Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.3985
Revised: April 25, 2024
Accepted: May 7, 2024
Published online: July 6, 2024
Processing time: 118 Days and 3.1 Hours
Myeloid sarcoma (MS), also referred to as granulocytic sarcoma or chloroma, is a rare type of extramedullary malignant tumor. MS comprises primitive granu
The present study reported the case of MS with gingival swelling in the maxillary region, with aleukemic presentation in a 32-year-old male patient. Following two courses of chemotherapy, computed tomography of the region demonstrated complete clearance of the tumor. At the 12-month follow-up appointment, the patient was in a stable condition with the absence of progression. The etiology, clinical features, diagnosis, and relevant treatment of MS are discussed in the present study.
Diagnosis of MS may be confirmed following histological and immunohistoche
Core Tip: We report a rare case of myeloid sarcoma (MS) with maxillary gingival swelling as the initial symptom. In this case, histopathological and immunohistochemical analyses showed diffuse infiltration of myeloperoxidase-positive neoplastic cells with 80% Ki-67 staining, indicating MS. Flow cytometry revealed a predominance of granulocytes without distinct B-cell or T-cell markers, supporting the diagnosis. Absence of CD20 and CD3 markers lessened lymphoma likelihood, confirming the lesion's hematopoietic nature.
- Citation: Li SH, Yang CX, Xing XM, Gao XR, Lu ZY, Ji QX. Myeloid sarcoma with maxillary gingival swelling as the initial symptom: A case report and review of literature. World J Clin Cases 2024; 12(19): 3985-3994
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/3985.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.3985
Myeloid sarcoma (MS), also referred to as granulocytic sarcoma or chloroma[1], is a solid malignant tumor associated with the infiltration of immature myeloid cells which occurs at extramedullary site[2,3]. The term chloroma is derived from the green color observed in some cases[4,5]. MS often occurs during myeloproliferative disorders or acute myeloid leukemia (AML)[6,7], and in some cases, may precede bone marrow or peripheral blood involvement[2]. Primary, iso
To the best of our knowledge, there are few intra-oral cases of MS[9-11]. A literature search using MS and oral cavity produced a total of 66 previous studies. Moreover, there are few cases of MS in the gums[10,12,13]. A literature search using MS and gingival produced a total of 49 previous studies; however, gum swelling was only described as the first symptom of MS in 21 of these cases. Thus, the occurrence of this tumor in the gingiva is not often described. The present study reported a case of gingival MS with gingival swelling comparable to periodontal disease in the posterior area.
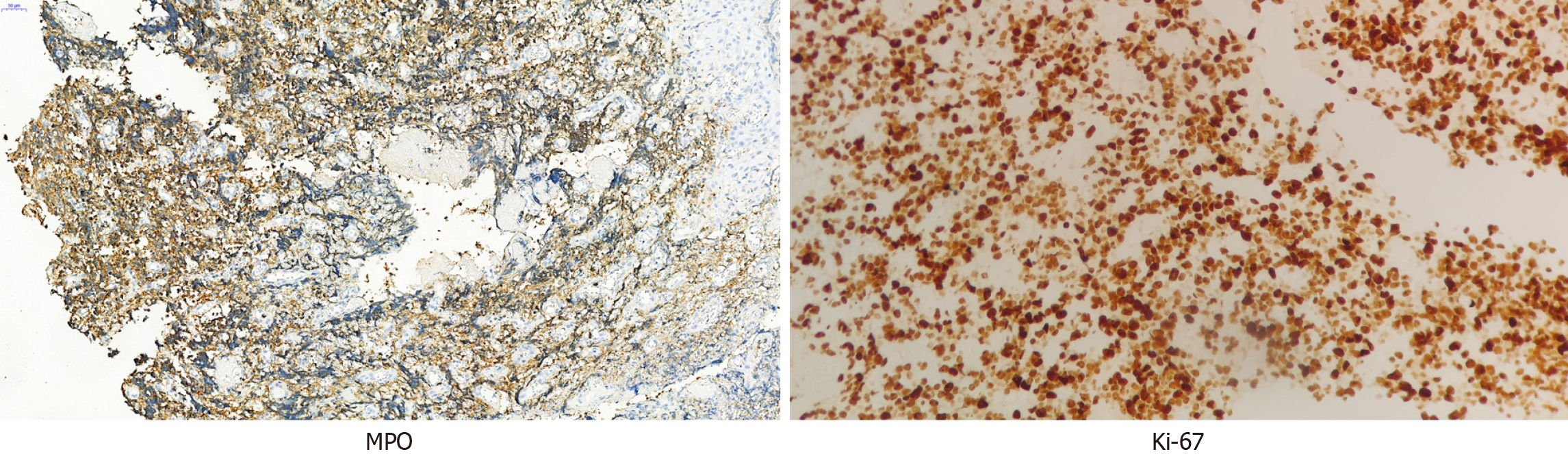
Notably, the biopsy performed on the maxillary mass of the patient and the subsequent histologic examination demonstrated the diffuse infiltration of abnormal cells. High levels of the tumor marker, Ki-67, revealed that the tumor exhibited a high potential for metastasis.
One-month history of indolent and stable swelling in the left maxillary molar buccal gingival region.
A 32-year-old male presented to Qingdao University Affiliated Hospital on August 31, 2015, with a one-month history of indolent and stable swelling in the left maxillary molar buccal gingival region. The patient did not present with prodrome symptoms, and the size of the mass was stable.
There was no history of systemic or hereditary disease, such as hematological disorders, and no history of previous medication.
Deny family genetic history.
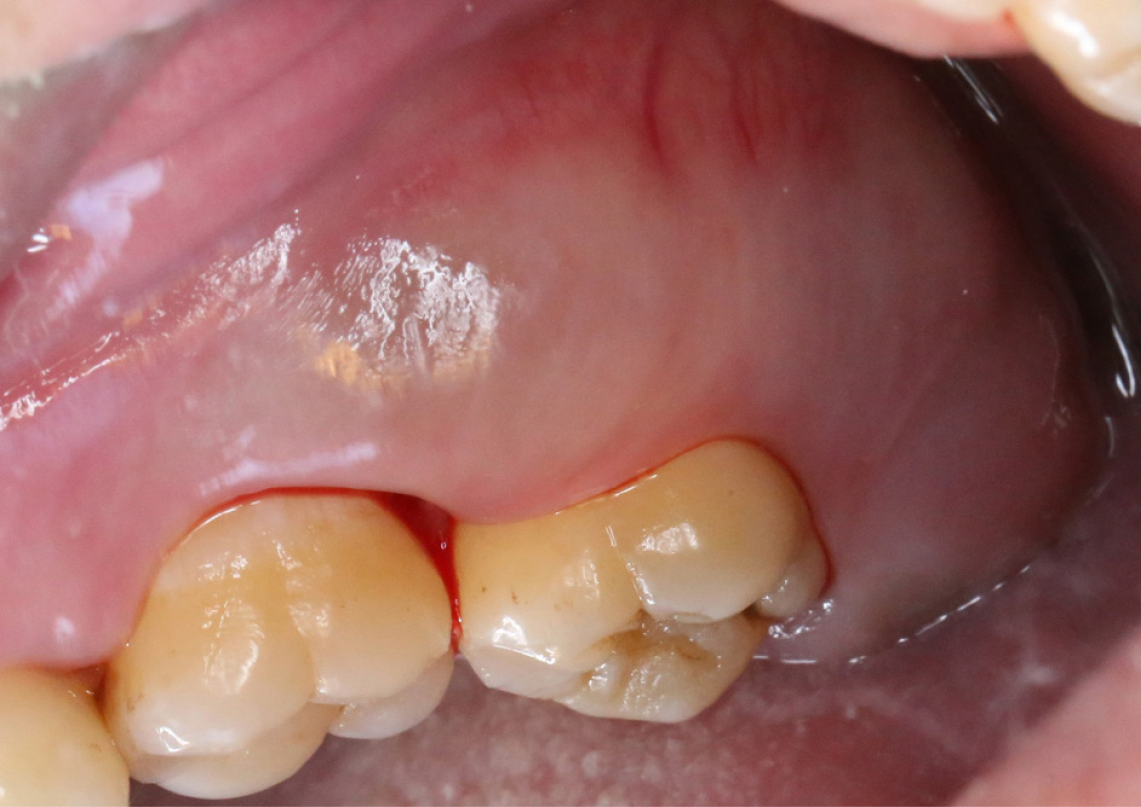
Based on an extra-oral examination, results of the present study revealed that the face of the patient was symmetrical, despite the presence of swelling on the left side of the molar buccal gingival region. This indicated that the swelling was localized to a specific area and did not cause any notable facial asymmetry. The left submandibular lymph nodes were not palpable. Intra-oral examination revealed a firm mass with tenderness that was gray-white in color with regular surfaces, a swelling mass in the left molar gingival area, and the largest dimension was 2 cm. Thus, results of these examinations demonstrated that the swelling or involvement of the gingiva extended from the distal aspect of the left maxillary first molar to the distal aspect of the left maxillary second molar. The observed swelling affected a specific area of the gingiva in the posterior region of the upper jaw (Figure 1). Notably, the probing depth was 4 mm and the probe bleeding degree was 2. In the periodontal pocket, subgingival plaques and attachment loss was observed.
Notably, results of laboratory tests revealed that the complete blood count of the patient was normal.
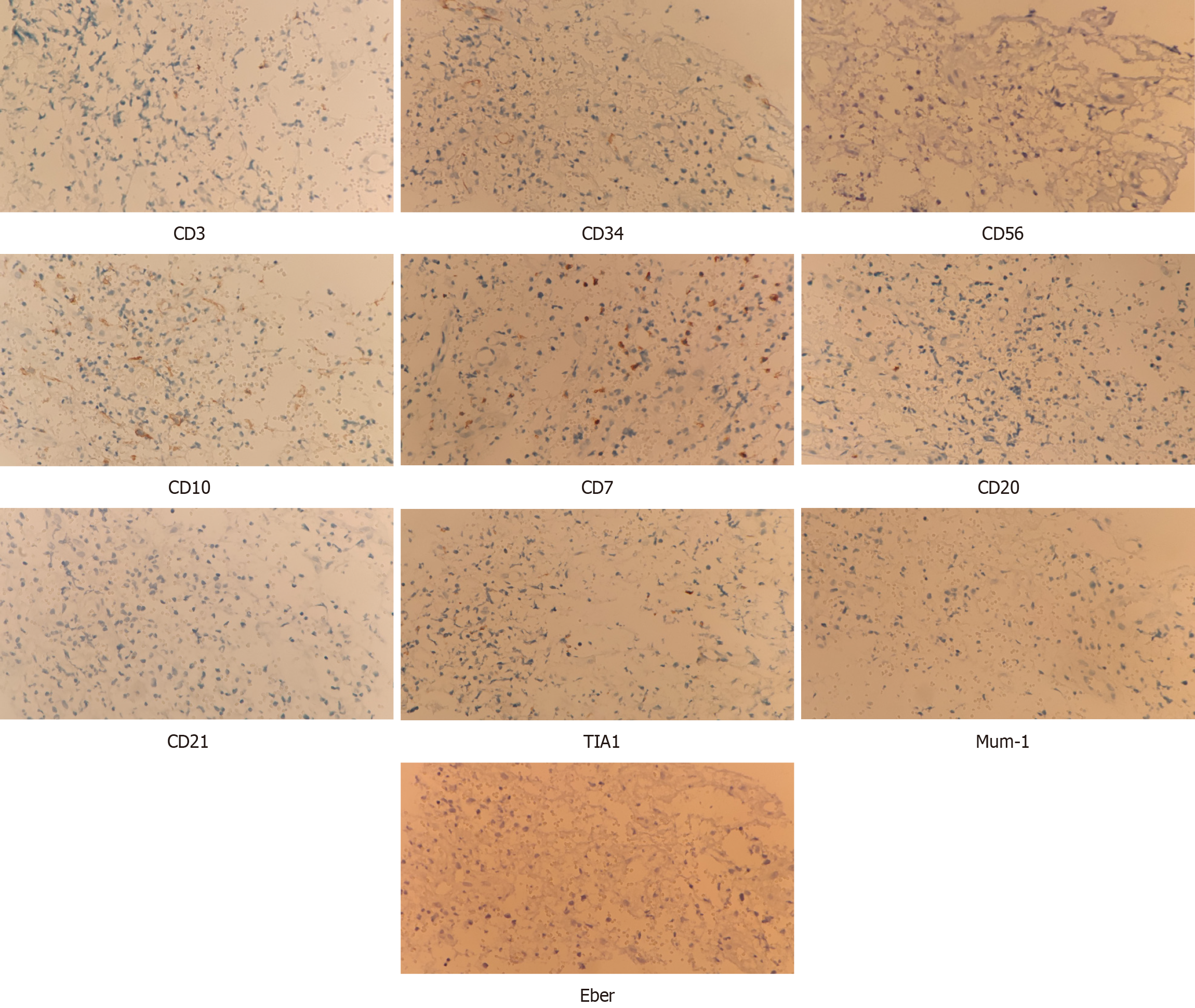
To further investigate the nature of the gray-white infiltrating mass, an incisional biopsy was performed using an intra-oral approach. After specimen collection, tissues were fixed in 10% PFA for 24 h and subsequently embedded in paraffin. Histological sections were obtained, dehydrated, and subjected to hematoxylin and eosin staining. Simultaneously, each section was incubated overnight at 4 °C in the presence of primary antibodies against myeloperoxidase-positive (MPO), Ki-67, CD3, CD34, CD56, CD10, CD7, CD20, CD21, TIA1, and Mum-1 (Maixin, China), followed by secondary immunohistochemistry labeling. After overnight hybridization with Eber probes (VENTANA, United States), the sections were washed and incubated at 37 °C for 30 min with the appropriate horseradish peroxidase-conjugated secondary antibodies. Finally, all sections were subjected to microscopic examination and histopathological diagnosis.
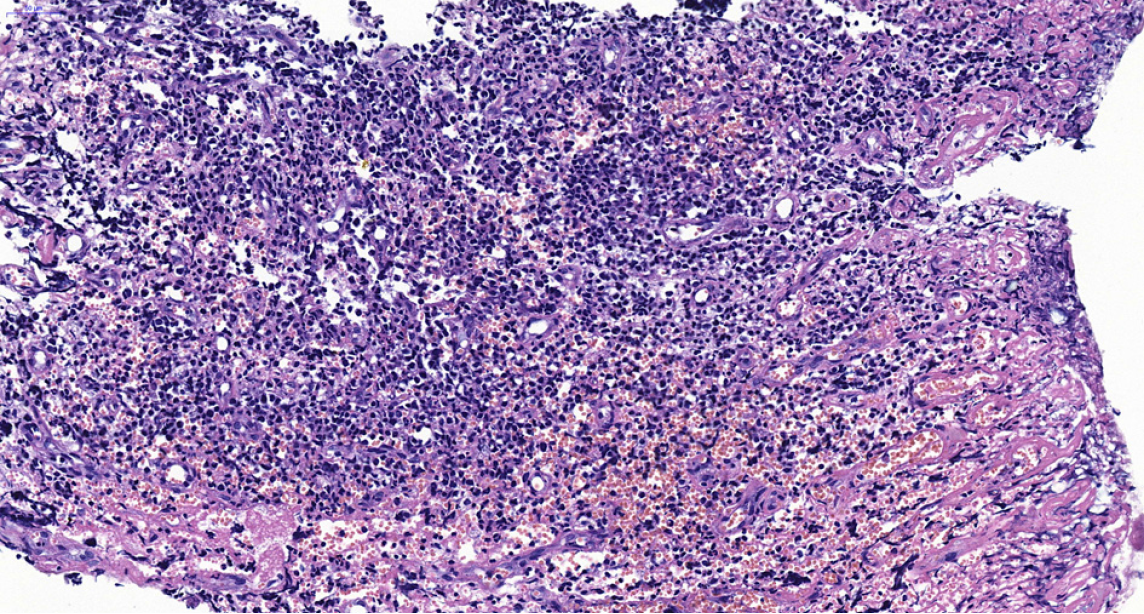
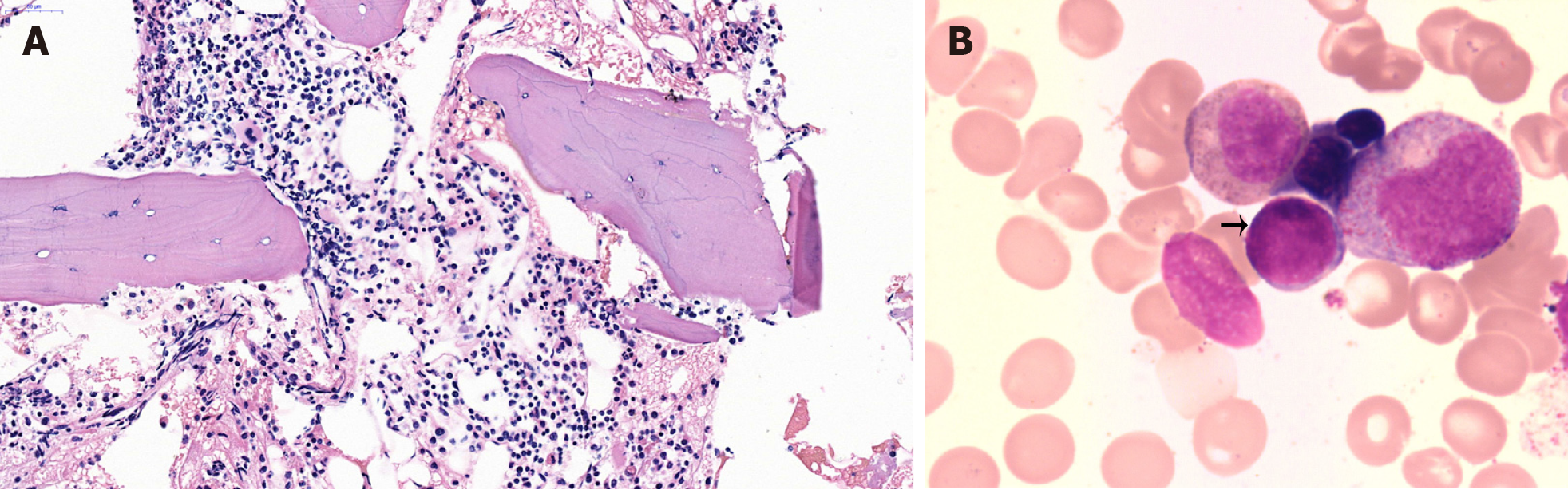
During the histological examination of the gingival biopsy specimen, diffuse infiltration of abnormal cells was ob
In the pathological bone marrow biopsy results of this case, the ratio of red blood cells to granulocytes is consistent with the expected values, and various stages of red blood cells and granulocytes are observed. The predominant cell population in the red blood cell lineage consists of mature cells. Megakaryocytes are visible, and their nuclei exhibit segmentation. Plasma cells are rarely observed in the lymphocytic lineage. Both fe and reticular fibers were positive staining pattern. There is no diffuse infiltration of abnormal leukemia cells in the bone marrow, indicating that the bone marrow is not involved. Alternatively, it is possible that the disease has not progressed to involve the bone marrow, and there is no cytological evidence of acute leukemia (Figure 5). Notably, granulocytes observed in the bone marrow slices demonstrated high levels of proliferation. Each stage of the granulocyte was visible, and 3% of the original granulocytes were in the late stage (Figure 6). In addition, eosinophilic granulocytes were visible.
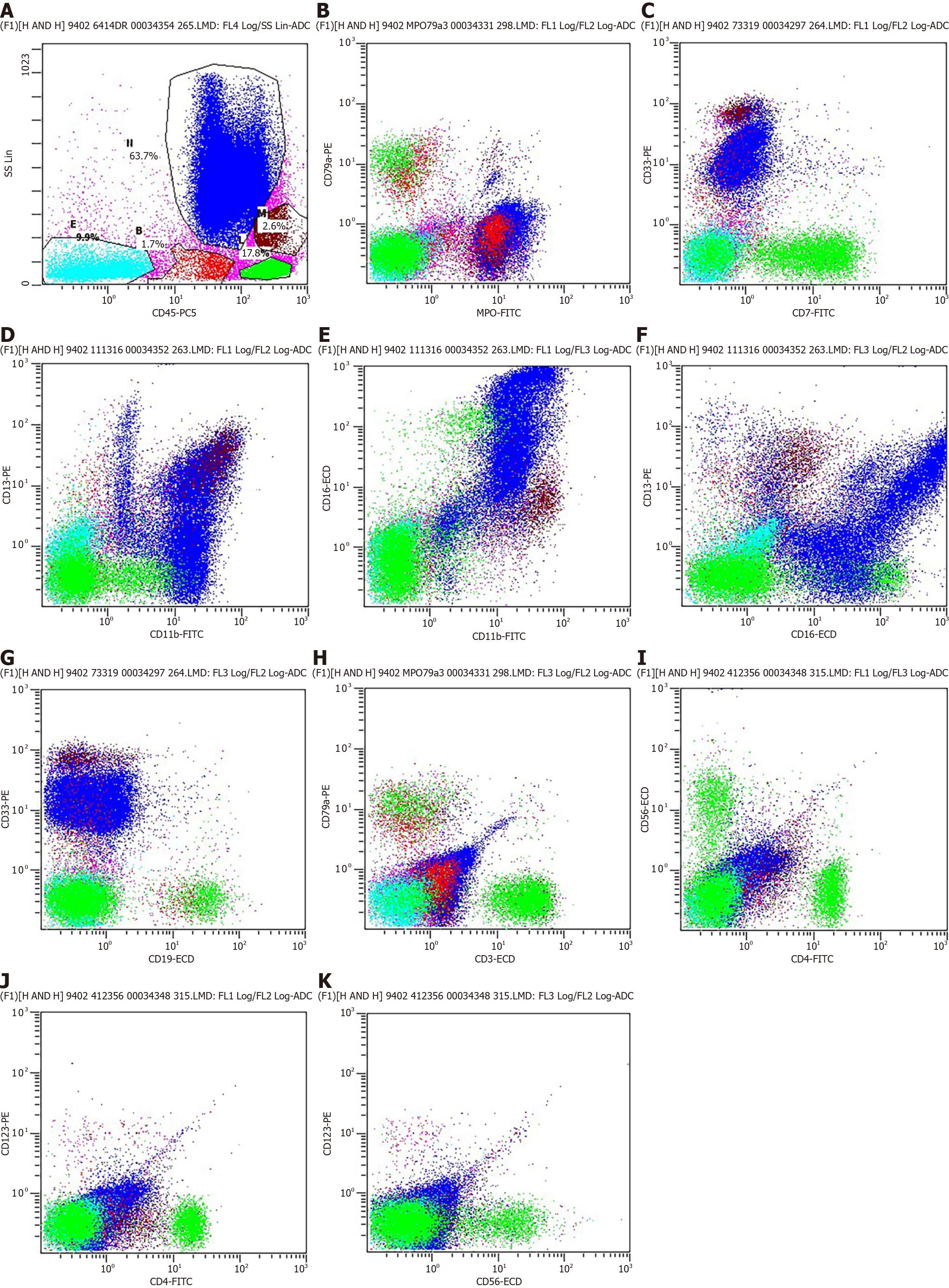
Flow cytometry was employed for the immunophenotypic analysis of the whole blood samples obtained to characterize immune cell subpopulations. After red blood cell lysis and washing, cells were centrifuged at 300 ×g for 10 min, the supernatant was completely aspirated, and a pre-mixture of antibodies against CD45, MPO, CD33, CD11b, CD13, CD16, CD19, CD3, CD4, CD56, and CD123 was added to the cells. The cells were resuspended and incubated in the dark at 4 °C for 10 min. After incubation, cells were centrifuged, and the cell pellet was resuspended and washed in PBS containing 1% BSA. Flow cytometric analysis was performed immediately.
Immunophenotyping using flow cytometry revealed that 63.7% of the cells were granulocytes, 17.8% of the cells were lymphocytes, 2.6% of the cells were monocytes, 1.7% of the cells were immature cells, and 9.9% of the cells were CD45-negative (Figure 6A). The granulocyte morphology of CD45, MPO, CD33, CD11b-CD13-CD16 is generally within normal parameters (Figure 6B-F). No distinct expressing CD4, CD56, and CD123 were observed within the B-cell group in the present study (Figure 6I-K).
The presence of the CD45 marker ruled out the potential for other tumors, and indicated that the lesion was a result of a hematopoietic disorder. Based on the absence of CD20 and CD3 markers, the patient was evaluated for potential lymphomas. Notably, these markers are commonly used in immunophenotyping to identify specific types of lymphocytes, and the absence of CD20 and CD3 markers are indicative that B-cell and T-cell lymphomas are less likely to be present. The lesion observed in the present case was confirmed as MS due to high MPO expression levels[14].
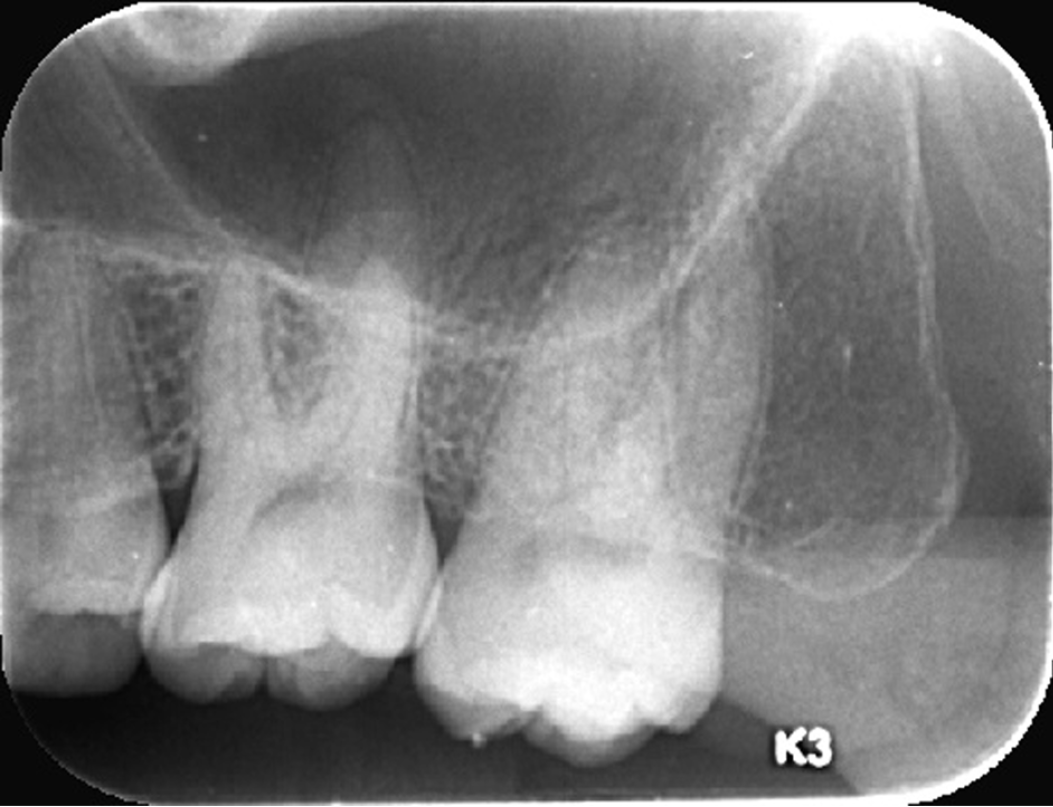
Radiological examination revealed no extensive bone loss (Figure 7). The gray-white color of the mass was indicative of localized chronic periodontal disease. The patient was referred to a hematologist, who confirmed the initial diagnosis of leukemia.
The lesion observed in the present case was confirmed as MS.
The patient initially received the idarubicin (IDA) regimen (IDA hydrochloride 10 mg/1 d-3 d, Cytarabine 100 mg/1 d-7 d) treatment, supplemented with gastric protection and antiemetic therapy. After one course of chemotherapy, the patient was switched to the Mitoxantrone (MA) regimen (MA 8 mg/1 d-3 d, Cytarabine 100 mg/1 d-7 d), with additional measures for gastric protection and antiemetic therapy.
After the two courses of chemotherapy, results of the computed tomography scan demonstrated complete clearance of the tumor. On days 7-14 after discontinuing chemotherapy, a bone marrow and blood sample examination indicated remission. On days 21-28 after discontinuing chemotherapy, a follow-up bone marrow and blood cell count showed complete disease remission. At the 12-month follow-up appointment, the patient was in a stable condition with the absence of MS progression, but long-term follow-up is still required in the later stages.
MS is a rare type of cancer that involves the growth of myeloid cells outside of the bone marrow. Notably, MS occurs as an extramedullary tumor mass in various parts of the body. This tumor is often associated with AML, and is estimated to develop in 2%-8% of patients with AML[2]. However, the specific pathogenesis of MS is not yet fully understood. Results of a previous study demonstrated that MS originates in the bone marrow and travels via the Haversian canals to reach the subperiosteal bone region[15].
Results of previous studies described increases in tissues with intact mucosa that varied in color, including brown, black, red and pale-gray. MS is often associated with other hematological disorders, such as myeloid leukemia and other myeloproliferative disorders. Previous studies described the presence of MS in the gingiva, palate, extraction sockets and buccal mucosa, despite the rare occurrence of MS in the oral cavity[16-18]. Notably, symptoms may be comparable with those of periodontitis, periodontal abscesses, pyogenic granuloma, carcinoma or lymphomas[10,19,20].
MS is not an aggressive tumor, and the lesion described in the present study demonstrated no extensive bone loss. Notably, the lesion described in the present study was gray-white in color, compared with the dark red color of the majority of periodontal diseases. As malignant, infectious and inflammatory lesions may exhibit overlapping clinical features, accurate diagnoses are challenging. Thus, the selection of appropriate histological and immunohistochemical analyses is required for the accurate clinical diagnosis of MS[11].
Histological examination carried out in a previous study revealed an infiltrate of primitive, poorly differentiated cells with round to oval nuclei, granular chromatin, well-defined nuclear membranes and variably prominent nucleoli[15]. Notably, granulocytic sarcomas are histologically comparable with lymphomas. Thus, immunohistochemical studies are ineffective in establishing an accurate diagnosis[21].
In the present study, an immunohistochemical examination was performed to exclude malignant lymphoma in the case of extramedullary myeloid cell infiltration. Myeloid cell markers were observed in the present study, including MPO and lysozyme, as well as B lineage markers and T lineage markers. The absence of these markers suggested that neoplastic cells involved in infiltration were not of lymphoid origin. Although lymphoid markers, such as CD3 and CD20, were used for differential diagnosis, the history of concurrent leukemia may have played a notable role in the final diagnosis of oral MS[22]. In addition, the presence of B-cell and T-cell lymphomas were excluded following negative staining for CD3 and CD20. However, a concise immunohistochemical panel, including markers such as CD20, CD43, CD68 and MPO are often used to identify the majority of extramedullary myeloid tumors. These markers are commonly used in the evaluation of myeloid neoplasms and may aid in distinguishing them from other types of tumors, including lymphomas[23]. The expression of these markers in conjunction with clinical history and histopathological features support the diagnosis of extramedullary myeloid tumors. Immunohistochemical staining may also aid in the final diagnosis of MS, as bone marrow cells respond to antibodies against lysozyme, MPO and chloroacetate esterase. Bone marrow mother cells in MS typically express antigens associated with bone marrow, such as CD43; however, these do not respond to lymphoid antigens[24].
A high Ki-67 staining rate of 80%-90% indicates a high proliferation rate of malignant cells in the tissue sample. Ki-67 is a protein marker that is often used to assess the proliferation or growth rate of cells in a tissue sample, particularly in the context of cancer[2]. The Ki-67 staining rate observed in the present study was 80%, indicative of malignant cells. MPO is an enzyme located in myeloid cells that is often used as an immunohistochemical marker for the identification of myeloid cells in tissue samples. MPO is used in the diagnosis of myeloid leukemia and other myeloproliferative disorders. MPO may be detected using various staining techniques, including Sudan Black B and peroxidase or diaminobenzidine[16]. Results of the present study indicated positive MPO staining, and negative CD3 and CD20 staining, indicative of a myeloid neoplasm.
The treatment of MS and AML are similar, as MS may develop into AML. Results of a previous study highlighted the slow progression to leukemia in patients with chloroma treated with chemotherapy. However, results of an additional previous study demonstrated that there was no association between the aforementioned treatment and the survival rates of patients[25]. Notably, the presence of MS in myelodysplastic syndromes is indicative of progression to a more aggressive disease stage[26,27]. At present, systemic chemotherapy and local radiotherapy are commonly used treatment options for patients with MS[28]. Notably, high-dose therapies are recommended as a front-line approach for patients with MS, in order to achieve complete remission. High-doses are used as aggressive treatment strategies[29]. Results of a previous study outlined positive outcomes in patients who received chemotherapy at the time of chloroma diagnosis. Specifically, these patients exhibited a lower rate of progression to leukemia, highlighting the potential benefits of early intervention[30]. During the remission period of hematological diseases, such as MS, allogeneic bone marrow or peripheral stem cell transplantation may be considered as treatment options. However, the efficacy of these methods remains unclear. Notably, the overall course of MS is acute, with the majority of MS-related deaths occurring within the first year following diagnosis. This highlights the aggressive nature of the disease, and the requirement for effective treatment strategies[25]. Moreover, there was no significant association between a specific treatment modality and survival.
Prognostic factors for MS may include patient age and genetic factors. Elderly individuals exhibit a higher risk of developing certain types of AML. The presence of AML in a patient with MS may indicate a poor prognosis[12,15]. Specific chromosomal alterations are associated with a more favorable prognosis. In addition, specific chromosomal alterations in patients with MS may be associated with either a more favorable or unfavorable prognosis. Thus, additional assessments of patients may improve the subsequent prognosis. Notably, patients with single lesion involvement exhibited a prolonged survival time compared with patients with multiple organ involvement. These results indicated that metastasis may play a role in determining the prognosis of the patient[2]. Moreover, patients with MS exhibit a poor prognosis, and MS disease progression is closely associated with the clinical course of AML. Thus, MS may significantly impact the progression and outcomes of AML in certain patients.
Following two courses of intensive chemotherapy without radiotherapy in the present case, complete clearance of MS was observed. Thus, early aggressive treatment may prevent or delay subsequent progression to AML; however, additional clinical observations are required in other cases. The overall survival rate of patients with MS remains unclear, as there are a small number of cases. Adequate diagnosis is required for the selection of appropriate treatment options, and the prevention of serious complications.
Mutation gene testing plays a crucial role in the treatment of MS and serves as important diagnostic and therapeutic evidence. Unfortunately, due to the relatively rare occurrence of such cases in the oral cavity, our lack of experience led us to not conduct these gene mutation tests in the initial sample cohort, including ASXL1, NPM1, DNMT3A, FLT3, TET2, IDH2, TP53, EZH2, and STAG2, among others. It is highly necessary to consider these gene tests as potential avenues for future research, thereby enhancing the quality and significance of our work.
In conclusion, the occurrence of MS in the oral cavity is rare. A diagnosis of MS should be considered in patients that present with atypical clinical features. Diagnosis of MS may be confirmed following histological and immunohistochemical examinations.
The present study reported the case of MS with gingival swelling in the maxillary region, with aleukemic presentation in a 32-year-old male patient. Diagnosis of MS may be confirmed following histological and immunohistochemical examinations.
| 1. | Hoversten K, Vallapureddy R, Lasho T, Finke C, Ketterling R, Hanson C, Gangat N, Tefferi A, Patnaik MM. Nonhepatosplenic extramedullary manifestations of chronic myelomonocytic leukemia: clinical, molecular and prognostic correlates. Leuk Lymphoma. 2018;59:2998-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Colella G, Tirelli A, Capone R, Rubini C, Guastafierro S. Myeloid sarcoma occurring in the maxillary gingiva: a case without leukemic manifestations. Int J Hematol. 2005;81:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Jin SH, Park G, Ko Y, Park JB. Myeloid sarcoma of the Gingiva with myelodysplastic syndrome: A Case Report. Medicine (Baltimore). 2016;95:e3897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Bassichis B, McClay J, Wiatrak B. Chloroma of the masseteric muscle. Int J Pediatr Otorhinolaryngol. 2000;53:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Wang X, Zhang L, Zhou D, Cai H, Wang X, Jiang X. A case report on concurrent occurrence of systemic mastocytosis and myeloid sarcoma presenting with extensive skin involvements and the results of genetic study. Medicine (Baltimore). 2020;99:e21948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Kumar M, Sonia F, Hamadani SM, Abbas SA. A Rare Case of Acute Cord Compression From Spinal Myeloid Sarcoma: A Complication of Acute Myeloid Leukemia. Cureus. 2020;12:e9502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Lu DH, Chen F, Zhang QG, Guan DN. [Granulocytic sarcoma of oral cavity: report of two cases]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2009;27:110-112. [PubMed] |
| 8. | Yilmaz AF, Saydam G, Sahin F, Baran Y. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013;3:265-270. [PubMed] |
| 9. | Mutalik VS, Neppalli V, Dale C, Penner C. Multiple painful ulcerated lesions in a 73-year-old patient. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:3-7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Wang D, He K, Sroussi H, Treister N, Luskin M, Villa A, Woo SB, Shazib MA. Oral myeloid sarcoma as an uncommon manifestation of acute myeloid leukemia: A case series and review of the literature. J Am Dent Assoc. 2021;152:393-400.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Mester A, Irimie A, Oprita L, Dima D, Petrushev B, Lucaciu O, Campian RS, Tanase A. Oral manifestations in stem cell transplantation for acute myeloid leukemia. Med Hypotheses. 2018;121:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | de Sena ACVP, de Arruda JAA, Costa FPD, Lemos APV, Kakehasi FM, Travassos DV, Abreu LG, Fonseca FP, Mesquita RA, Silva TA. Leukaemic infiltration in the oral and maxillofacial region: An update. J Oral Pathol Med. 2021;50:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Shen Y, Zhao L, Wu Y, Huang P. Multifocal occurrence of intraoral isolated MS in a patient without leukemic presentation: A case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e42-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Abdulsalam AH. "Arbitrary" criterion for the diagnosis of acute leukemia. Turk J Haematol. 2011;28:149-150. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Kim K, Velez I, Rubin D. A rare case of granulocytic sarcoma in the mandible of a 4-year-old child: a case report and review of the literature. J Oral Maxillofac Surg. 2009;67:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Ponnam SR, Srivastava G, Jampani N, Kamath VV. A fatal case of rapid gingival enlargement: Case report with brief review. J Oral Maxillofac Pathol. 2014;18:121-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Vázquez-martínez C, Redondo-alamillos M, Romance-garcía AI, Sánchez- Aniceto G. Sarcoma mieloide intraoral: forma poco común de debut de la leucemia aguda mieloide. Rev Esp Cir Oral Maxilofac. 2019;41:41-43. [DOI] [Full Text] |
| 18. | Quispe RA, Aguiar EM, de Oliveira CT, Neves ACX, Santos PSDS. Oral manifestations of leukemia as part of early diagnosis. Hematol Transfus Cell Ther. 2022;44:392-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Xie Z, Zhang F, Song E, Ge W, Zhu F, Hu J. Intraoral granulocytic sarcoma presenting as multiple maxillary and mandibular masses: a case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e44-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Lee DY, Baron J, Wright CM, Plastaras JP, Perl AE, Paydar I. Radiation Therapy for Chemotherapy Refractory Gingival Myeloid Sarcoma. Front Oncol. 2021;11:671514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Schroers-martin JG, Soo J, Brisou G, Scherer F, Kurtz DM, Sworder B, Khodadoust MS, Jin MC, Bru A, Liu CL, Stehr H, Vineis P, Natkunam Y, Nadel B, Diehn M, Roulland S, Alizadeh AA. Recurrent Crebbp Mutations in Follicular Lymphoma Appear Localized to the Committed B-Cell Lineage. Blood. 2020;136:30-31.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology. 1999;34:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Traweek ST, Arber DA, Rappaport H, Brynes RK. Extramedullary myeloid cell tumors. An immunohistochemical and morphologic study of 28 cases. Am J Surg Pathol. 1993;17:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Papamanthos MK, Kolokotronis AE, Skulakis HE, Fericean AM, Zorba MT, Matiakis AT. Acute myeloid leukaemia diagnosed by intra-oral myeloid sarcoma. A case report. Head Neck Pathol. 2010;4:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Meis JM, Butler JJ, Osborne BM, Manning JT. Granulocytic sarcoma in nonleukemic patients. Cancer. 1986;58:2697-2709. [PubMed] [DOI] [Full Text] |
| 26. | Strupp C, Germing U, Trommer I, Gattermann N, Aul C. Pericardial effusion in chronic myelomonocytic leukemia (CMML): a case report and review of the literature. Leuk Res. 2000;24:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Ganzel C, Lee JW, Fernandez HF, Paietta EM, Luger SM, Lazarus HM, Cripe LD, Douer D, Wiernik PH, Rowe JM, Tallman MS, Litzow MR. CNS involvement in AML at diagnosis is rare and does not affect response or survival: data from 11 ECOG-ACRIN trials. Blood Adv. 2021;5:4560-4568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Srinivasan B, Ethunandan M, Anand R, Hussein K, Ilankovan V. Granulocytic sarcoma of the lips: report of an unusual case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e34-e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Mei KD, Lin YS, Chang SL. Myeloid sarcoma of the cheek and the maxillary sinus regions. J Chin Med Assoc. 2013;76:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Imrie KR, Kovacs MJ, Selby D, Lipton J, Patterson BJ, Pantalony D, Poldre P, Ngan BY, Keating A. Isolated chloroma: the effect of early antileukemic therapy. Ann Intern Med. 1995;123:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 5.0] [Reference Citation Analysis (0)] |