Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1019
Peer-review started: November 7, 2022
First decision: December 20, 2022
Revised: December 26, 2022
Accepted: January 10, 2023
Article in press: January 10, 2023
Published online: February 16, 2023
Processing time: 99 Days and 5.8 Hours
Lacunes are the manifestations of lacunar infarction which can lead many patients to the clinical outcome of disability or dementia. However, the relationship between lacune burden, cognitive function and blood glucose fluctuation in patients with type 2 diabetes mellitus (T2DM) complicated with lacunes is not very clear.
To explore the correlation between glucose variability, lacune burden and cognitive function in patients with lacunes complicated with T2DM.
The clinical and imaging data of 144 patients with lacunes combined with T2DM were reviewed retrospectively. 72 h continuous glucose monitoring was perfor
The standard deviation (SD) of the average blood glucose concentration, percentage coefficient of variation (%CV) and time of range (TIR) were significantly different between the low and the high load groups (P < 0.05). The SD, %CV and TIR of the cognitive impairment group and non-cognitive impairment group were significantly different (P < 0.05). SD (odds ratio (OR): 3.558, 95% confidence interval (CI): 1.268-9.978, P = 0.006), and %CV (OR: 1.192, 95%CI: 1.081-1.315, P < 0.05) were the risk factors for an increased infarct burden in lacunes patients complicated with T2DM. TIR (OR: 0.874, 95%CI: 0.833-0.928, P < 0.05) is a protective factor. In addition, an increased SD (OR: 2.506, 95%CI: 1.008-6.23, P = 0.003), %CV (OR: 1.163, 95%CI: 1.065-1.270, P < 0.05) were the risk factors for cognitive impairment in patients with lacunes complicated with T2DM, TIR (OR: 0.957, 95%CI: 0.922-0.994, P < 0.05) is a protective factor. A nomogram prediction model of the risk of cognitive impairment was established based on SD, %CV and TIR. Decision curve analysis and the internal calibration analysis were used for internal verification and showed that the model was clinical benefit. The area under the ROC curves for predicting cognitive impairment in patients with lacunes complicated with T2DM was drawn were %CV: 0.757 (95%CI :0.669-0.845, P < 0.05), TIR: 0.711 (95%CI: 0.623-0.799, P < 0.05).
Blood glucose variability is closely associated with the level of lacune burden and cognitive dysfunction in lacune patients combined with T2DM. %CV, TIR have a certain predictive effect in cognitive impairment in lacune patients.
Core Tip: Standard deviation, percentage of coefficient of variation, largest amplitude of glucose, and time of range were used in this study describe the degree of glucose variability (GV) more comprehensively and effectively in patients. Statistical analysis confirmed the close relationship between GV, lacune burden and cognitive function in lacune patients with type 2 diabetes mellitus (T2DM). In addition, a nomogram prediction model of GV index and lacunes complicated with T2DM patients with cognitive impairment was established, which could concisely and intuitively reflect the relationship between the risk of cognitive impairment in lacune patients complicated with T2DM and various risk factors.
- Citation: Meng QZ, Wang Y, Li B, Xi Z, Wang M, Xiu JQ, Yang XP. Relationship between glycemic variability and cognitive function in lacune patients with type 2 diabetes. World J Clin Cases 2023; 11(5): 1019-1030
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1019.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1019
Cerebral infarction is the major reason of the disability and the second major cause of death worldwide[1]. Ischemic stroke has different subtypes including cerebral small vessel disease (CSVD), aortic atherosclerosis and cardiac embolism. Although CSVD has many imaging and clinical characteristics, cavitary infarction (LI) is the most typical accounting for 20%-30% of ischemic stroke cases[2]. LI encompasses recent small subcortical infarcts and lacunes. Lacunes are the most common imaging findings of LI[3]. Lacune is defined as a lumen filled with a circular or elliptical subcortical space 3-15 diameter and is consistent with previous acute subcortical infarction in the perforated arteriole area[4]. Lacunes represent large problems that can drive patients toward a clinical outcome of disability or dementia. Lacune requires special attention because it has a recurrence rate of 20%, a 5-year mortality rate of 25%, and related diseases such as vascular cognitive impairment[5]. T2DM is a common disease in older adults; although determination of glycated hemoglobin (HbA1c) is considered to be the standard for evaluation of glycemic control in diabetic patients, it does not consider changes in blood glucose levels, that is, blood glucose variability (GV)[6]. GV, the cause of glycemic fluctuations, is a sensitive indicator of blood sugar, and advances in continuous blood glucose monitoring (CGM) technology have become an established evaluation tool[7]. CGM technology can further understand daily fluctuations and changes in the daily glucose concentration. The measurement of blood GV includes the standard deviation (SD) of the average blood glucose concentration, the percentage of coefficient of variation (%CV), the largest amplitude of glucose (LAGE), and the determination of the time within the blood glucose range, a newly discovered indicator in recent years, as the result of blood glucose control. Lacunes and type 2 diabetes (T2DM) are both common diseases in the population. Recently, it is clarified that the degree of GV will affect the cognitive function of elderly T2DM patients[8]. In previous studies, changes in blood glucose also affected pathological changes in small arterioles[9]. This indicates that the blood glucose level in lacune patients with T2DM may be closely associate with infarction load and cognitive dysfunction. However, the association between GV, the severity of lacunes and the cognitive impairment in lacune patients with T2DM is still unknown. If we can understand the correlation between GV and cognitive function in lacune patients combined with T2DM, we can easily identify high-risk groups, and judge the severity and prognosis of the disease.
The purpose of this research was to investigate the association between GV, lacune burden and cognitive function in patients with lacunes complicated with T2DM, and to find out the relevant factors for cognitive dysfunction in lacune patients with T2DM. Specificity, sensitivity, and indicators for early clinical diagnosis will allow clinicians to formulate effective preventive strategies and treatment measures as soon as possible.
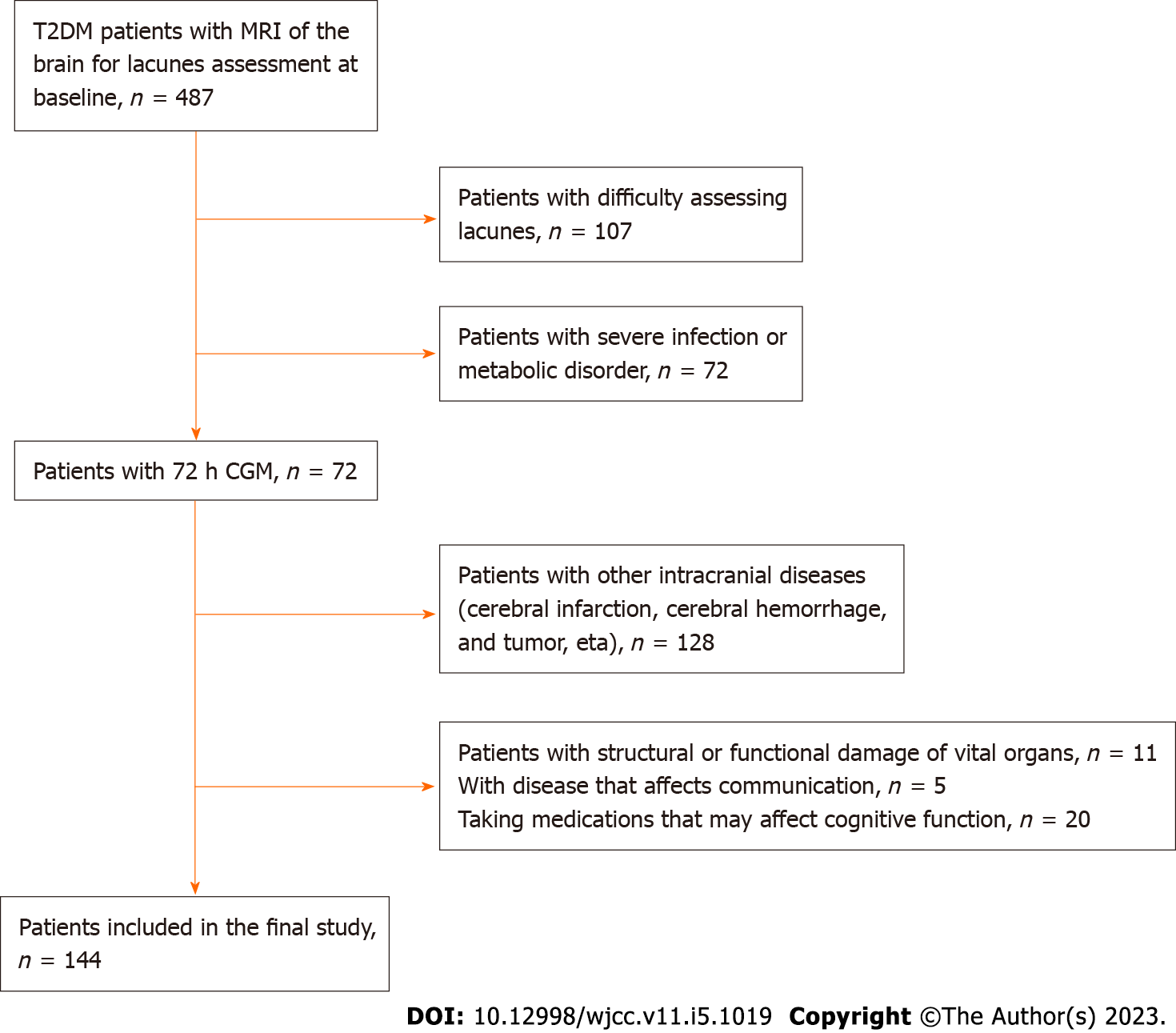
PASS11 was used to estimate the sample size (α = 0.05, β = 0.1, two-sided test, balanced group design). Based on the assumption of homogeneity of variances, pooled variance was used to calculate the sample size. According to previous literature reports and preliminary experiments, substituting the test level, test efficiency, mean and standard deviation of the experimental group and the control group, we calculated that at least 120 patients need to be enrolled. Considering the loss of follow-up of the subjects, at least 140 patients need to be included. In total, 144 patients with lacunes combined with T2DM treated at the second hospital of Zhengzhou University from January 2021 to June 2022 were retrospectively screened (Figure 1). According to the appearance of lacunes on imaging, patients were divided into the high-load group (lacune lesions > 3, n = 50) and low load group (lacune lesions 1-3, n = 94)[10]. Cognitive function disorder (n = 37) and normal cognitive function (n = 107) patients were classified according to whether or not cognitive dysfunction occurred. The inclusion criteria were as follows: (1) Patients compatible with T2DM diagnostics criteria established by the ‘China Diabetes Prevention and Treatment Guideline (2017 Edition)”[11]; (2) patients who met the “Diagnostic Criteria of Lacunes” in the 2021 “Chinese Common Cerebrovascular Disease Medical Specialist Common Recognition” guidelines[12]; (3) routine blood tests performed on admission, and baseline data such as blood lipids and blood sugar levels were complete; and (4) head magnetic resonance imaging (MRI) was recorded on admission. The exclusion criteria were as follows: (1) Other intracranial diseases such as intracranial infection, massive cerebral infarction, cerebral hemorrhage, and tumor, trauma; (2) taking medications that may affect cognitive function; (3) combined with severe infectious and metabolic diseases; (4) combined with structural or functional damage of vital organs; (5) age < 18 years; and (6) no informed consent was signed. This study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Zhengzhou University (2022031).
The following data was collected from all patients who participated in this study: Montreal cognitive assessment (MoCA) score, body mass index (BMI), history of hypertension, history of coronary heart disease, age, gender, fasting blood glucose, blood lipids, homocysteine (Hcy), HbA1c, C-reactive protein (CRP), smoking history (more than 12 mo in total, > 3 cigarettes/d) and drinking history (intake of alcohol content > 50 g/d, > 6 mo in total).
Patients underwent CGM (Abbott Diabetes Care Inc, England) for 72 h after admission, and GV evaluation indicators were calculated, which included: standard deviation (SD) of the mean blood glucose concentration, %CV, LAGE and glucose target range time (TIR). The %CV = SD/mean blood sugar × 100%; LAGE = the maximum minus minimum blood glucose, and the glucose TIR was defined as the percentage time that glucose was in the target range (3.9-7.8 mmol/L) within 72 h.
All selected patients completed an MRI of the head. In this study, the number of lacunes is used to evaluate the infarct burden of lacunes. Lacune was defined as a cavity that appeared on MRI as a subcortical, round or oval, fluid-filled cavity resembling a cerebrospinal fluid signal that was low on T1-weigthed imaging (WI) sequences, high on T2WI sequences, and low in the center surrounded by a high signal ring on fluid-attenuated inversion recover sequences; the diameter was 3-15 mm. Patients were divided into a high-load group (lacunes > 3) and a low-load group (lacunes: 1-3) according to the number of lacunes,. Evaluations were performed by two neurologists, and in the event of disagreement, both were consulted.
All enrolled patients completed the MoCA. A total score < 26 was defined as a patient with cognitive impairment, whereas a score of 26 and above was defined as normal.
SPSS (v.26.0) software was used for statistical analysis. The measurement data that conformed to the normal distribution was expressed as the mean ± standard deviation (mean ± SD) and a comparison between the two groups was made using the Student’s t-test. The measurement data that was not normally distributed was expressed as the median with the 25th and 75th percentiles (M; P25, P75); the Mann-Whitney U test was used for comparison between the two groups. The χ2 test was used for the analysis of enumeration data. Multivariate Logistic regression analysis was used to explore the influencing factors of lacune burden and cognitive function in patients with lacunes complicated with T2DM. Using the R 4.2.1 software package, a nomogram prediction model of blood GV indicators for cognitive impairment in patients with lacunes complicated with T2DM was established. The internal correction analysis was used, and the clinical benefit was verified with decision curve analysis (DCA). A receiver operating characteristic (ROC) curve was used curve to evaluate the predictive value of each index on cognitive function in patients with lacunes complicated with T2DM. P < 0.05 was statistically significant.
In patients with lacunes, the low-load groups and high-load groups showed no significant difference in BMI, hypertension history, coronary sclerosis history, age, lipid levels, smoking history, drinking history, fasting blood sugar, and CRP (all P > 0.05). Among the GV-related indicators, SD and %CV in the high-load group were higher than those in the low load group, whereas TIR was lower in the high-load; and the differences were statistically significant (P < 0.05; Table 1). The GV indexes of the two groups of patients defined by cognitive impairment were compared. The SD and %CV of the cognitive impairment group were higher than those of the normal cognitive group, whereas the TIR was lower in the cognitive impairment group; the differences were statistically significant (P < 0.05; Table 2). Multivariate logistic regression analysis was performed for lacune complicated with T2DM patients with lacune load and degree of cognitive impairment as dependent variables, and SD, %CV, and TIR as independent variables. Elevated SD and %CV were the risk factors of a high lacune burden and cognitive impairment in lacune patients complicated with T2DM (P < 0.05). In addition, elevated glucose TIR was a protective factor for high lacune burden and cognitive impairment in lacune patients complicated with T2DM (P < 0.05; Table 3 and 4).
| Variables | Low load group | High load group | Statistical parameters | P value |
| Age [M (P25, P75), yr] | 66 (57, 73) | 70 (63, 76) | -1.632 | 0.105 |
| Gender (Male/Female) | 51/43 | 28/22 | 0.040a | 0.841 |
| Fasting blood glucose [M (P25, P75), mmol/L] | 5.42 (4.94, 6.18) | 5.6 (5.01, 7.33) | -1.154 | 0.249 |
| TG [M (P25, P75), mmol/L] | 1.6 (1.23, 1.88) | 1.55 (1.36, 1.86) | -0.520 | 0.603 |
| TC (mean ± SD, mmol/L) | 3.88 ± 0.9 | 3.69 ± 1.11 | 1.054b | 0.295 |
| HDL-C (mean ± SD, mmol/L) | 1.63 ± 0.78 | 1.85 ± 1.07 | -1.276b | 0.206 |
| LDL-C (mean ± SD, mmol/L) | 2.25 ± 0.66 | 2.11 ± 0.8 | 1.168b | 0.245 |
| HbA1c [M (P25, P75), %] | 8.20 (7.12, 9.66) | 8.99 (7.48, 9.90) | -1.452 | 0.147 |
| Hcy [M (P25, P75) umol/L] | 10.6 (8.33, 13.57) | 11 (9.17, 13.93) | -0.913 | 0.361 |
| CRP [M (P25, P75) mg/L] | 2.35 (0.8, 4.3) | 2.98 (1.1, 5.9) | -1.408 | 0.159 |
| History of hypertension (n, %) | 30 (31.9%) | 24 (48%) | 3.603a | 0.058 |
| History of coronary heart disease (n, %) | 20 (21.3%) | 14 (28%) | 0.818a | 0.366 |
| Smoking history (n, %) | 16 (17%) | 11 (22%) | 0.531a | 0.466 |
| Drinking history (n, %) | 17 (18.1%) | 12 (24%) | 0.710a | 0.399 |
| BMI (mean ± SD, mmol/L) | 24.78 ± 2.82 | 25.27 ± 2.29 | -1.065b | 0.289 |
| SD (mean ± SD, mmol/L) | 2.79 ± 0.37 | 3.15 ± 0.57 | -3.949b | < 0.001 |
| %CV (mean ± SD, %) | 25.74 ± 4.1 | 31.02 ± 5.94 | -5.615b | < 0.001 |
| LAGE [M (P25, P75), mmol/L)] | 9.94 (6.16, 12.27) | 10.38 (6.79, 13.33) | -0.89 | 0.373 |
| TIR [M (P25, P75), %)] | 59.95 (49.18, 72.64) | 43.2 (35.3, 51.24) | 6.682 | < 0.001 |
| Group | Number | SD | %CV | LAGE | TIR |
| Cognitive dysfunction | 37 | 3.15 ± 0.53 | 31.28 ± 5.38 | 10.35 (6.51, 13.49) | 47.04 (37.61, 53.52) |
| Normal cognitive function | 107 | 2.84 ± 0.44 | 26.29 ± 4.83 | 10.01 (6.34, 12.27) | 56.02 (45.78, 69.46) |
| Statistical parameters | -3.513 | -5.254 | -0.738 | 3.825 | |
| P value | < 0.001 | < 0.001 | 0.461 | < 0.001 |
| β | SD | Wald | P value | OR | 95%Cl | |
| SD | 1.269 | 0.526 | 2.412 | 0.016 | 3.558 | [1.268, 9.978] |
| %CV | 0.176 | 0.05 | 3.527 | < 0.001 | 1.192 | [1.081, 1.315] |
| TIR | -0.129 | 0.028 | -4.648 | < 0.001 | 0.874 | [0.833, 0.928] |
| β | SD | Wald | P value | OR | 95%CI | |
| SD | 0.919 | 0.465 | 1.976 | 0.048 | 2.506 | [1.008, 6.230] |
| %CV | 0.151 | 0.045 | 3.365 | 0.001 | 1.163 | [1.065, 1.270] |
| TIR | -0.044 | 0.019 | -2.253 | 0.024 | 0.957 | [0.922, 0.994] |
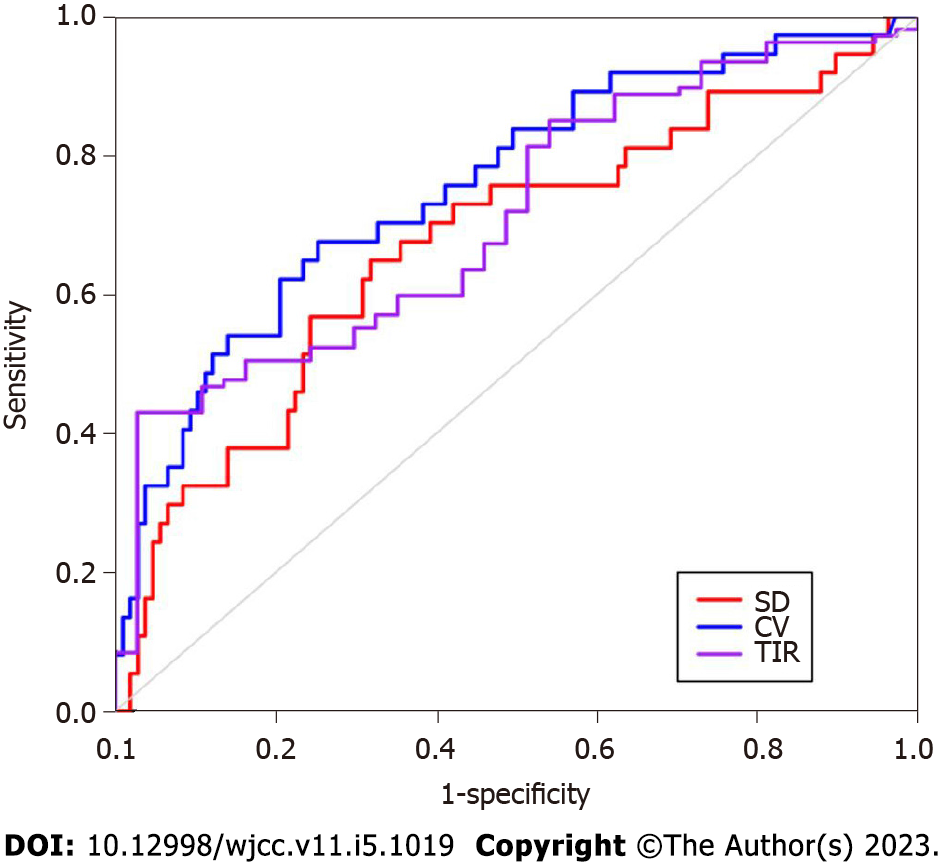
The ROC curve of cognitive impairment was drawn based on SD, %CV, and TIR (Figure 2), The area under the curve (AUC) of SD to predict cognitive impairment in patients with lacunes complicated with T2DM was 0.673 (95% confidence interval (CI): 0.565-0.781, P < 0.01). The optimal cut-off value was 3.018, the sensitivity was 64.9%, and the specificity was 68.2%. The AUC of %CV for predicting cognitive impairment was 0.757 (95%CI: 0.669-0.845, P < 0.01). The optimal cutoff value was 29.485%, the sensitivity was 67.6%, and the specificity was 74.8%. The AUC of TIR was 0.711 (95%CI: 0.623-0.799, P < 0.01). The optimal cut-off value was 60.5, the sensitivity was 97.3%, and the specificity was 43.0%. %CV and TIR had good clinical predictive value.
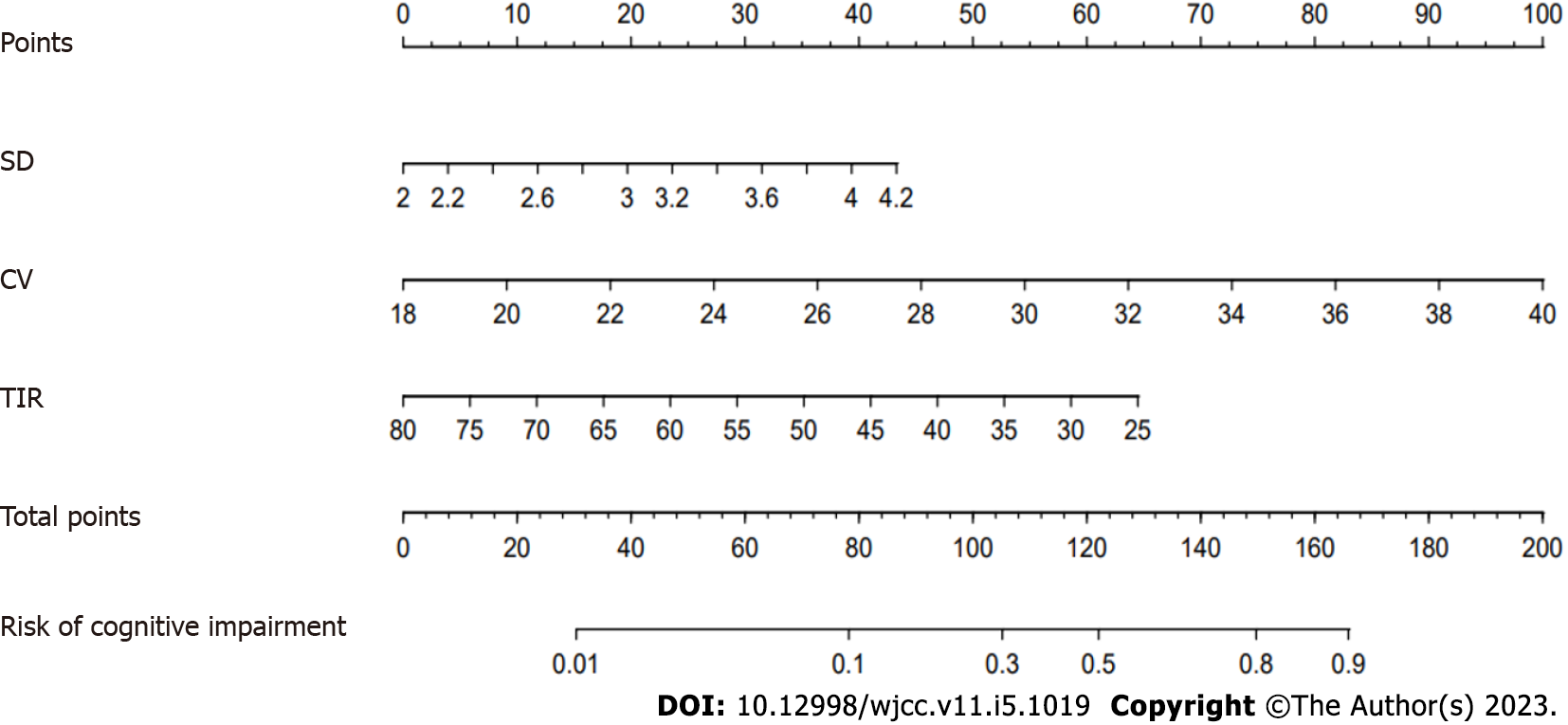
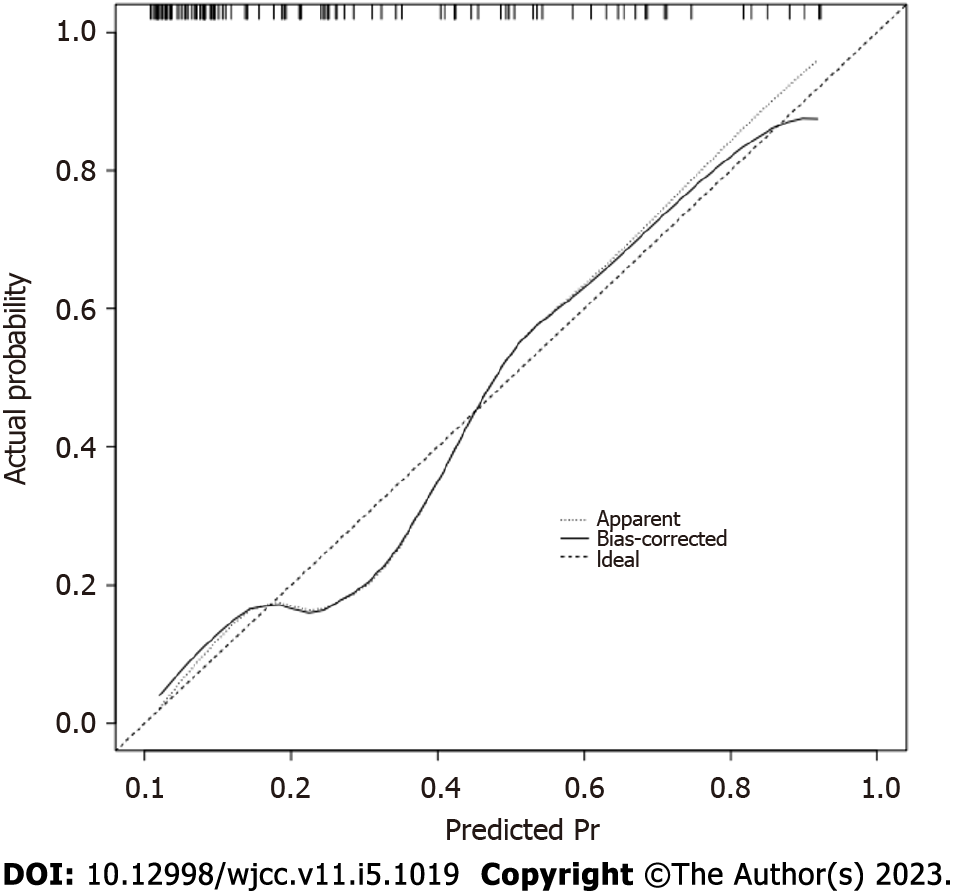
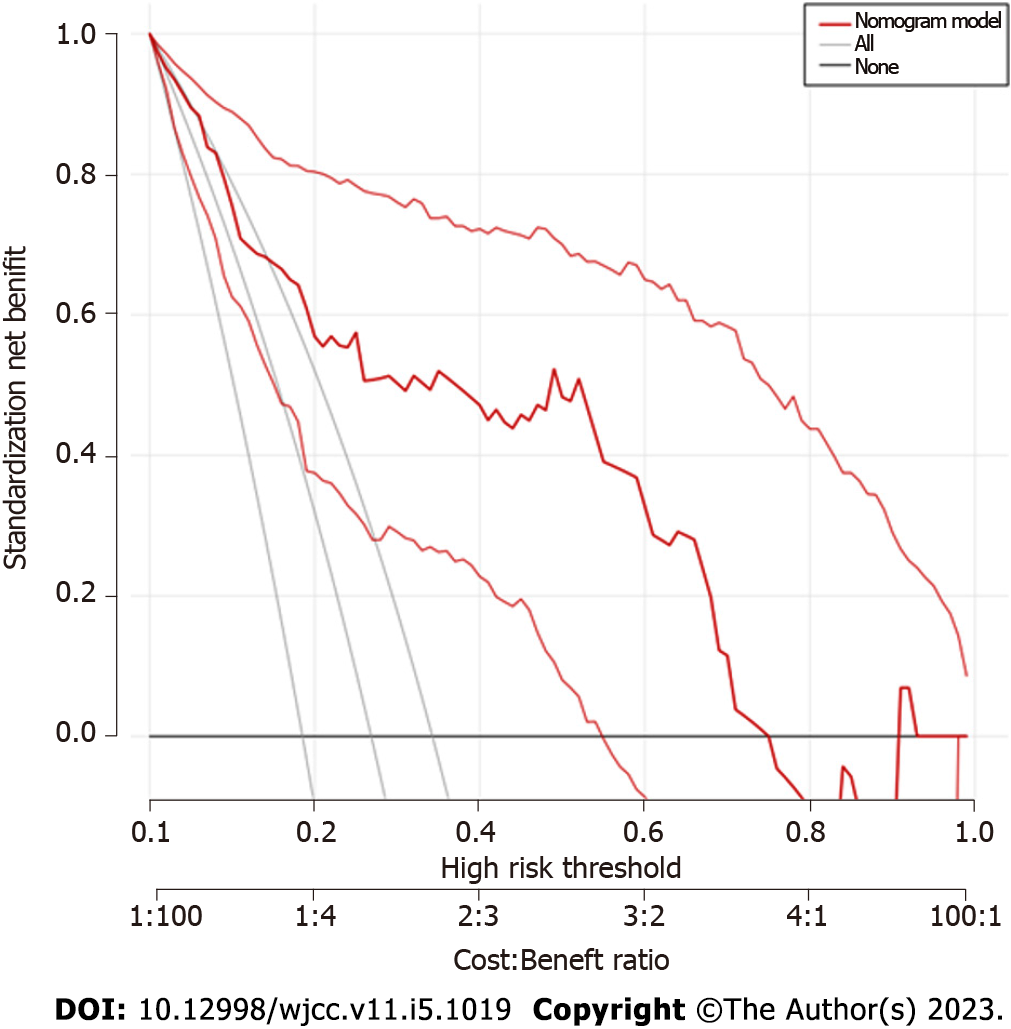
Based on SD, %CV, and TIR, a nomogram prediction model for cognitive impairment in patients with lacunes complicated with T2DM was established (Figure 3). The internal calibration analysis indicated how far the actual result is offset from the ideal result. An internal calibration analysis for this model showed that the actual result curve was similar to the corrected result curve and the ideal result curve, indicating that the actual prediction result of the model was similar to the ideal prediction result (Figure 4). The DCA for verification of the clinical benefit was performed and it could evaluate the clinical benefit and clinical predictive value of the model. The DCA of the model showed that the nomogram model curve was located on the upper right side of the two solid lines (the ‘none’ line and the ‘all’ line), indicating that the model had a greater clinical benefit and better clinical predictive value, (Figure 5).
According to previous studies, the severity of lacunes is related to various factors, although the specific mechanisms remain unclear. It has been suggested that the main pathogenesis of lacunes is endothelial disorder and disruption of blood brain barrier (BBB)[2]; endothelial disorder is indicated to be the most important mechanism for lacunes[13]. The endothelium can regulate the tension of blood vessels, adjust fiber-dissolution or aggregation, participate in inflammatory responses, and participate in vascular wall formation[14]. Dysfunction of the endothelium reflects a shift towards promoting vasoconstriction, coagulation, inflammation, and increasing vessel thickness[15]. This can result in structural and functional damage to the endothelium, allowing the vessel walls to become leaky and inflamed. The autoregulatory function of the vessel wall is thereby impaired, preventing the vessel from dilating and reducing perfusion[16]. The normal vessel wall structure is gradually replaced by connective tissue, and the vessel wall thickens, resulting in lumen stenosis, thrombosis, and vascular occlusion[15,17]. GV is defined as variations in the blood glucose levels in a day or on different days, but the time remains the same. It contains elevated daily blood glucose levels, episodes of hyperglycemia, and hypoglycemia[18]. TIR is a new convenient, intuitive, and easy to measure and understand indicator, that has been shown to predict the risk of long-term complications in diabetes. Diabetic complications can be caused by hyperglycemia, abnormal blood sugar levels, and fluctuations in blood sugar levels[19,20]. GV is closely related to arterial stiffness[9]. Furthermore, a cohort study in Taiwan, China, showed that GV had a positive correlation with ischemic stroke risk[21]. Japanese researchers reported that, especially in the postprandial state, excessive glucose fluctuations were positively associated with diabetic retinopathy and atherosclerosis[22]. These studies all suggest that changes in blood glucose fluctuation may affect the pathological progression of in lacune patients. T2DM is related to an increased risk of cognitive impairment. T2DM patients with high blood GV had increased cognitive decline[8]. According to this study, GV was an influencing factor for increased infarct burden in lacune patients combined with T2DM, and increased GV increased the risk of cognitive impairment in lacune patients complicated with T2DM. This suggests that the GV level of lacune patients with T2DM on admission should be detected to determine whether it will increase the burden of lacunes, increase the risk of cognitive impairment, and whether early clinical identification can be performed. Early treatment could prevent the occurrence and development of lacunes, and reduce the severity of lacunes, to improve prognosis and achieve glucose homeostasis in individuals with T2DM by regulating blood glucose fluctuations to reduce overall complications.
The influence of GV in the pathogenesis of infarct burden in lacune patients complicated with T2DM is not well understood. According to the previous research, blood GV has a significant impact on the progression and development of arterial endothelial disorder and atherosclerosis. Both hyperglycemia and hypoglycemia can damage brain structures. First, hyperglycemia can lead to changes in cerebral hemodynamics. One study found that the cerebral blood flow of hyperglycemic rats decreased by 37% compared with normal rats[23]. Second, vasodilation is mainly mediated by endothelial-derived nitric oxide, synthesized from endothelial nitric oxide synthase, the expression of which decreases in hyperglycemic environments[24]. Hyperglycemia can also increase reactive oxygen species (ROS) through a protease C mediated pathway increasing NADPH, resulting in neuronal deaths[25]. Furthermore, hyperglycemia is related to increased nuclear factor κB, which is found to stimulate inflammatory cell function, the production of inflammatory cytokines and endothelial damage, ultimately leading to increased damage and infarct area[26]. Hypoglycemia is also a factor that can aggravate cerebral ischemia. Severe hypoglycemia and mild hypoglycemia independently increased total mortality in patients with ischemic stroke[27]. Under hypoglycemic conditions (hunger-induced or insulin-induced), Ischemic brain cells showed increased energy metabolism and increased oxidative stress exposure[28]. Hyperglycemia and hypoglycemia worsen brain damage; however, importantly, when present with more dynamic glycemic control index, such as GV, it can be used to predict the lacune burden in lacune patients with T2DM more accurately and become an appropriate therapeutic target. The effect of GV on the burden of lacunes may be due to oxidative stress caused by elevated GV, triggering a surge of superoxide, leading to atherosclerosis[29]. Secondly, the increase of GV can lead to the enhanced adsorption of inflammatory factors and macrophages to vascular endothelial cells, aggravating endothelial dysfunction[30]. Prolonged exposure to glucose instability can promote the ROS, aggravate oxidative stress and damage cellular DNA[31]. Finally, people with higher GV also have other dangerous elements such as hypertension and insulin resistance. The existence of these combined risk factors increases the risk of cerebrovascular events. The mechanism of GV affecting cognitive function may be that the above pathological changes result in damage to brain cells areas of cognitive function such as cerebellum, hippocampus, and frontal cerebral lobe. Furthermore the increase in blood GV leads to the damage of the BBB[32,33],and the increased production of ROS induces mitochondrial dysfunction, impaired oxidative stress, increased release of pro-inflammatory factors, and apoptosis[34,35], these pathological changes lead to vascular injury in the central system of nerve[36]. Lacunes due to GV disrupts the network connections in the basal ganglia and dorsolateral prefrontal cortex. The lesions cleaved the fiber connections between several subcortical regions and the frontal cortex, prefrontal cortex and cingulate gyrus, which in turn inhibits functions related to cognition in the frontal cortex[37]. The destruction of cerebral vascular structure causes cerebral vascular autoregulation dysfunction, and can lead to the occurrence of amyloid angiopathy, which continuously reduces the cognitive function of patients, resulting in cognitive dysfunction[38]. In addition, increasing evidence has shown that the cerebellum is related to cognitive function, the prefrontal and the cerebellum cortex have extensive connections, and the cell damage of these connection could explain the cognitive decline in patients with cerebellar ischemia[8].
SD, %CV, LAGE, and TIR were used in this study describe the degree of GV more comprehensively and effectively in patients. Statistical analysis confirmed the close relationship between GV, lacunes burden and cognitive function in lacune patients with T2DM. In addition, a nomogram prediction model of GV index and lacunes complicated with T2DM patients with cognitive impairment was established, which could concisely and intuitively reflect the relationship between the risk of cognitive impairment in lacune patients complicated with T2DM and various risk factors.
However, this study has certain limitations. First, the calculation of indicators mainly relied on a single blood test after admission; therefore, the relative accuracy may be slightly lower, and there may be systematic errors, resulting in biased results. Second, the predictive model was only validated with internal data and lacks external validation. Finally, this study is a regression, single-center study, which may have selection bias.
In conclusion, there is a close relationship between GV and lacune burden in lacune patients with T2DM. Cognitive impairment in lacune patients complicated with T2DM is closely related to GV. In clinical practice, early assessment of GV indicators in patients with lacunes complicated with T2DM can increase early recognition of the severity and prognosis of lacunes, and improve TIR, which may be the key to preventing the occurrence and development of lacunes. By observing changes in the %CV, TIR and other indicators, they may aid in predicting the occurrence and development of cognitive disorder in patients. By strengthening the management of risk factors, we could significantly slow down the development of lacunes or reduce their severity and improve the prognosis.
Lacunes and glucose variability (GV) are gaining growing attention.
The association between GV, the severity of lacunes and the cognitive impairment in lacune patients with type 2 diabetes mellitus (T2DM) is still unknown.
To explore the correlation between GV, lacune burden and cognitive function in patients with lacunes complicated with T2DM.
Seventy-two h continuous blood glucose monitoring was performed. The Montreal cognitive assessment was used to assess cognitive function. The burden of lacunes was evaluated using magnetic resonance imaging findings. Multifactorial logistic regression analysis was used to study the affecting the lacune load and cognitive impairment in patients. To predict the value of patients' cognitive impairment with lacunes complicated with T2DM, a receiver operating characteristic curve and a nomogram prediction model were constructed.
Standard deviation and percentage coefficient of variation (%CV) were the risk factors for an increased infarct burden in lacune patients complicated with T2DM. Time of range (TIR) is a protective factor. In addition, an increased SD, %CV were the risk factors for cognitive impairment in patients with lacunes complicated with T2DM. TIR is a protective factor.
Blood glucose variability is closely associated with the level of lacune burden and cognitive dysfunction in lacune patients combined with T2DM. %CV, TIR have a certain predictive effect in cognitive impairment in lacune patients.
Prospective studies are further needed to verify the results of this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Byeon H, South Korea; Pandey NM, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Regenhardt RW, Das AS, Stapleton CJ, Chandra RV, Rabinov JD, Patel AB, Hirsch JA, Leslie-Mazwi TM. Blood Pressure and Penumbral Sustenance in Stroke from Large Vessel Occlusion. Front Neurol. 2017;8:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Caplan LR. Lacunar infarction and small vessel disease: pathology and pathophysiology. J Stroke. 2015;17:2-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in Understanding the Pathophysiology of Lacunar Stroke: A Review. JAMA Neurol. 2018;75:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 4. | Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge Rv, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2813] [Cited by in RCA: 3978] [Article Influence: 331.5] [Reference Citation Analysis (0)] |
| 5. | Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003;2:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Umpierrez GE, P Kovatchev B. Glycemic Variability: How to Measure and Its Clinical Implication for Type 2 Diabetes. Am J Med Sci. 2018;356:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 7. | Sparks JR, Kishman EE, Sarzynski MA, Davis JM, Grandjean PW, Durstine JL, Wang X. Glycemic variability: Importance, relationship with physical activity, and the influence of exercise. Sports Med Health Sci. 2021;3:183-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Tang X, Xiao X, Yin J, Yang T, Zeng B. An Assessment of the Relationship between Structural and Functional Imaging of Cerebrovascular Disease and Cognition-Related Fibers. Comput Math Methods Med. 2020;2020:4347676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Wu S, Li M, Wang T, Xu M, Lu J, Wang S, Zhang J, Bi Y, Wang W, Ning G, Xu Y, Chen Y. Long-Term Glycemic Variability Is Associated With Arterial Stiffness in Chinese Adults. Front Endocrinol (Lausanne). 2021;12:711540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Che YW, Miao YW, Jiang YH, Chang PP, Song QW. A preliminary study of MRI of cerebral small vessel lesions in patients with systemic lupus erythematosus.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Chinese Diabetes Society. Guidelines for the prevention and control of type 2 diabetes in China (2017 edition). Zhonghua Shiyongbingxue Zazhi. 2018;38:292-344. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 12. | Hu WL, Yang L, Li XT, Huang YH. Expert consensus on diagnosis and treatment of cerebral small vessel disease in China 2021.. [DOI] [Full Text] |
| 13. | Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1205] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 14. | Wardlaw JM, Doubal FN, Valdes-Hernandez M, Wang X, Chappell FM, Shuler K, Armitage PA, Carpenter TC, Dennis MS. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke. 2013;44:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J Cereb Blood Flow Metab. 2016;36:72-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 16. | Giwa MO, Williams J, Elderfield K, Jiwa NS, Bridges LR, Kalaria RN, Markus HS, Esiri MM, Hainsworth AH. Neuropathologic evidence of endothelial changes in cerebral small vessel disease. Neurology. 2012;78:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Bagi Z, Brandner DD, Le P, McNeal DW, Gong X, Dou H, Fulton DJ, Beller A, Ngyuen T, Larson EB, Montine TJ, Keene CD, Back SA. Vasodilator dysfunction and oligodendrocyte dysmaturation in aging white matter. Ann Neurol. 2018;83:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, Heatlie G, Loke Y, Rutter MK, Mamas MA. Long-term Glycemic Variability and Risk of Adverse Outcomes: A Systematic Review and Meta-analysis. Diabetes Care. 2015;38:2354-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 376] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 19. | Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, Close KL. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes Care. 2019;42:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 521] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 20. | Agiostratidou G, Anhalt H, Ball D, Blonde L, Gourgari E, Harriman KN, Kowalski AJ, Madden P, McAuliffe-Fogarty AH, McElwee-Malloy M, Peters A, Raman S, Reifschneider K, Rubin K, Weinzimer SA. Standardizing Clinically Meaningful Outcome Measures Beyond HbA(1c) for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40:1622-1630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 294] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 21. | Lin CC, Yang CP, Li CI, Liu CS, Chen CC, Lin WY, Hwang KL, Yang SY, Li TC. Visit-to-visit variability of fasting plasma glucose as predictor of ischemic stroke: competing risk analysis in a national cohort of Taiwan Diabetes Study. BMC Med. 2014;12:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23 Suppl 2:B21-B29. [PubMed] |
| 23. | Quast MJ, Wei J, Huang NC, Brunder DG, Sell SL, Gonzalez JM, Hillman GR, Kent TA. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab. 1997;17:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Ding Y, Vaziri ND, Coulson R, Kamanna VS, Roh DD. Effects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. Am J Physiol Endocrinol Metab. 2000;279:E11-E17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 26. | Bémeur C, Ste-Marie L, Desjardins P, Vachon L, Butterworth RF, Hazell AS, Montgomery J. Dehydroascorbic acid normalizes several markers of oxidative stress and inflammation in acute hyperglycemic focal cerebral ischemia in the rat. Neurochem Int. 2005;46:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35:2262-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 459] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 28. | Chang YS, Park WS, Ko SY, Kang MJ, Han JM, Lee M, Choi J. Effects of fasting and insulin-induced hypoglycemia on brain cell membrane function and energy metabolism during hypoxia-ischemia in newborn piglets. Brain Res. 1999;844:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 947] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 30. | Ceriello A, Ihnat MA. 'Glycaemic variability': a new therapeutic challenge in diabetes and the critical care setting. Diabet Med. 2010;27:862-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Schisano B, Tripathi G, McGee K, McTernan PG, Ceriello A. Glucose oscillations, more than constant high glucose, induce p53 activation and a metabolic memory in human endothelial cells. Diabetologia. 2011;54:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 33. | Carey CL, Kramer JH, Josephson SA, Mungas D, Reed BR, Schuff N, Weiner MW, Chui HC. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke. 2008;39:397-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Quincozes-Santos A, Bobermin LD, de Assis AM, Gonçalves CA, Souza DO. Fluctuations in glucose levels induce glial toxicity with glutamatergic, oxidative and inflammatory implications. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Xue B, Wang L, Zhang Z, Wang R, Xia XX, Han PP, Cao LJ, Liu YH, Sun LQ. Puerarin may protect against Schwann cell damage induced by glucose fluctuation. J Nat Med. 2017;71:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci Rep. 2019;9:840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 37. | Yoon CW, Seo SW, Park JS, Kwak KC, Yoon U, Suh MK, Kim GH, Shin JS, Kim CH, Noh Y, Cho H, Kim MJ, Kim JH, Roh JH, Lee JM, Na DL. Cerebellar atrophy in patients with subcortical-type vascular cognitive impairment. Cerebellum. 2013;12:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Qi Q, T Yi, Jia JP. P1-181: Amyloid beta oligomers involved in cognitive impairment by ngr-rhoa-rock pathway mediated inhibition of t-type calcium channels. Alzheimer's & Dementia. 2019;15:7S. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |