who met the relevant diagnostic criteria for T2DM[16], had never used hypoglycemic drugs, and had a glycosylated hemoglobin level < 9% were included in our study.
Patients with serious infectious diseases, coagulopathy, cardiocerebrovascular diseases, malignancies, or severe heart, lung, and kidney dysfunction; drug contraindications; history or family history of medullary thyroid cancer; history of acute/chronic pancreatitis; and pregnant or lactating females were excluded.
The control group received short-term IIT via subcutaneous injection with Novolin R, Novolin N, Penfill 30R, or Penfill 50R (Novo Nordisk, Denmark) before their three main daily meals and at bedtime for 2 weeks.
The research group received 0.6 mg liraglutide via daily subcutaneous injection. The dose was increased to 1.2 mg daily after 1 week of continuous treatment. If the hypoglycemic effect was suboptimal, the dose was further increased to 1.8 mg daily. The treatment course was 2 weeks.
Improvement in IPFD: IPFD was assessed according to computed tomography (CT) values. After fasting for 8-12 hours, the upper abdomen was scanned using GE16-slice spiral CT (volumetric CT). The scanning parameters were as follows: scanning time: 0.5–0.8 s/cycle, pitch: 1.375:1, tube voltage: 120 KV, and tube current: 380 mA. The CT values at the maximum level of the head, neck, body, and tail of the pancreas and three areas of the spleen were measured separately and used to calculate the average CT values of the pancreas and spleen. Because the spleen is not susceptible to adipose tissue infiltration, the fatty pancreas group was defined as having a spleen-pancreas CT value difference > 5 HU, and the nonfatty pancreas group was defined as having a spleen-pancreas CT value difference ≤ 5 HU.
Blood glucose indices: Fasting venous blood and 2-h postprandial venous blood samples (volume, 3 mL) were collected before and after treatment to determine fasting blood glucose (FBG) and 2-h postprandial blood glucose (2hPBG) levels.
Insulin sensitivity indicators: Fasting insulin levels were measured using an automated biochemical analyzer. Insulin resistance was evaluated using the homeostasis model assessment of insulin resistance (HOMA-IR), which was calculated as insulin × FBG/22.5.
The mean ± SEM was used to describe categorical data, with intergroup comparisons performed using the independent sample t-test. Numbers and percentages were used to describe continuous data, and intergroup comparisons were performed using χ2 test. The correlation between T2DM remission and IPFD was determined using Pearson correlation coefficients. SPSS v 18.0 statistical software was used for data analysis. P values < 0.05 were considered statistically significant.
The two groups were similar in terms of sex, age, height, weight, disease course, and visceral fat area (P > 0.05; Table 1).
| Characteristics | Research group (n = 40) | Control group (n = 40) | χ2/t | P value |
| Sex, n (%) | 2.452 | 0.117 | ||
| Male | 23 (57.50) | 16 (40.00) | ||
| Female | 17 (42.50) | 24 (60.00) | ||
| Age (years) | 41.95 ± 7.94 | 43.02 ± 7.37 | 0.625 | 0.534 |
| Height (cm) | 168.48 ± 10.46 | 164.72 ± 13.71 | 1.379 | 0.172 |
| Weight (kg) | 69.25 ± 13.74 | 73.05 ± 12.91 | 1.275 | 0.206 |
| Disease course (years) | 2.90 ± 1.01 | 2.80 ± 0.82 | 0.486 | 0.628 |
| Visceral fat area (cm2) | 184.65 ± 22.20 | 176.30 ± 28.87 | 1.450 | 0.151 |
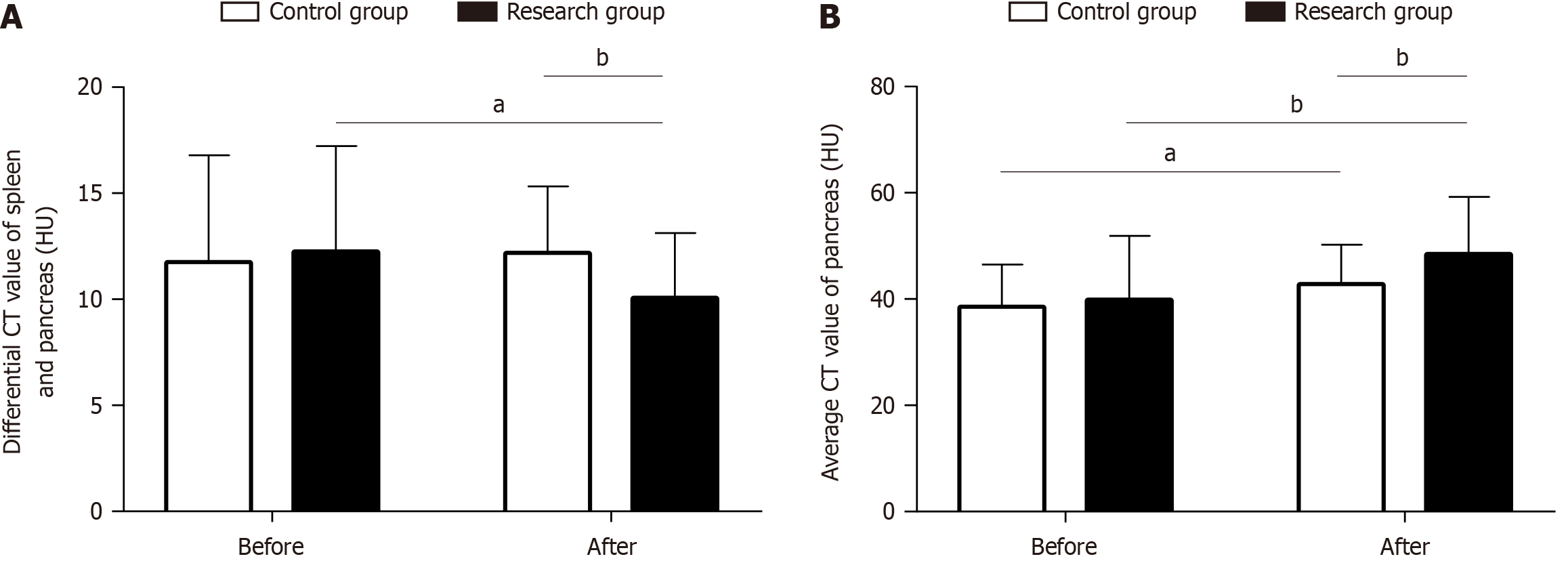
Improvements in IPFD in the two groups were evaluated by determining the differential CT values of the spleen and pancreas and the mean CT value of the pancreas. Differences in these values between the research and control groups before treatment were not statistically significant (P > 0.05). However, the differential CT values of the spleen and pancreas were markedly reduced in both groups after treatment (Figure 1A), and the mean CT value of the pancreas increased (Figure 1B), with more significant changes in the research group than in the control group (P < 0.05).
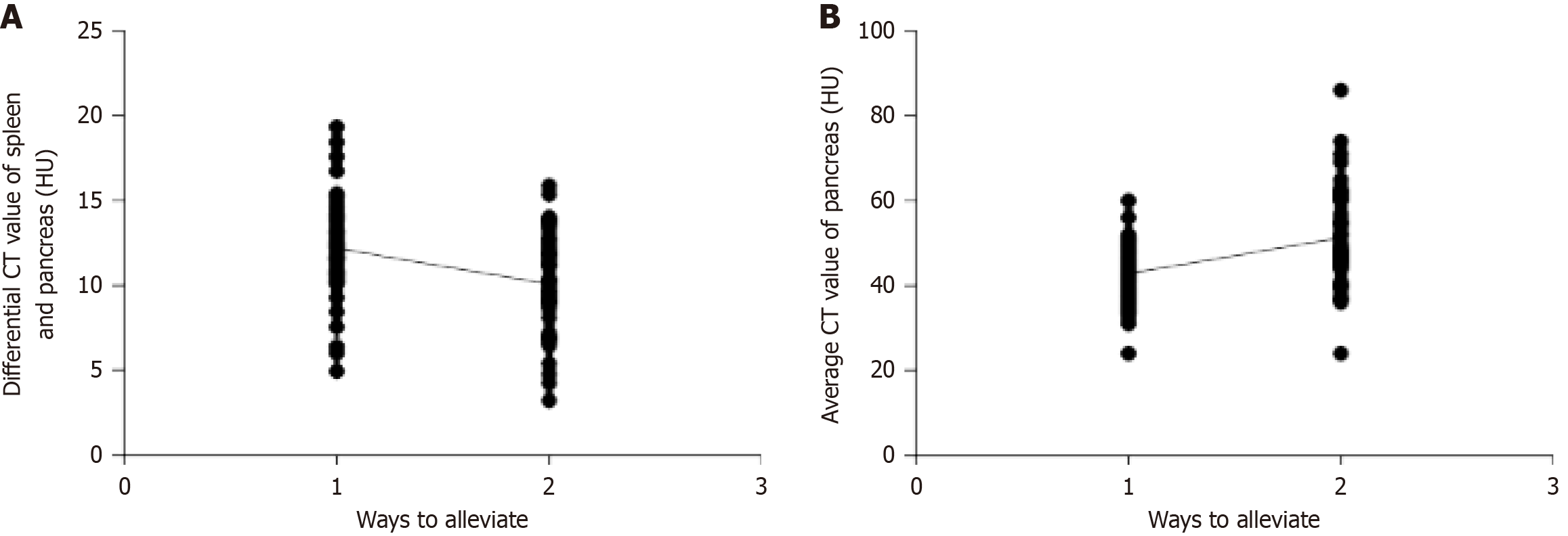
For the correlation analysis, we set the control group as 1 and the research group as 2 and analyzed the correlation between the two remission methods and the improvement in IPFD. T2DM remission was inversely correlated with IPFD (Figure 2 and Table 2).
| Indicators | r value | P value |
| Correlation between T2DM remission and differential CT values of the spleen and pancreas | -0.327 | 0.003 |
| Correlation between T2DM remission and average CT value of the pancreas | 0.397 | < 0.001 |
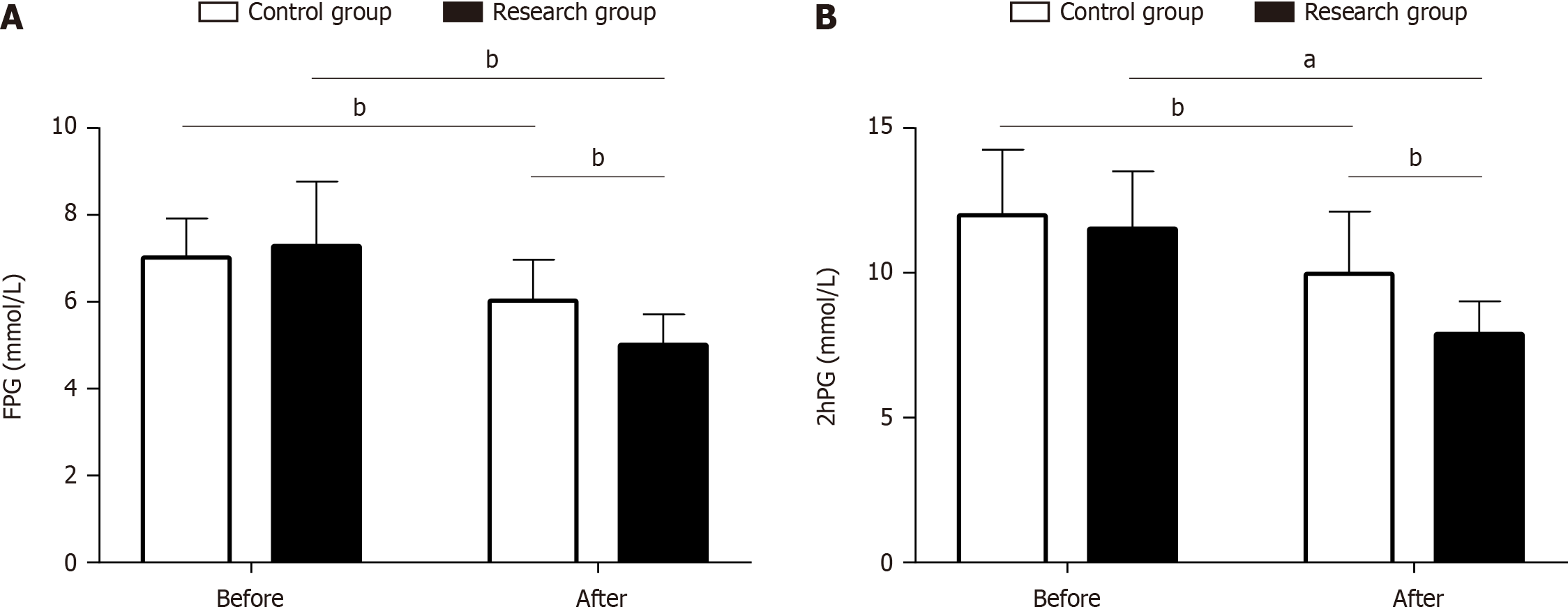
We determined FBG and 2hPBG levels to compare and analyze the effects of the two remission methods on blood glucose metabolism. There were no significant intergroup differences in FBG and 2hPBG levels before treatment (P > 0.05). After treatment, FBG and 2hPBG levels decreased significantly in both groups, and this change was more prominent in the research group (P < 0.05; Figure 3).
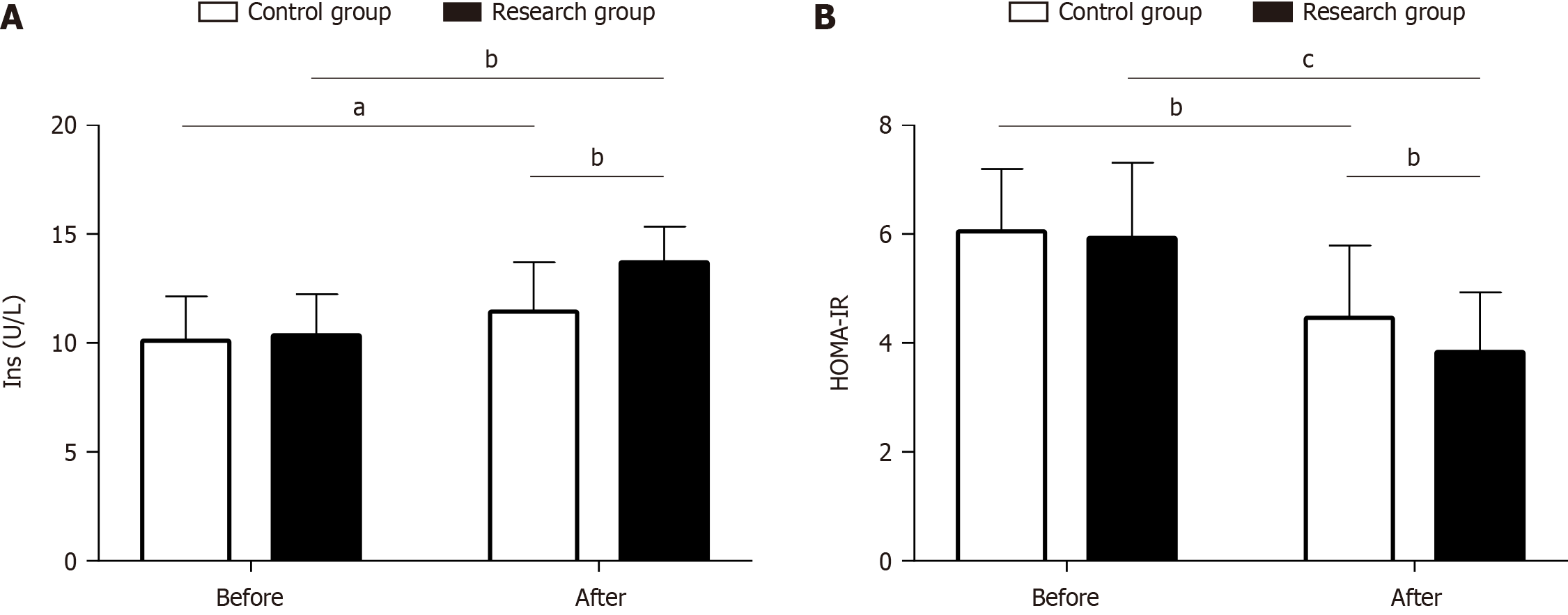
We tested insulin levels and calculated HOMA-IR in the two groups to evaluate the effects of the two remission methods on islet function. There were no significant intergroup differences in either parameter before treatment (P > 0.05). After treatment, both groups showed increased insulin levels and decreased HOMA-IR, which was more pronounced in the research group (P < 0.05; Figure 4).
IPFD is a common histopathological change that often occurs in patients who are aging and obese[17]. Abdominal ultrasound findings of enhanced pancreatic echo, which is closely related to β-cell damage and function failure, are observed in patients with T2DM and IPFD[18]. We analyzed the correlation of two remission methods with IPFD in T2DM and explored the changes in relevant metabolic indices to add to the body of research in this area, with the aim of enhancing the diagnosis and treatment level of the endocrinology department of our hospital in this field.
The posttreatment differential CT values of the spleen and pancreas in the research group were significantly lower than the pretreatment levels and the control group, and the average CT value of the pancreas was significantly higher, suggesting that liraglutide significantly relieved IPFD in patients with T2DM. Correlation analysis revealed a significant negative correlation between T2DM remission and IPFD. A rodent model study reported a strong association between IPFD and a high-fat/high-glucose diet, which not only abnormally increased plasma glucose levels but also predisposed the body to adverse effects, such as oxidative stress, inflammation, and islet cell apoptosis[19,20]. Excessive intra-abdominal fat deposition has also been suggested to increase the risk of cardiovascular disease and diabetic nephropathy in T2DM[21]. Neeland et al[22] reported that liraglutide effectively reduced visceral fat and ectopic fat deposition in overweight and obese adults at high cardiovascular risk, similar to our findings. Liraglutide has a long-lasting anti-T2DM effect that is closely related to its slow absorption after subcutaneous injection and low elimination rates due to slow metabolism and renal filtration[23]. The mechanism by which liraglutide relieves IPFD is associated with its effective regulation of the endoplasmic reticulum stress pathway and downstream apoptotic signals[24]. In our study, FBG and 2hPBG levels were markedly reduced in the research group after treatment and were lower than the pretreatment level and the control group, suggesting that liraglutide intervention was more effective than short-term IIT in controlling blood glucose in patients with T2DM. Assessment of pancreatic islet function revealed that insulin levels in the research group after treatment were significantly higher than those before treatment and in the control group, and HOMA-IR was significantly lower, indicating that liraglutide intervention in T2DM was more effective than short-term IIT in enhancing islet function. Liraglutide effectively controls the levels of blood lipids and blood glucose and effectively regulates insulin sensitivity, thereby helping to reduce body fat and maintain muscle tropism[25,26]. Liraglutide also prevents adverse cardiometabolic events and improves health-related quality of life in patients with DM[27].
This study has several limitations that must be addressed. First, the sample size is inadequate, which may inevitably lead to data collection bias that affects the accuracy of our results. Second, we did not perform a basic experiment to explore the anti-IPFD mechanism of the two remission methods in depth. We plan to perform supplementary analyses that will focus on these aspects to improve the credibility of our research results.
In summary, we found a negative correlation between T2DM remission and IPFD, and liraglutide was beneficial for glycemic control and islet function repair in patients with T2DM. Our findings are of great significance for promoting the development of research on the relationship between DM and fat and constructing effective treatment models for patients with T2DM.
| 1. | Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 2. | Ma Q, Li Y, Li P, Wang M, Wang J, Tang Z, Wang T, Luo L, Wang C, Wang T, Zhao B. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed Pharmacother. 2019;117:109138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 256] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 3. | Kao KT, Sabin MA. Type 2 diabetes mellitus in children and adolescents. Aust Fam Physician. 2016;45:401-406. [PubMed] |
| 4. | Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37:278-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1205] [Article Influence: 133.9] [Reference Citation Analysis (0)] |
| 5. | Dugani SB, Mielke MM, Vella A. Burden and management of type 2 diabetes in rural United States. Diabetes Metab Res Rev. 2021;37:e3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 6. | Chansela P, Potip B, Weerachayaphorn J, Kangwanrangsan N, Chukijrungroat N, Saengsirisuwan V. Morphological alteration of the pancreatic islet in ovariectomized rats fed a high-fat high-fructose diet. Histochem Cell Biol. 2022;157:427-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | van der Zijl NJ, Goossens GH, Moors CC, van Raalte DH, Muskiet MH, Pouwels PJ, Blaak EE, Diamant M. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab. 2011;96:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Saisho Y. Pancreas Volume and Fat Deposition in Diabetes and Normal Physiology: Consideration of the Interplay Between Endocrine and Exocrine Pancreas. Rev Diabet Stud. 2016;13:132-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Kramer CK, Zinman B, Retnakaran R. Short-term intensive insulin therapy in type 2 diabetes mellitus: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013;1:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 10. | Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. JAMA. 2014;311:2315-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Kramer CK, Choi H, Zinman B, Retnakaran R. Determinants of reversibility of β-cell dysfunction in response to short-term intensive insulin therapy in patients with early type 2 diabetes. Am J Physiol Endocrinol Metab. 2013;305:E1398-E1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Retnakaran R, Yakubovich N, Qi Y, Opsteen C, Zinman B. The response to short-term intensive insulin therapy in type 2 diabetes. Diabetes Obes Metab. 2010;12:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Ferrari F, Fierabracci P, Salvetti G, Jaccheri R, Vitti J, Scartabelli G, Meola A, Magno S, Ceccarini G, Santini F. Weight loss effect of liraglutide in real-life: the experience of a single Italian obesity center. J Endocrinol Invest. 2020;43:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Gupta V. Glucagon-like peptide-1 analogues: An overview. Indian J Endocrinol Metab. 2013;17:413-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Kuriyama T, Ishibashi C, Kozawa J, Baden MY, Horii T, Niki A, Ozawa H, Hosokawa Y, Fujita Y, Sadahiro K, Satoh T, Hamaguchi T, Shimomura I. Effects of liraglutide on intrapancreatic fat deposition in patients with type 2 diabetes. Clin Nutr ESPEN. 2024;59:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2621] [Article Influence: 655.3] [Reference Citation Analysis (0)] |
| 17. | Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137:111315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 440] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 18. | Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2010;10:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Cheng X, Jiang S, Pan B, Xie W, Meng J. Ectopic and visceral fat deposition in aging, obesity, and idiopathic pulmonary fibrosis: an interconnected role. Lipids Health Dis. 2023;22:201. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Murakami R, Saisho Y, Watanabe Y, Inaishi J, Tsuchiya T, Kou K, Sato S, Kitago M, Kitagawa Y, Yamada T, Itoh H. Pancreas Fat and β Cell Mass in Humans With and Without Diabetes: An Analysis in the Japanese Population. J Clin Endocrinol Metab. 2017;102:3251-3260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, Zhang W, Zhang H, Xia F, Wang N, Lu Y. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol. 2020;19:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 22. | Neeland IJ, Marso SP, Ayers CR, Lewis B, Oslica R, Francis W, Rodder S, Pandey A, Joshi PH. Effects of liraglutide on visceral and ectopic fat in adults with overweight and obesity at high cardiovascular risk: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Diabetes Endocrinol. 2021;9:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 23. | Jacobsen LV, Flint A, Olsen AK, Ingwersen SH. Liraglutide in Type 2 Diabetes Mellitus: Clinical Pharmacokinetics and Pharmacodynamics. Clin Pharmacokinet. 2016;55:657-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Fang T, Huang S, Chen Y, Chen Z, Chen J, Hu W. Glucagon Like Peptide-1 Receptor Agonists Alter Pancreatic and Hepatic Histology and Regulation of Endoplasmic Reticulum Stress in High-fat Diet Mouse Model. Exp Clin Endocrinol Diabetes. 2021;129:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Perna S, Guido D, Bologna C, Solerte SB, Guerriero F, Isu A, Rondanelli M. Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res. 2016;28:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Rondanelli M, Perna S, Astrone P, Grugnetti A, Solerte SB, Guido D. Twenty-four-week effects of liraglutide on body composition, adherence to appetite, and lipid profile in overweight and obese patients with type 2 diabetes mellitus. Patient Prefer Adherence. 2016;10:407-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Fujioka K, O'Neil PM, Davies M, Greenway F, C W Lau D, Claudius B, Skjøth TV, Bjørn Jensen C, P H Wilding J. Early Weight Loss with Liraglutide 3.0 mg Predicts 1-Year Weight Loss and is Associated with Improvements in Clinical Markers. Obesity (Silver Spring). 2016;24:2278-2288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |