Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1188
Peer-review started: November 14, 2022
First decision: November 30, 2022
Revised: December 9, 2022
Accepted: January 16, 2023
Article in press: January 16, 2023
Published online: February 16, 2023
Processing time: 91 Days and 22.2 Hours
Littoral cell angioma (LCA) is a rare benign vascular tumor of the spleen. Given its rarity, standard diagnostic and therapeutic recommendations have yet to be developed for reported cases. Splenectomy is the only method of obtaining a pathological diagnosis and providing treatment to obtain a favorable prognosis.
A 33-year-old female presented with abdominal pain for one month. Computed tomography and ultrasound revealed splenomegaly with multiple lesions and two accessory spleens. The patient underwent laparoscopic total splenectomy and accessory splenectomy, and splenic LCA was confirmed by pathology. Four months after surgery, the patient presented with acute liver failure, was readmitted, rapidly progressed to multiple organ dysfunction syndrome and died.
Preoperative diagnosis of LCA is challenging. We systematically reviewed online databases to identify the relevant literature and found a close relationship between malignancy and immunodysregulation. When a patient suffers from both splenic tumors and malignancy or immune-related disease, LCA is possible. Due to potential malignancy, total splenectomy (including accessory spleen) and regular follow-up after surgery are recommended. If LCA is diagnosed after surgery, a comprehensive postoperative examination is needed.
Core Tip: Littoral cell angioma (LCA) is a rare benign vascular tumor of the spleen. No standard diagnostic and therapeutic recommendations are available. We report a patient with LCA and without comorbidities who died of multiple organ dysfunction syndrome 4 mo after surgery, which is extremely rare. Systematic analysis of relevant cases in the PubMed, Embase, Web of Science and the Cochrane Library databases revealed that LCA has a close relationship with malignancy and immunodysregulation. The possibility of LCA should not be overlooked when a patient presents with splenic tumors and malignancy or immune-related disease. Considering its potential malignant behavior, total splenectomy (including accessory spleen) and regular follow-up after surgery are recommended.
- Citation: Jia F, Lin H, Li YL, Zhang JL, Tang L, Lu PT, Wang YQ, Cui YF, Yang XH, Lu ZY. Early postsurgical lethal outcome due to splenic littoral cell angioma: A case report. World J Clin Cases 2023; 11(5): 1188-1197
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1188.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1188
Littoral cell angioma (LCA) is a very rare benign nonhematological vascular neoplasm of the spleen that originates from the cells lining the splenic red pulp sinuses[1]. Only approximately 300 cases have been published since LCA was first described in 1991 by Falk et al[2]. Standard diagnostic and therapeutic recommendations are not available to date. As imaging examination is insufficient for diagnosing LCA, splenectomy remains the only method of obtaining a pathological diagnosis and providing treatment to achieve a favorable prognosis. However, we describe an LCA patient without obvious preoperative comorbidities who died of multiple organ dysfunction syndrome (MODS) four months after splenectomy, which is extremely rare.
A 33-year-old female complained of abdominal pain in the left upper quadrant and discomfort without any obvious cause for 1 mo prior.
The pain presented as intermittent dull pain. Occasionally, she felt abdominal distention and experienced acid regurgitation without nausea, vomiting, fever, jaundice, diarrhea, or constipation. Other complaints included poor sleep and loss of appetite. There was no change in weight or bowel habits.
The patient reported that she had no history of past illness.
The patient reported no family history of malignant tumors.
Physical examination revealed an enlarged spleen with an irregular edge and tough quality; the inferior margin of the spleen extended to 4 cm below the left costal margin.
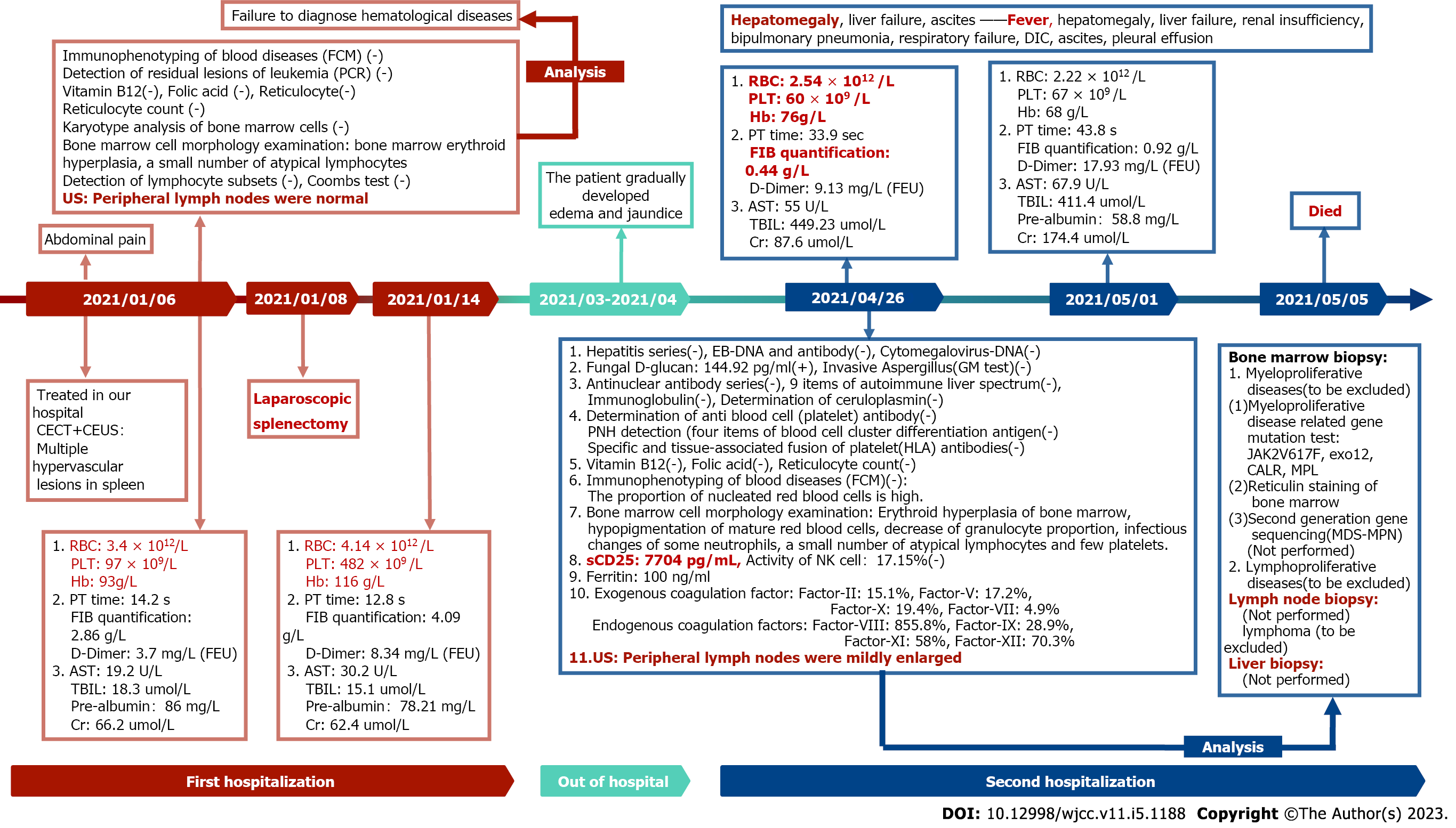
Laboratory findings included a low red blood cell count (3.4 × 1012/L) and low hemoglobin level (93 g/L), which suggested anemia, without evidence of thrombocytopenia (platelet count: 9.7 × 1010/L) and hypersplenism. Other blood tests, including those for tumor markers, liver and renal function, and coagulation, were within normal limits.
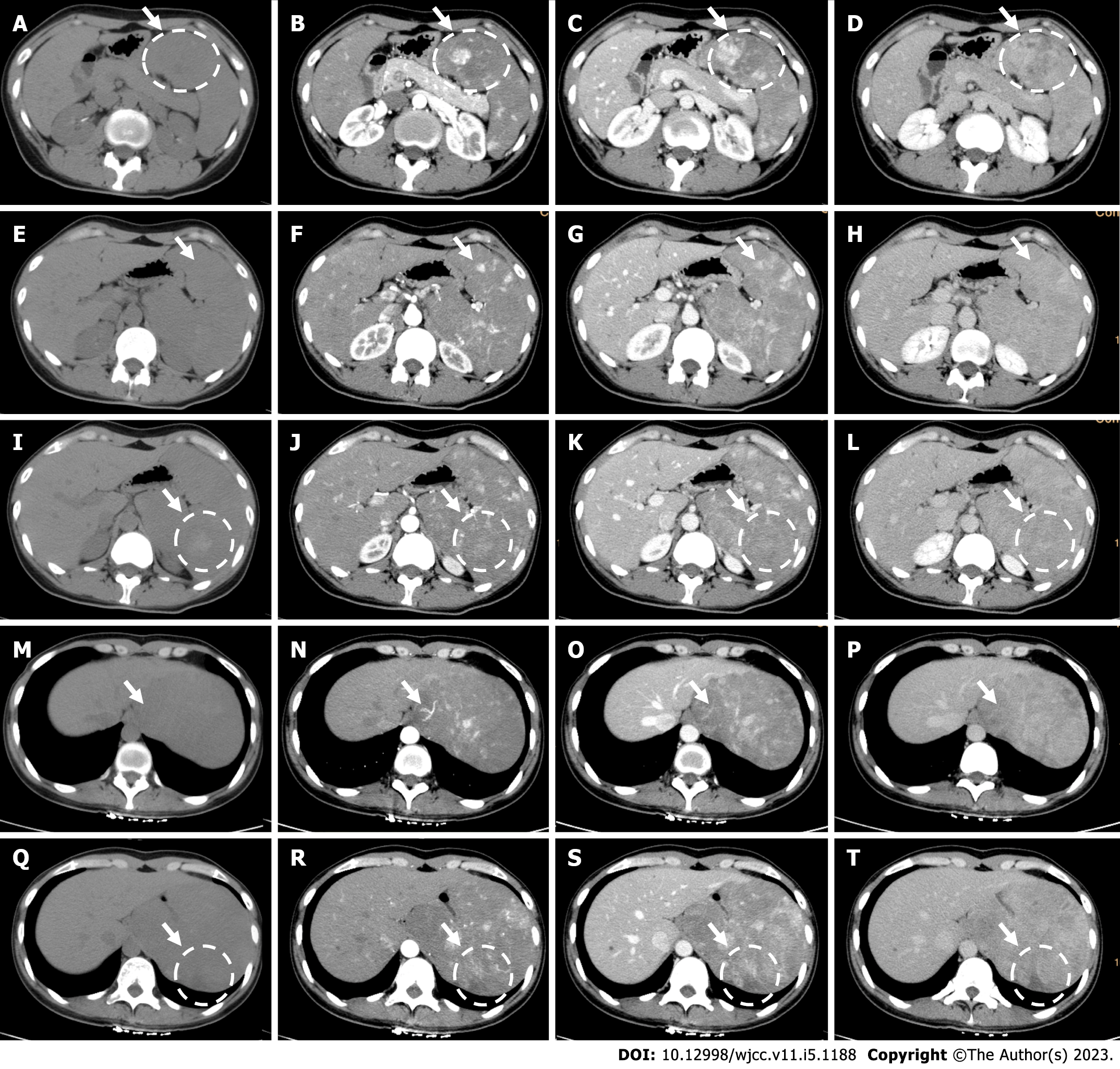
Imaging examination showed splenomegaly with an irregular shape. Computed tomography (CT) showed that the density of the spleen was uneven. The lesions exhibited hypodensity (31 HU), isodensity (40 HU) or hyperdensity (55 HU). Contrast-enhanced CT (CECT) revealed that the degree of enhancement ranged from moderate to high (100-150 HU), gradually decreased in the venous or delayed phase (100-120 HU), and was nearly equal to the splenic parenchyma (78 HU) around the lesions (80-90 HU) in the delayed phase. There were also some lesions with no obvious enhancement (Figure 1).
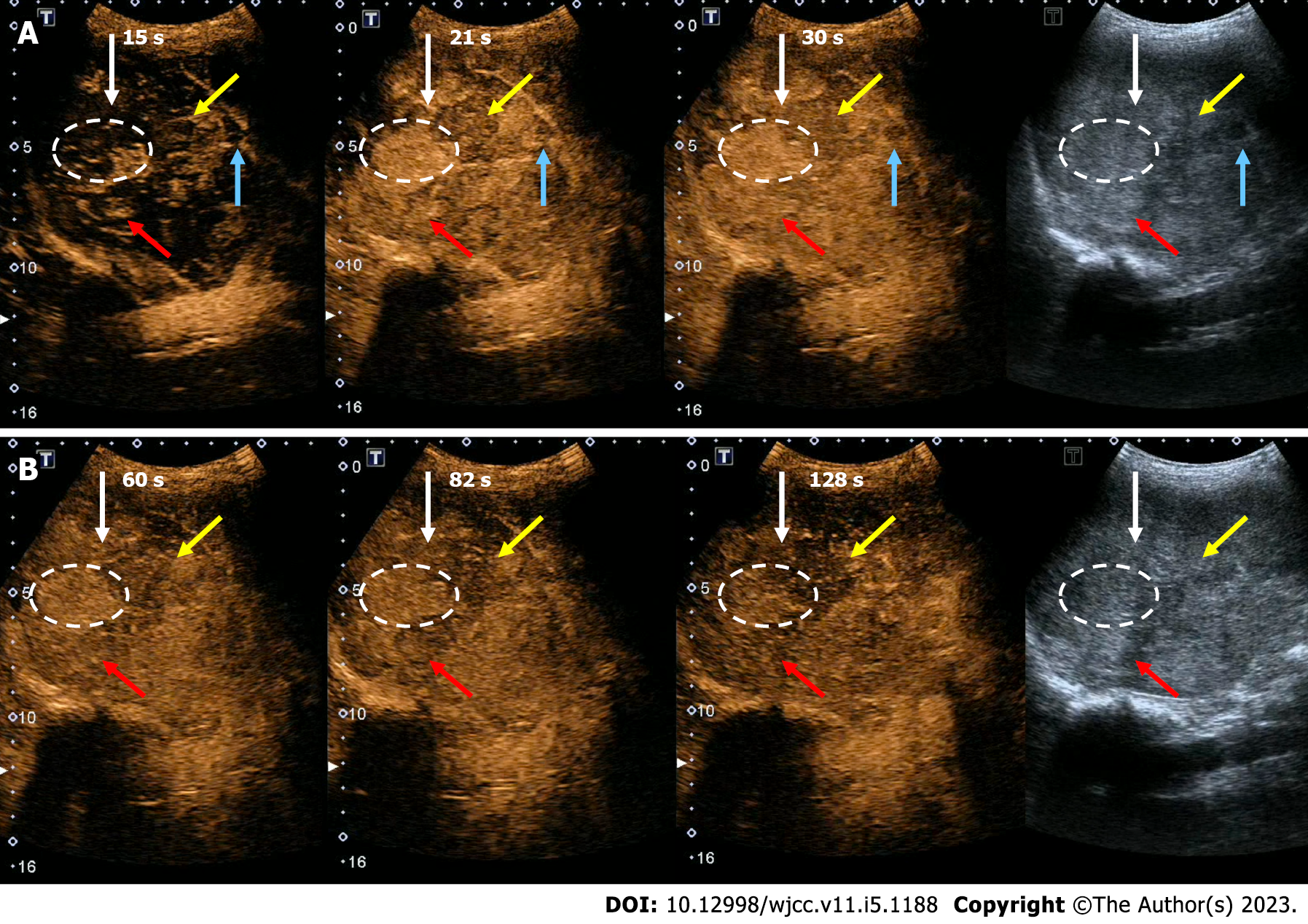
Ultrasound (US) showed multiple hyperechoic lesions with unclear boundaries. Some were integrated, and some were heterogeneous. The largest lesion was 5.4 cm × 5.0 cm, protruding to the outside of the spleen. Two accessory spleens were displayed, and the diameter of the larger accessory spleen was 1.1 cm. The features of the lesions varied on contrast-enhanced US (CEUS) (Figure 2).
Given the negative results of cervical, axillary, inguinal and abdominal lymph node ultrasounds and the bone marrow biopsy, no typical sign of hematologic malignancy was observed.
As malignant disease could not be excluded, laparoscopic total splenectomy and accessory splenectomy were performed for diagnosis and treatment. The whole operation time was 3.5 h, and the blood loss was 50 mL.
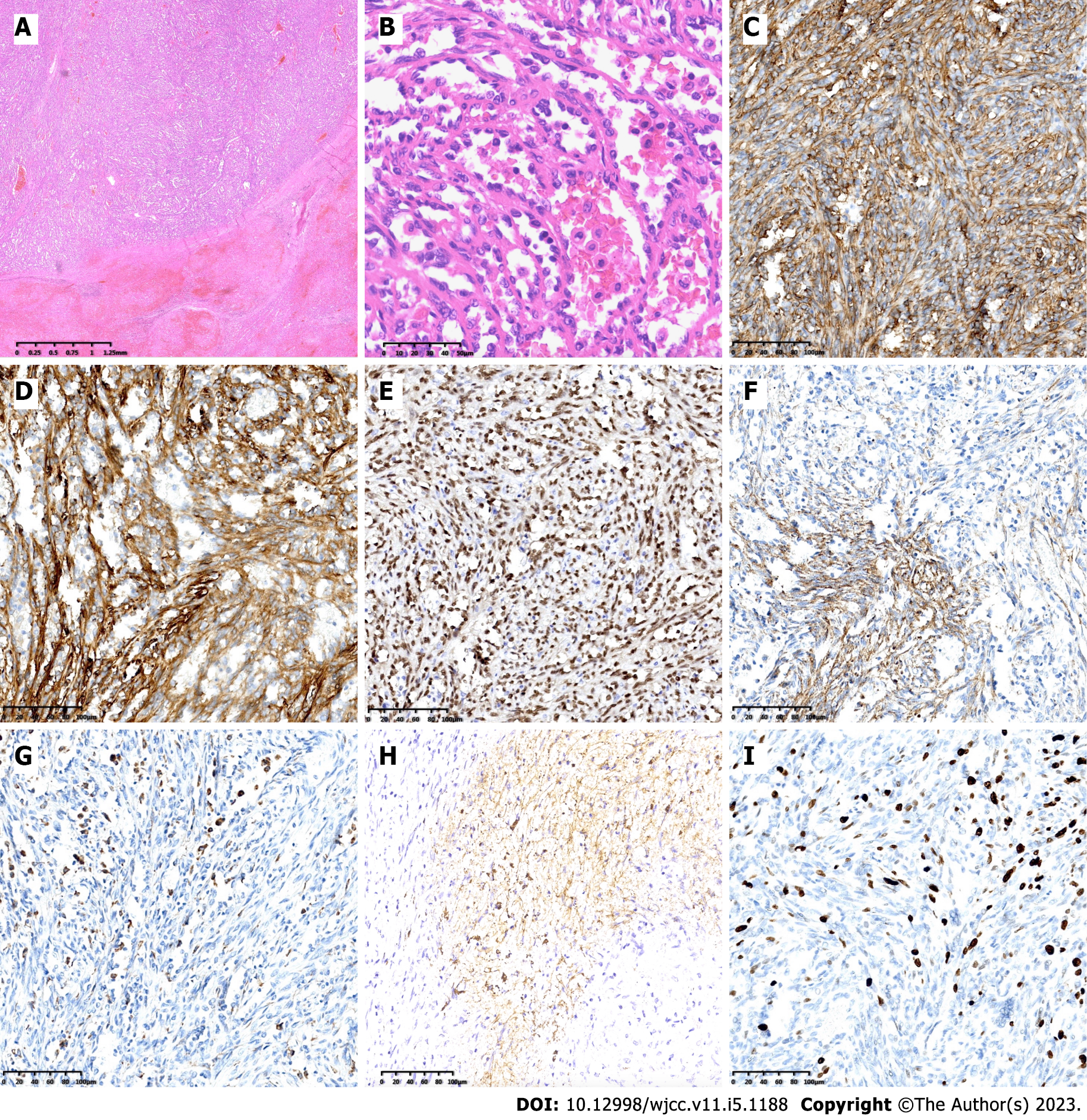
Pathological examination confirmed splenic LCA. Grossly, the spleen measured 17 cm × 13 cm × 7 cm. A longitudinal section exhibited multiple brownish-red nodules, and the texture was soft. Microscopically, the lesions were in the red pulp of the spleen and consisted of multiple vascular cavities. The normal structures of the splenic parenchyma among the lesions could not be detected in the hematoxylin and eosin (HE)-stained section, and almost all the tissue was invaded by tumor cells. Immunohistochemical analysis confirmed benign LCA, with the cells positive for the endothelial markers factor VIII (FVIII), CD31, CD34, ETS-related gene and the histiocytic markers CD68 and CD21. The Ki-67 Labeling index was no more than 20%, and the cells were negative for cytokeratin (Figure 3).
Red blood cell counts and hemoglobin levels were within normal limits 6 d after the surgery. The patient was discharged on the 8th d and underwent regular follow-up after the surgery.
After 4 mo, the patient was readmitted to the hospital with systemic edema and jaundice. The patient was diagnosed with acute liver failure (ALF). Multiple examinations failed to detect atypical results. In addition, the clinicians were unable to acquire pathological results of the liver tissue because of the poor condition of the patient, which made it more difficult to acquire a definite diagnosis and carry out effective etiological treatment. During the course of the disease, the patient suffered from hypocalcemia and hypokalemia. Calcium gluconate and potassium chloride were given to correct the ionic disorders. Blood gas analysis was performed to determine whether there was acid-base imbalance. Given the coagulation dysfunction, the patient was supplemented with plasma. Hypoproteinemia was treated with supplemental albumin. To treat ALF, liver-protecting and cholagogic drugs were administered. For pulmonary infection and respiratory failure, spectrum antibiotic treatment and oxygen support treatment were given. Although multiple treatments were administered, the patient’s condition deteriorated rapidly, and she developed renal insufficiency and respiratory failure; ultimately, she died of MODS. Laboratory examination results and disease progression are shown in detail in Figure 4.
LCA is a rare benign vascular tumor of the spleen. To obtain a better understanding of LCA, we systematically searched the PubMed, Embase, Web of Science and Cochrane Library databases for relevant data between 1991 and November 1st, 2022. The search terms for benign tumors included “littoral cell angioma” [MeSH Terms] OR “littoral cell angioma” [All Fields]. Other relevant records were also retrieved, including conference abstracts, references of the eligible studies and clinical trials that mentioned LCA. Two reviewers (Fan Jia and Han Lin) independently screened the literature according to the inclusion and exclusion criteria and extracted relevant data from the articles. Disagreements were resolved by consensus; if consensus was not achieved, then a third author (Yi-Long Li) provided an assessment of eligibility.
We included 167 studies containing 319 cases of LCA. According to our review, LCA usually occurs in middle-aged adults (range of age at onset: 28 d to 86 years)[3,4], with no obvious sex differences. The lesions may be solitary or multiple, and the diameters range from 0.1 cm to 21.0 cm[5,6]. Symptoms and signs are usually not specific and include splenomegaly, abdominal pain, thrombocytopenia, anemia, fatigue, and fever.
Some patients are asymptomatic; in such patients, LCA is usually detected by routine physical examination or incidentally. LCA can be easily misdiagnosed as hemangioma or (sometimes) as hamartoma before surgery. The CECT imaging features of our case presented characteristics similar to hemangioma, which was why the lesions were misdiagnosed at the local hospital. Thus, when a patient presents with several of the following features, the possibility of LCA should not be overlooked: splenomegaly, hypersplenism, multiple lesions, hypodensity in CT and hypo/hyperenhancement in CECT, and hypo/isoechoic in US as well as specific magnetic resonance imaging features (i.e., low signals in T1-weighted images, high signals in T2-weighted images and diffusion-weighted images (DWI), and/or sign of “freckles”). Splenic infarction may occur[7,8], especially when hemangioma is highly suspected because of an absence of typical features.
LCA is closely related to malignancy (78/319 cases) (hematological malignancy, gastrointestinal cancer, genitourinary cancer, and endocrine cancer as well as immunodysregulation (47/319 cases) (vital hepatitis, liver cirrhosis[9], Crohn’s disease, and immune thrombocytopenia, etc.), and has the potential for recurrence and malignant transformation.
During postoperative follow-up, some LCA cases showed potential malignancy. One case had recurrence of LCA in the accessory spleen 7 years later[10]. Two cases had hepatic recurrence and metastases of LCA after 8 and 10 years[3,11]. A patient who was diagnosed with LCA after splenectomy died of multiple metastases of littoral cell hemangioendothelioma (LCHE) 4 years later[12]. One patient had both LCA and primary splenic angiosarcoma (PSAS) cells in the same lesion[13].
Eleven (3.6%) patients, including our patient, died after splenectomy; details are listed in Table 1. No case was similar to ours. In our case, a young woman without obvious preoperative comorbidities died of MODS 4 mo after surgery, emphasizing the complexity and potential lethality of LCA and the importance of regular postoperative follow-up. Unfortunately, due to the local customs and beliefs of the patient’s family members, autopsy results could not be obtained, and the cause of MODS was unknown.
| Ref. | Age (yr) | Gender (M/F) | Weight (g) | Length (cm) | Number (S/M) | Diameter (cm) | Splenomegaly (Yes/No) | Died after splenectomy | Cause of death | The details of the dead patient |
| Falk et al[2], 1991 | 42 | F | 145 | NA | NA | NA | No | 3 yr | Disseminated lymphoma | The patient suffered from malignant lymphoma, and died of disseminated lymphoma 3 yr after splenectomy |
| Falk et al[2], 1991 | 77 | M | 2404 | NA | M | 18 | Yes | 2 wk | NA | The patient’s splenic lesion were discovered incidentally during surgical repair of a dissecting aneurysm, and died 2 wk after splenectomy |
| Priego et al[26], 2008 | 83 | F | NA | NA | M | NA | Yes | 2 d | Great stress of operation | The patient suffered from myelodysplastic syndrome, and died 2 d after splenectomy because of the great stress of operation |
| Hansen et al[27], 2010 | 67 | M | 213 | 14 | S | 2.1 | Yes | 6 mo | Advanced liver failure, additional renal failure | The patient suffered from colon carcinoma, hepatocellular carcinoma and LCA, simultaneously. Hemicolectomy and splenectomy were performed first, and partial hepatectomy was performed 6 mo later. The patient died of advanced liver failure and additional renal failure a few weeks after surgery |
| Kranzfelder et al[13], 2012 | 62 | M | 110 | NA | M | NA | No | 8 mo | NA | Splenectomy was performed because of rupture of the spleen, and the pathology was LCA and PSAS. The patient died 8 mo after splenectomy |
| Peckova et al[3], 2016 | 62 | F | 310 | 19 | M | 1 | Yes | 6 yr | NA | The patient suffered from endometrioid endometrial adenocarcinoma and died of unknown cause 6 yr after splenectomy |
| Peckova et al[3], 2016 | 83 | M | 200 | 14 | M | 1.2 | Yes | NA | Multiple myeloma | The patient was performed renal resection because of renal cell carcinoma in 2005, and splenectomy because of LCA in 2006, and was diagnosed as multiple myeloma in 2011, adenocarcinoma of ascending colon (the time was unclear). The patient died of multiple myeloma |
| No authors listed[28], 2017 | NA | NA | NA | NA | NA | NA | NA | NA | Recurrence of malignant tumors | Two patients died of recurrence of malignant tumors (1 patient with gastric diffuse large B-cell lymphoma, 1 patient with ovarian serous cancer) after splenectomy |
| Takayoshi et al[11], 2018 | 61 | F | NA | NA | S | NA | NA | 10 yr | Hemorrhagic cerebral infarction | After 10 yr of splenectomy, the patient sufferred from metastatic LCA recurrence and multiple liver metastases, and died of hemorrhagic cerebral infarction |
| Our patient | 33 | F | NA | 17 | M | 5.4 | Yes | 4 mo | MODS | The patient died of MODS after 4 mo after splenectomy without any obvious evidence of comorbidities |
Clinicians from different departments convened to analyze the diagnostic experience and discuss the possible causes of MODS. Based on the clinical characteristics, disease progression (Figure 4) and the results of the literature review, the possibility of lymphoma was proposed.
Common causes of ALF are viral infection (especially hepatitis virus), drugs, toxins, bacteria and parasites, other liver diseases (such as autoimmune liver disease), biliary diseases, metabolic disorders and circulatory failure. However, given the results of the examination, none of them could explain the patient’s condition. Hepatic invasion of malignancies can also lead to ALF, which is an independent predictive factor for 30-d mortality[14]. Considering that no typical images were observed, solid tumors could be excluded. However, hematologic malignancies cannot be ignored, especially lymphoma. Hepatic infiltration of tumor cells can be observed in 15%-22% of hematological malignancies[15], rarely leading to significant liver dysfunction[16-20], which usually occurs in the terminal stage of the disease[16,17,21,22] and is closely related to high mortality (67%-100%)[16]. Although rare, some case reports have described this course of disease. Liver biopsy was not acquired because of serious coagulopathy, but given the positive bone marrow biopsy and expected clinical features (ALF, hepatomegaly, and elevated lactate dehydrogenase), lymphoma should be highly suspected[15-18,23]; among malignancies, it has a high rate of cooccurrence with LCA.
Our patient had fever, decreased blood cells and fibrinogen, and increased sCD25 (Figure 4). Thus, four of the eight diagnostic criteria[24] for hemophagocytic lymphohistiocytosis (HLH) were met. Not all symptoms are displayed at the early stage, and many diseases can lead to HLH, including malignancies (leukemia, lymphoma, and other solid tumors), infections and rheumatoid disorders[24]. HLH should be highly suspected. Combined with the analysis in the previous paragraph and negative results of the bone marrow biopsy, the possibility of lymphoma was proposed again.
In our experience, if the patient is diagnosed with ALF after splenectomy without other obvious causes, lymphoma should be considered, and liver biopsy should be performed as soon as possible for early diagnosis and etiological treatment if the patient’s situation allows. If LCA is diagnosed after surgery, standard postoperative long-term follow-ups should be strictly observed[25].
These recommendations are based on the disease characteristics of the patient and a literature review; thus, more studies are needed. Although we cannot provide clear diagnostic criteria for LCA because its imaging features are similar to those of other splenic tumors, when a patient suffers from both splenic tumors and malignant or immune-related diseases, LCA should be considered given the close relationship of LCA with malignancy and immunodysregulation. In view of the potential recurrence and malignant transformation of LCA, the recommended treatment is total splenectomy (including the accessory spleen). If LCA is diagnosed after surgery, a comprehensive postoperative examination is needed. In addition, standard postoperative long-term monitoring should be strictly observed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Cioffi U, Italy; Shen TC, Taiwan; Vyshka G, Albania S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Opatrny V, Treska V, Waloschek T, Molacek J. Littoral cell angioma of the spleen: A case report. SAGE Open Med Case Rep. 2020;8:2050313X20959874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15:1023-1033. [PubMed] |
| 3. | Peckova K, Michal M, Hadravsky L, Suster S, Damjanov I, Miesbauerova M, Kazakov DV, Vernerova Z. Littoral cell angioma of the spleen: a study of 25 cases with confirmation of frequent association with visceral malignancies. Histopathology. 2016;69:762-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Gakenheimer-Smith L, Mohlman J, VandenHeuvel K, Jackson WD, Thomsen W, Stevenson A, Cipriano F. A Novel Presentation of Littoral Cell Angioma and Lymphatic Malformations in a Neonate. Pediatrics. 2018;141:S520-S525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Truong V, Finch R, Martin B, Buzacott K, Singh M, Patel B. Littoral cell angioma of spleen. ANZ J Surg. 2019;89:E158-E159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Veillon DM, Williams RB, Sardenga LJ, Harrison GK, Cotelingam JD. ‘Little’ littoral cell angioma of the spleen. Am J Surg Pathol. 2000;24:306-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Ozer N, Sozutek A. Littoral Cell Angioma of the Spleen presenting with Thrombocytosis and Splenic Infarct. J Coll Physicians Surg Pak. 2021;31:986-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Du J, Shen Q, Yin H, Zhou X, Wu B. Littoral cell angioma of the spleen: report of three cases and literature review. Int J Clin Exp Pathol. 2015;8:8516-8520. [PubMed] |
| 9. | Kim HG, Park IS, Lee JI, Jeong S, Lee JW, Kwon KS, Lee DH, Kim PS, Kim HG, Shin YW, Kim YS, Ahn IS, Lee KY. Littoral cell angioma (LCA) associated with liver cirrhosis. Yonsei Med J. 2005;46:184-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Venkatanarasimha N, Hall S, Suresh P, Williams MP. Littoral cell angioma in a splenunculus: a case report. Br J Radiol. 2011;84:e11-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Takayoshi K, Doi G, Tsuruta N, Yoshihiro T, Nio K, Tsuchihashi K, Ariyama H, Odawara J, Shimoda S, Kohashi K, Oda Y, Itoh S, Harimoto N, Maehara Y, Kusaba H, Akashi K, Baba E. Successful chemotherapeutic treatment for metastatic littoral cell angioma: A case report. Medicine (Baltimore). 2018;97:e0378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Fernandez S, Cook GW, Arber DA. Metastasizing splenic littoral cell hemangioendothelioma. Am J Surg Pathol. 2006;30:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Kranzfelder M, Bauer M, Richter T, Rudelius M, Huth M, Wagner P, Friess H, Stadler J. Littoral cell angioma and angiosarcoma of the spleen: report of two cases in siblings and review of the literature. J Gastrointest Surg. 2012;16:863-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Thanapirom K, Treeprasertsuk S, Soonthornworasiri N, Poovorawan K, Chaiteerakij R, Komolmit P, Phaosawasdi K, Pinzani M. The incidence, etiologies, outcomes, and predictors of mortality of acute liver failure in Thailand: a population-base study. BMC Gastroenterol. 2019;19:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Morali GA, Rozenmann E, Ashkenazi J, Munter G, Braverman DZ. Acute liver failure as the sole manifestation of relapsing non-Hodgkin’s lymphoma. Eur J Gastroenterol Hepatol. 2001;13:1241-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Williams MO, Akhondi H, Khan O. Primary Hepatic Follicular Lymphoma Presenting as Sub-acute Liver Failure: A Case Report and Review of the Literature. Clin Pathol. 2019;12:2632010X19829261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Shibata J, Kurahashi S, Naito T, Sugiura I. Diffuse large B cell lymphoma primarily presenting as acute liver failure in a surviving patient. J Community Hosp Intern Med Perspect. 2019;9:135-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Lettieri CJ, Berg BW. Clinical features of non-Hodgkins lymphoma presenting with acute liver failure: a report of five cases and review of published experience. Am J Gastroenterol. 2003;98:1641-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Saló J, Nomdedeu B, Bruguera M, Ordi J, Ginès P, Castells A, Vilella A, Rodés J. Acute liver failure due to non-Hodgkin’s lymphoma. Am J Gastroenterol. 1993;88:774-776. [PubMed] |
| 20. | Ghosh P, Fox IJ, Rader AM, Sorrell MF. Fulminant hepatic failure as the initial manifestation of non-Hodgkins lymphoma. Am J Gastroenterol. 1995;90:2207-2209. [PubMed] |
| 21. | Rowbotham D, Wendon J, Williams R. Acute liver failure secondary to hepatic infiltration: a single centre experience of 18 cases. Gut. 1998;42:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Colby TV, LaBrecque DR. Lymphoreticular malignancy presenting as fulminant hepatic disease. Gastroenterology. 1982;82:339-345. [PubMed] |
| 23. | Thompson DR, Faust TW, Stone MJ, Polter DE. Hepatic failure as the presenting manifestation of malignant lymphoma. Clin Lymphoma. 2001;2:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3597] [Article Influence: 199.8] [Reference Citation Analysis (1)] |
| 25. | Arcuri PP, Taglianetti S, Vavalà B, Battaglia C, Laganà D, Manti F. Incidental littoral cell angioma of the spleen: cross-sectional imaging findings and review of the literature. Radiol Case Rep. 2022;17:3545-3550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 26. | Priego P, Rodríguez Velasco G, Griffith PS, Fresneda V. Littoral cell angioma of the spleen. Clin Transl Oncol. 2008;10:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Hansen T, Habekost M, Flieger D, Kirkpatrick CJ. [Littoral cell angioma of the spleen. Association with colon and hepatocellular carcinoma]. Pathologe. 2010;31:290-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | [13 cases of littoral cell angioma in spleens]. Beijing Da Xue Xue Bao Yi Xue Ban. 2017;49:495-500. [PubMed] |