Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1513
Peer-review started: October 24, 2022
First decision: January 20, 2023
Revised: February 2, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 6, 2023
Processing time: 129 Days and 6.3 Hours
Multiple primary malignant neoplasms (MPMNs) are rare, while synchronous MPMNs (SMPMNs) are even less common. Owing to the progression of medical technology and the extension of life expectancy, its incidence is gradually increasing.
Although reports of breast and thyroid dual cancers are common, cases of an additional diagnosis of kidney primary cancer within the same individual are rare.
We present a case of simultaneous MPMN of three endocrine organs, reviewing the relevant literature to enhance our understanding of SMPMNs while emphasizing the increasingly important need for accurate diagnosis and multidisciplinary management whenever this challenging situation arises.
Core Tip: Multiple primary malignant neoplasms (MPMNs) are rare, while synchronous MPMNs are even less common. Owing to the progression of medical technology and the extension of life expectancy, its incidence is gradually increasing. Although reports of breast and thyroid dual cancers are common, cases of an additional diagnosis of kidney primary cancer within the same individual are rare.
- Citation: Jia MM, Yang B, Ding C, Yao YR, Guo J, Yang HB. Synchronous multiple primary malignant neoplasms in breast, kidney, and bilateral thyroid: A case report. World J Clin Cases 2023; 11(7): 1513-1520
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1513.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1513
Multiple primary malignant neoplasms (MPMNs) are generally defined as the occurrence of two or more pathologically diagnosed primary malignant tumors in ≥ 1 organ or tissue in the same person[1]. Although inconsistent among various studies, an additional diagnosis that occurs within six months of the primary tumor diagnosis is defined as synchronous MPMN (SMPMN); otherwise, they are classified as metachronous cancers (MMPMNs)[2].
The number of confirmed cases of MPMNs is increasing, accompanied by increasing availability of screening, diagnosis, and treatment. Most of these are diagnosed as dual primary cancers, while triple is relatively rare, and triple SMPMNs are even rarer. However, a kidney cancer that occurs concurrently with breast and thyroid cancers has rarely been described in the literature. Therefore, we report a case of SMPMNs to further understand its diagnosis and management.
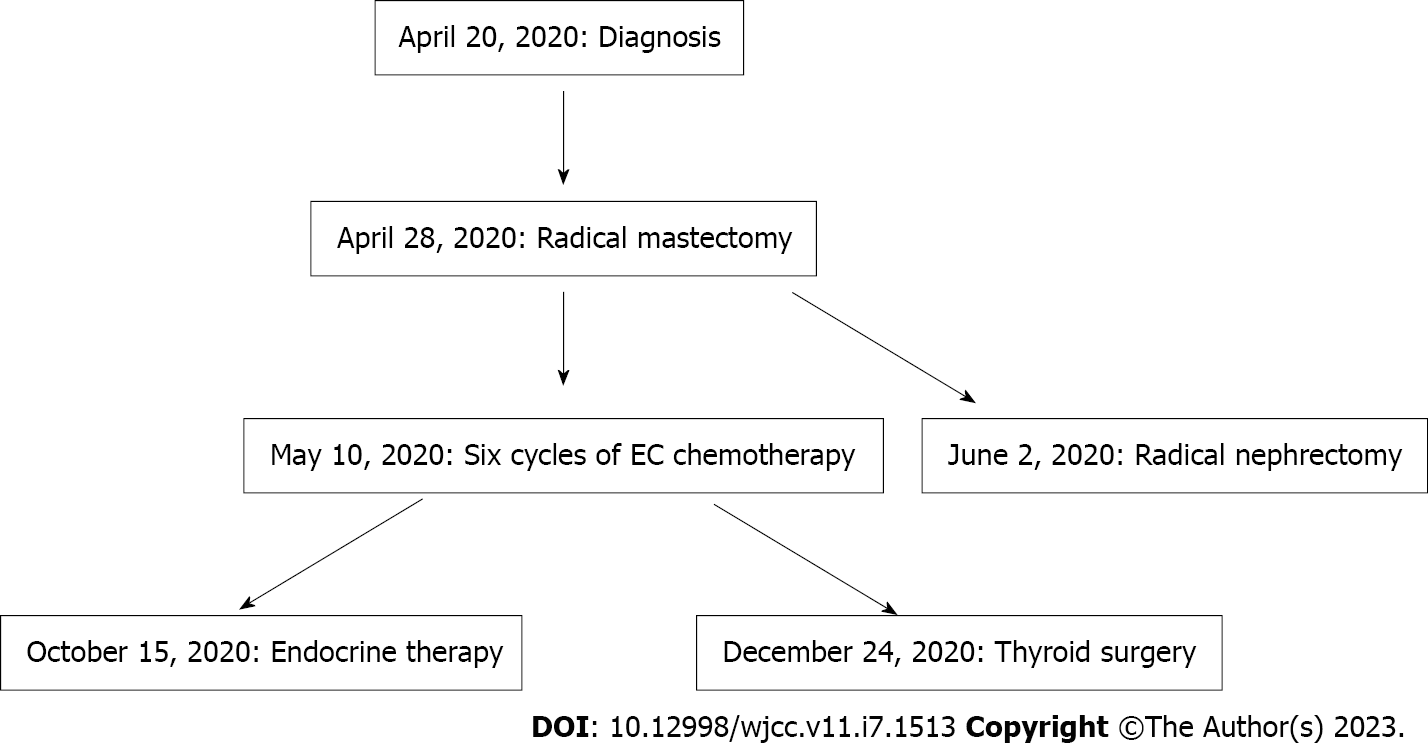
A 48-year-old female patient was admitted to our department of breast surgery at a tertiary hospital, presenting with "the right breast mass and pain for more than four months" on April 20, 2020. Body mass index (BMI) is 22.8.
The patient had no previous medical history.
No adverse health habits such as smoking and alcohol.
No family disease history. We did not palpate any other positive signs except for the right breast mass.
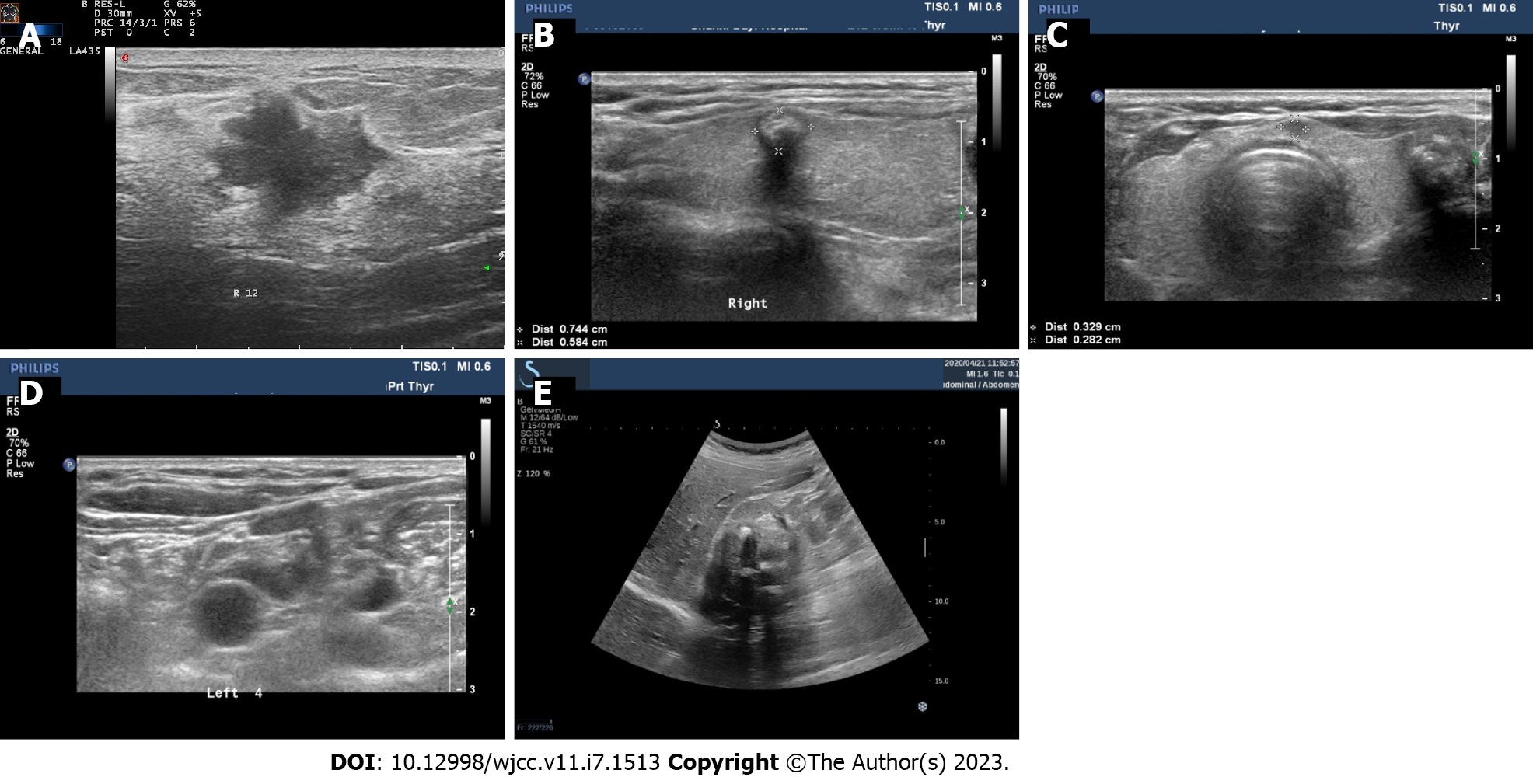
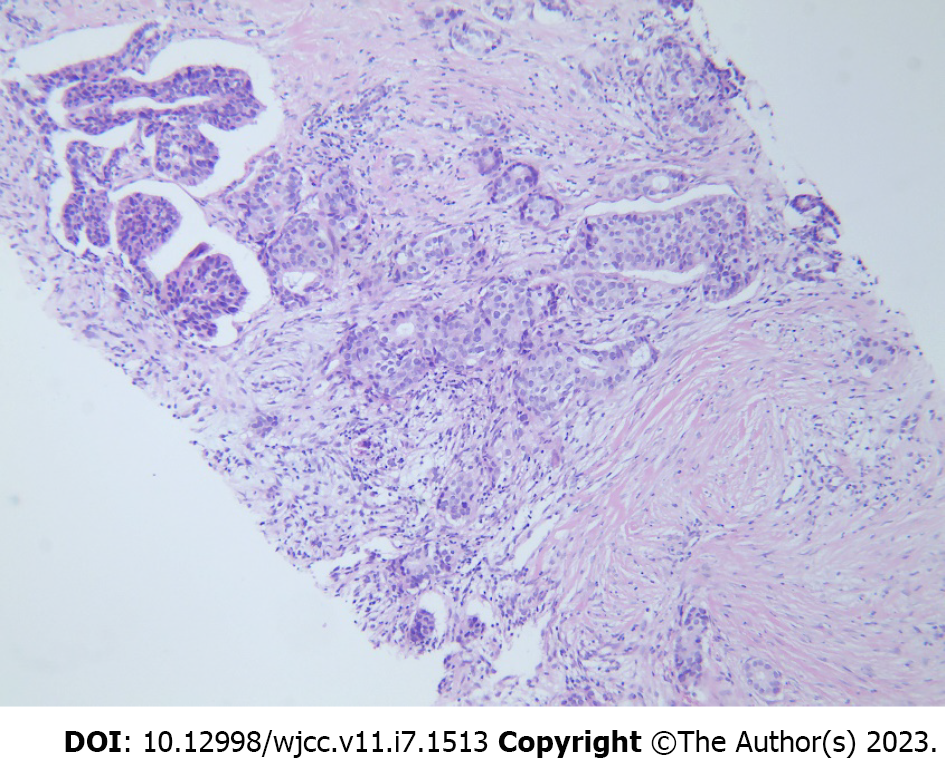
We performed an imaging evaluation, and breast ultrasound revealed a mass in the gland approximately 2.0 cm × 1.3 cm × 1.7 cm in size, positioned at 12 o'clock in the right breast, 1.8 cm away from the nipple. The mass had an unclear border and an angled edge and was classified into five categories according to the breast imaging reporting and data system (Figure 1A). No abnormal enlargement of lymph nodes was observed in the axillary, supraclavicular, or infraclavicular regions. Both mammogram and breast magnetic resonance imaging (MRI) indicated that the mass was malignant. In addition, no other abnormal changes were observed in the patient’s bilateral breasts. Immediate ultrasound-guided core needle biopsy confirmed the presence of invasive ductal carcinoma of the breast by pathology (Figure 2).
Therefore, we performed a further systemic evaluation to rule out metastatic disease and found thyroid- and kidney-occupying lesions. Thyroid ultrasound revealed solid nodules in the middle and upper of the thyroid right lobe, the inferior proximal isthmus, and the middle of the left lobe with sizes of approximately 0.7 cm × 0.6 cm, 0.8 cm × 0.5 cm, and 1.0 cm × 0.8 cm, respectively (Figure 1B-D). With unclear boundaries, multiple-sized calcification foci were observed in the nodules and classified as category 4c according to the thyroid imaging reporting and data system. In the IV area of the left neck, an enlarged lymph node with a size of 1.8 cm × 0.6 cm and uneven cortical thickening can be seen.
In addition to this, an abdominal ultrasound revealed a 6.4 cm × 5.9 cm solid hypoechoic mass on the upper pole of the right kidney and an uneven internal echo. Furthermore, computed tomography (CT) of the urinary system displayed that the lesion was cystic low-density, with massive calcification and shadows of flocculent soft tissues, which appeared slightly thickened on an enhanced scan and was therefore considered to be cystic kidney cancer (Figure 1E).
After a multidisciplinary team discussion, the decision was made to temporarily exclude metastasis because of the earlier manifestations of the patient's various examinations. Surgical indications were clear. At the request of the patient and family members and considering the patient's tolerance, separate surgeries were performed.
Owing to the variety of breast cancer treatments available, this surgery was performed in advance. Second, kidney surgery was advised as soon as possible to reduce the tumor burden. Considering the inertia of thyroid cancer, thyroid surgery was performed.
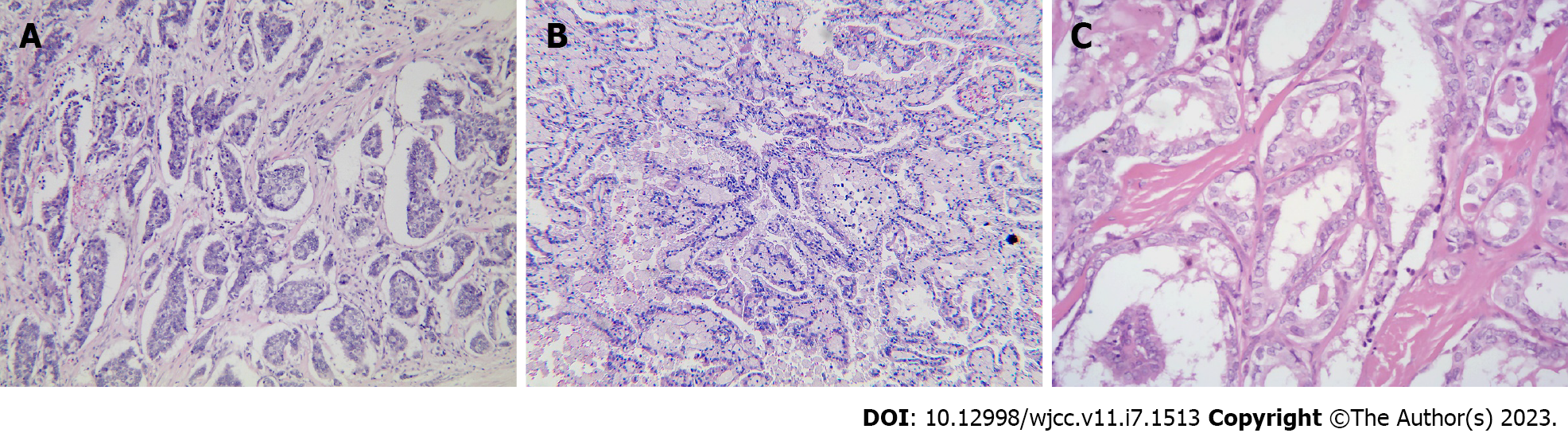
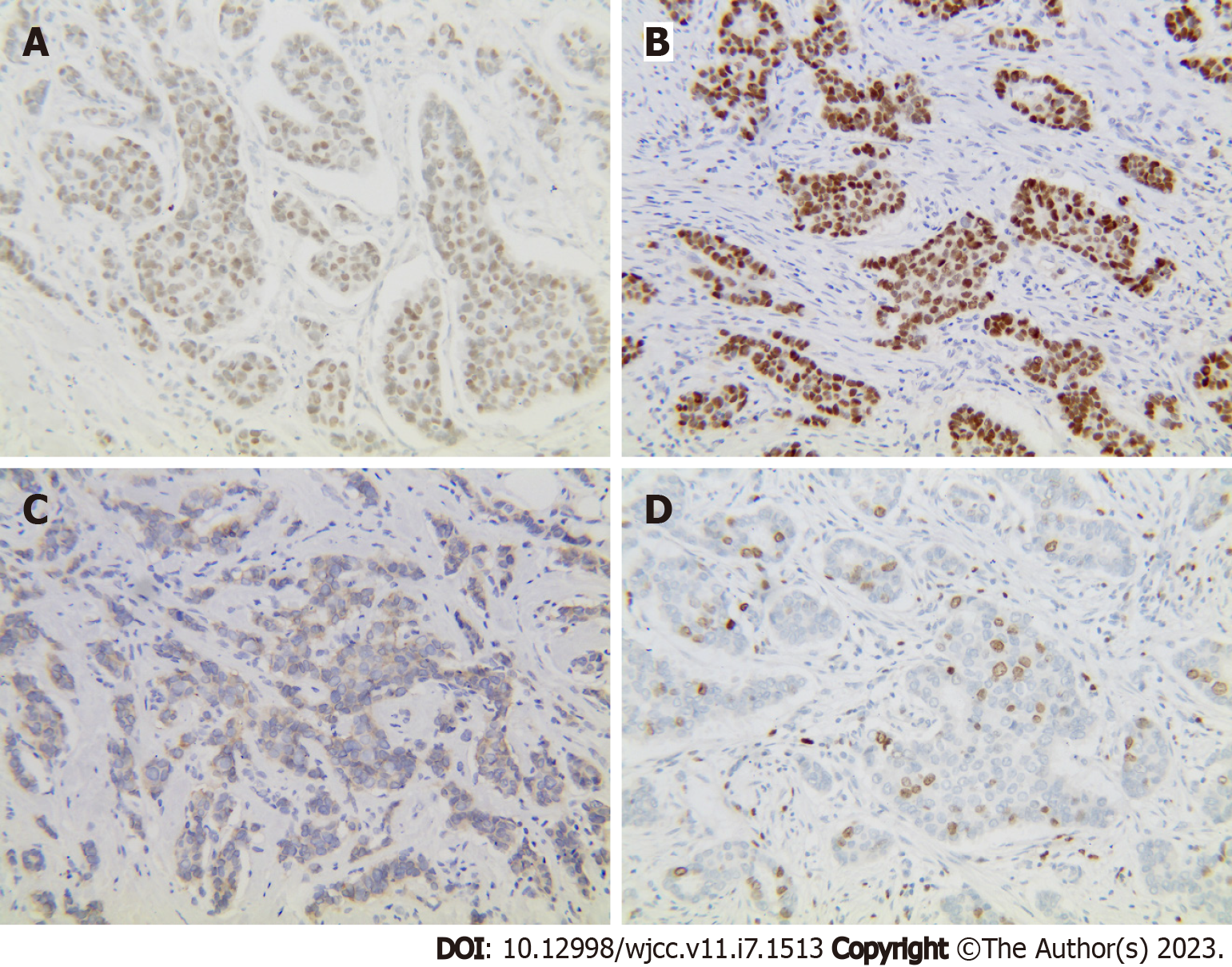
Breast-conserving surgery was performed; however, the patient refused surgery. Thus, a right radical mastectomy was performed on April 28, 2020. Postoperative pathology revealed moderately differentiated invasive ductal carcinoma (Figure 3A), with a tumor volume of 2 cm × 1.5 cm × 1.2 cm, where the vessels and nerves were not invaded. The margins were negative. Immunohistochemistry revealed the following results: Estrogen receptor (+, medium intensity, approximately 80%, 1604/2000 cells), progesterone receptor (+, medium-strong intensity, approximately 80%, 1596/2000 cells), CerbB-2 (1+), and Ki67 (approximately 30%+) (Figure 4). A 0.2 mm metastasis (1/5) was detected in the sentinel lymph node, while (0/23) no cancer cells were detected in the axillary lymph node dissection. According to the American Joint Committee on Cancer version 8.0, the pathological stage was pT1N0(i+). Therefore, six cycles of endometrial cancer regimen chemotherapy were delivered postoperatively per National Comprehensive Cancer Network guidelines for breast cancer, followed by endocrine therapy with tamoxifen.
A radical nephrectomy was performed at our hospital on June 2, 2020. Right kidney pathological revealed (Figure 3B) renal cell carcinoma grade 3, with a mass of 6.5 cm × 6 cm × 5 cm. Combined with immunohistochemistry, it was found prone to type 2 papillary renal cell carcinoma. The vessels, nerves, renal sinuses, and perirenal fat sacs are clear. No tumor was found in the severed ureters or blood vessels. The pathological result was pT1bN0, and no medication was administered following surgical excision.
Due to personal reasons, thyroid surgery was postponed to December 24, 2020. Postoperative pathology found that the left, right, and isthmus tumors of the thyroid were all papillary carcinoma (Figure 3C), each with a diameter of about 1, 0.9, and 0.6 cm, consistent with the imaging results observed eight months prior. The remaining cancer tissue invaded the thyroid capsule and reached the striated muscle tissue, and a tumor thrombus was observed in the vessel without lymphatic complications. No lymphovascular invasion was observed in the right lobe. Lymph node infiltration was observed around the thyroid (1/1), right IV (1/3), and left III (1/8) areas. I131 and levothyroxine sodium tablets were administered postoperatively.
Thus far, SMPMNs of the breast, kidney and bilateral thyroid have been diagnosed if surgery is performed as planned (Figure 5). The patient followed the doctor's advice for the treatment, and no evidence of recurrence or metastasis has been observed in the follow-ups to date. Both the doctors’ team and patients were satisfied with the treatment results.
In 2003, Billroth[3] first described dual primary cancers of the thyroid and breast. The situation has gradually increased since then in the case presented herein. Increasing evidence has demonstrated that progress in medicine has contributed to the detection rate of MPMNs in this aging population era. However, the morbidity of MPMNs varies greatly among different cancer types, ranging from 0.73% to 20% aboard[4,5], which is significantly higher than China's 0.4% to 2.4%[6]. This discrepancy could be attributed to medical level and financial factors. Salem et al[7] reported that the morbidity rates of MPMNs were 2.3%, and triple cancer was only 0.16%, respectively[7]. Rosso et al[8] found that MPMNs accounted for 6.3%, of which 2%–12% were triple cancer and above[8]. In addition, SMPMNs, accounting for 18.4%–30.1% of MPMNs[2,9], are associated with a lower incidence than MMPMNs. Therefore, the three synchronous primary cancers are extremely rare.
Early diagnosis and proper treatment are essential for obtaining the best prognosis. Based on postoperative proprietary pathological features and immunohistochemistry to exclude mutual metastasis, our patient was confirmed to have SMPMNs following Warren and Gates’s criteria[1,2]. Early staging further strengthens the diagnostic evidence. With a breast and kidney cancer diagnosis within two months, if treated as planned, the patient will be diagnosed simultaneously with bilateral thyroid cancer. Clinical data have demonstrated that MPMNs are more likely to occur in the same organ, system or pairs of organs, such as the lung, breast, thyroid, and kidney[10]. Interestingly, SMPMNs occurred in three pairs of organs.
However, the pathogenesis of MPMNs remains unclear. Compared with the general population, the increased risk appears to be complicated by genetics, host autoimmune and hormonal, external environment, and living habits (smoking, alcohol abuse, and excessive fat intake). Therefore, some patients have increased susceptibility to tumors. In addition, carcinogenic factors, such as radiation and cytotoxic drugs, contribute to the prolonged survival of patients. Thyroid cancer is commonly observed in breast cancer patients. A large retrospective study in South Korea demonstrated that the most common malignant tumor is thyroid cancer in breast cancer patients[11,12]. The breast and thyroid glands are controlled by the hypothalamic-pituitary endocrine axis. There are certain commonalities in the absorption of sex hormones, iodine, and thyroid hormones, which can potentially develop MPMNs[13,14]. Several studies have depicted that breast, thyroid, and renal cancers have bidirectional associations, likely shared genetic and environmental risk factors, and treatment effects[15,16]. Gordon syndrome is an autosomal dominant inherited disease caused by mutations in phosphatase and tensin homolog (PTEN), which often accompanies thyroid and breast cancers[17]. In addition, papillary renal cancer is increased in individuals with PTEN mutations[18,19]. The reasons for this remain unknown based on available data. Many studies have revealed that obesity is related to these three cancer types[20,21]; however, as stated, the patient’s BMI was not high. Moreover, they had no family history of tumors or unhealthy habits. Concurrent suffering from breast, thyroid, and kidney cancers may be closely related to gene mutations, immunodeficiency, and hormones. Unfortunately, the patient declined genetic testing for financial reasons. The pathogenesis and risk factors of MPMNs require further exploration.
As completely different treatment options exist, it is particularly important to distinguish primary lesions from metastatic lesions. Advances in imaging technology have contributed to the MPMNs diagnosis rate, and genetic testing has further improved its accuracy. On imaging, metastasis is often multiple, and MPMNs are often singular. When there is no peripheral lymph node metastasis in the first malignant tumor or other tumors, we should be aware of the presence of MPMNs. In addition, most patients only undergo traditional primary cancer imaging examinations and ignore MPMNs, which leads to missed diagnosis, delays in treatment, and affects prognosis. Recently, whole-body MRI and positron-emission tomography-CT are highly sensitive in discovering new cancer foci and observing the progression of the disease in asymptomatic carriers, which has great consequences for diagnosis[22]. In addition, accuracy can be ensured by increasing the number of inspection methods.
It is difficult to identify SMPMNs and metastasis; however, it affects treatment and prognosis in sequence. Treating MPMNs is similar to that of early single primary cancers and tends to be a comprehensive treatment based on surgery, while recurring and metastatic tumors lose the opportunity for surgery based on comprehensive treatment. Currently, recommendations for the treatment of MPMN are limited. A multidisciplinary team discussion is optimal, following the principles of individualized and comprehensive treatment[23]. Almost all literature reports support the selection of radical surgery resection based on tumor location, pathological characteristics, staging, and the patient’s system conditions. Subsequent treatments should be performed separately for each MPMN tumor. If the situation permits, simultaneous surgery is recommended, especially for young and healthy patients. Otherwise, in principle, the treatment of highest malignant degree should be undertaken first, followed by an appropriate interval that is beneficial for observing the treatment effect and rehabilitation[24]. Therefore, independent staging of each tumor should be performed as soon as possible after diagnosing MPMNs[25]. Inoperable patients should be actively administered chemotherapy, targeted therapy, and other treatment methods to improve their quality of life and prolong survival as much as possible. The advantages and disadvantages must be weighed because of the risk of secondary radio-induced, chemo-induced, and endocrine-induced cancers. After multidisciplinary team discussion, we performed sequential surgery based on the above principles in combination with the patient's situation and provided corresponding postoperative adjuvant treatment.
As we all know, primary cancer has a significantly better prognosis than recurrence or metastasis. For MPMNs, prognosis based on the patient's physical condition is mainly related to cancer characteristics, radical resection, and the interval between different tumors. The shorter the interval between the second and first primary cancers, the worse the prognosis[11]. Multiple cancers that occur simultaneously in the same system generally progress quickly and have a poor prognosis[26,27]. However, there are also reports that a second primary cancer does not affect the disease-free survival and overall survival of patients with a single primary cancer[11,28]. For such patients, early detection, diagnosis, and provision of reasonable and effective treatment are key to improving survival rates and prognosis. In addition, compared with single primary cancers, patients with MPMNs experience more psychological pain, financial stress, and worse treatment compliance, which has a negative impact on the outcome[29]. Therefore, it is necessary to pay attention to the psychological burden of these patients.
Herein, we report a rare case of early SMPMNs in the breast, kidney, and bilateral thyroid, where early recognition and management of this disease is important. Early-stage malignant tumor symptoms are subtle and difficult to diagnose. Therefore, careful physical examination can help to identify tumors located elsewhere. When it is difficult to distinguish between primary and metastatic tumors, immunohistochemistry and genetic analysis may aid the diagnosis. Second, a highly individualized and comprehensive treatment plan should be developed for every MPMN patient, preferably by multidisciplinary joint diagnosis and treatment, with an urgent need to establish evidence-based methods for managing them. Finally, the patient is encouraged to abandon unhealthy lifestyles, such as smoking, drinking, and excessive fat intake, to aid in successful prevention and recovery. Appropriate follow-up cannot only detect the recurrence and metastasis of the first primary cancer earlier but also other primary cancers simultaneously, which can ultimately improve patient outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dimofte GM, Romania; Mehdipour P, Iran S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Swaroop VS, Winawer SJ, Kurtz RC, Lipkin M. Multiple primary malignant tumors. Gastroenterology. 1987;93:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Powell S, Tarchand G, Rector T, Klein M. Synchronous and metachronous malignancies: analysis of the Minneapolis Veterans Affairs (VA) tumor registry. Cancer Causes Control. 2013;24:1565-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Billroth T. Pathology and therapeutics, in fifty lectures. 1871. Clin Orthop Relat Res. 2003;4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Forjaz G, Howlader N, Scoppa S, Johnson CJ, Mariotto AB. Impact of including second and later cancers in cause-specific survival estimates using population-based registry data. Cancer. 2022;128:547-557. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Morton LM, Onel K, Curtis RE, Hungate EA, Armstrong GT. The rising incidence of second cancers: patterns of occurrence and identification of risk factors for children and adults. Am Soc Clin Oncol Educ Book. 2014;e57-e67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Liu Z, Liu C, Guo W, Li S, Bai O. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One. 2015;10:e0125754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Salem A, Abu-Hijlih R, Abdelrahman F, Turfa R, Amarin R, Farah N, Sughayer M, Almousa A, Khader J. Multiple primary malignancies: analysis of 23 patients with at least three tumors. J Gastrointest Cancer. 2012;43:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Rosso S, De Angelis R, Ciccolallo L, Carrani E, Soerjomataram I, Grande E, Zigon G, Brenner H; EUROCARE Working Group. Multiple tumours in survival estimates. Eur J Cancer. 2009;45:1080-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Etiz D, Metcalfe E, Akcay M. Multiple primary malignant neoplasms: A 10-year experience at a single institution from Turkey. J Cancer Res Ther. 2017;13:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Chirila DN, Turdeanu NA, Constantea NA, Coman I, Pop T, Popp RA, Balacescu O, Vesa SC, Ciuce C. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502. [PubMed] |
| 11. | Kim BK, Oh SJ, Song JY, Lee HB, Park MH, Jung Y, Park WC, Lee J, Sun WY; Korean Breast Cancer Society. Clinical Characteristics and Prognosis Associated with Multiple Primary Cancers in Breast Cancer Patients. J Breast Cancer. 2018;21:62-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Huang NS, Chen XX, Wei WJ, Mo M, Chen JY, Ma B, Yang SW, Xu WB, Wu J, Ji QH, Guo XM, Liu GY, Shao ZM, Wang Y. Association between breast cancer and thyroid cancer: A study based on 13 978 patients with breast cancer. Cancer Med. 2018;7:6393-6400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Kim YA, Kim YA, Cho SW, Song YS, Min HS, Park IA, Park DJ, Hwang KT, Park YJ. Increased expression of thyroid hormone receptor alpha and estrogen receptor alpha in breast cancer associated with thyroid cancer. Eur J Surg Oncol. 2021;47:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hall LC, Salazar EP, Kane SR, Liu N. Effects of thyroid hormones on human breast cancer cell proliferation. J Steroid Biochem Mol Biol. 2008;109:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Van Fossen VL, Wilhelm SM, Eaton JL, McHenry CR. Association of thyroid, breast and renal cell cancer: a population-based study of the prevalence of second malignancies. Ann Surg Oncol. 2013;20:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Lee KD, Chen SC, Chan CH, Lu CH, Chen CC, Lin JT, Chen MF, Huang SH, Yeh CM, Chen MC. Increased risk for second primary malignancies in women with breast cancer diagnosed at young age: a population-based study in Taiwan. Cancer Epidemiol Biomarkers Prev. 2008;17:2647-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Pilarski R. PTEN Hamartoma Tumor Syndrome: A Clinical Overview. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 18. | Shuch B, Ricketts CJ, Vocke CD, Komiya T, Middelton LA, Kauffman EC, Merino MJ, Metwalli AR, Dennis P, Linehan WM. Germline PTEN mutation Cowden syndrome: an underappreciated form of hereditary kidney cancer. J Urol. 2013;190:1990-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Mester JL, Zhou M, Prescott N, Eng C. Papillary renal cell carcinoma is associated with PTEN hamartoma tumor syndrome. Urology. 2012;79:1187.e1-1187.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Recalde M, Davila-Batista V, Díaz Y, Leitzmann M, Romieu I, Freisling H, Duarte-Salles T. Body mass index and waist circumference in relation to the risk of 26 types of cancer: a prospective cohort study of 3.5 million adults in Spain. BMC Med. 2021;19:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Vithayathil M, Carter P, Kar S, Mason AM, Burgess S, Larsson SC. Body size and composition and risk of site-specific cancers in the UK Biobank and large international consortia: A mendelian randomisation study. PLoS Med. 2021;18:e1003706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 22. | Mihatsch PW, Beissert M, Bley TA, Buck AK, Lapa C. Thyroid incidentalomas with increased focal (18)F-FDG uptake in (18)F-FDG PET/CT of a patient with multiple primary cancers. Endocrine. 2021;73:491-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 23. | Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2:e000172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 365] [Article Influence: 45.6] [Reference Citation Analysis (1)] |
| 24. | Gu HL, Zeng SX, Chang YB, Lin Z, Zheng QJ, Zheng XQ, Peng ZW, Zhan SQ. Multidisciplinary treatment based on surgery leading to long-term survival of a patient with multiple asynchronous rare primary malignant neoplasms: A case report and literature review. Oncol Lett. 2015;9:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Zhai C, Cai Y, Lou F, Liu Z, Xie J, Zhou X, Wang Z, Fang Y, Pan H, Han W. Multiple Primary Malignant Tumors - A Clinical Analysis of 15,321 Patients with Malignancies at a Single Center in China. J Cancer. 2018;9:2795-2801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, Chen Z, Li P, Li S. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine (Baltimore). 2017;96:e6799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Shan S, She J, Xue ZQ, Su CX, Ren SX, Wu FY. Clinical characteristics and survival of lung cancer patients associated with multiple primary malignancies. PLoS One. 2017;12:e0185485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Hamza MA, Kamiya-Matsuoka C, Liu D, Yuan Y, Puduvalli VK. Outcome of patients with malignant glioma and synchronous or metachronous non-central nervous system primary neoplasms. J Neurooncol. 2016;126:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Andrykowski MA, Goedendorp MM. Distress and mental health care and medication use among survivors of multiple primary cancer diagnoses: Findings from the 2016 National Health Interview Survey. J Psychosom Res. 2020;134:110137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |