Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3248
Peer-review started: September 3, 2022
First decision: February 14, 2023
Revised: February 28, 2023
Accepted: March 24, 2023
Article in press: March 24, 2023
Published online: May 16, 2023
Processing time: 174 Days and 3 Hours
Tuberculous uveitis caused by tuberculosis infection factors is common, but tuberculous uveitis caused by Mycobacterium tuberculosis found in the intraocular fluid is rare. This report describes the use of intraocular fluid in the diagnosis of tuberculous uveitis in a patient and reviews the relevant literature.
A 24-year-old woman who was 31-wk pregnant visited Hebei Chest Hospital due to intermittent chest pain, fever, and decreased vision for 3 mo. The hydrothorax test suggested “tuberculous pleurisy”, and yellow effusion was extracted from the chest tube twice resulting in a total volume of approximately 800 mL. The patient chose to continue the pregnancy without treatment, and was hospitalized again due to high fever. Following 2 mo of anti-tuberculosis treatment, a healthy boy was delivered by cesarean section. Tuberculous uveitis was diagnosed using tuberculosis Xpert, and intraocular infection was detected by second-generation gene sequencing. Following systemic treatment, the patient gradually improved, and the corrected visual acuity of the left eye gradually increased from 0.08 to 1.0.
The etiology of uveitis is complex, and it is necessary to assess the patient’s general condition and apply molecular biology methods to determine the patho
Core Tip: Tuberculous uveitis caused by tuberculosis infection factors is common, but tuberculous uveitis caused by Mycobacterium tuberculosis found in intraocular fluid is rare. This report describes a 24-year-old pregnant patient who was diagnosed using tuberculosis Xpert and ophthalmologic multimodal imaging after 2 mo of anti-tuberculosis treatment and cesarean delivery of a healthy baby boy. Detection of intraocular infections can be performed by second-generation genetics. Folowing systemic treatment, the patient’s vision recovered.
- Citation: Zhang YK, Guan Y, Zhao J, Wang LF. Diagnosis of tuberculous uveitis by the macrogenome of intraocular fluid: A case report and review of the literature. World J Clin Cases 2023; 11(14): 3248-3255
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3248.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3248
Uveitis is a common ophthalmic disease, and is an important cause of visual impairment in humans, accounting for 10% of blindness worldwide[1]. Tuberculous ocular lesions account for 1.40%-5.74% of systemic tuberculosis[2], among which tuberculous uveitis caused by tuberculosis infection is common. However, tuberculous uveitis in which Mycobacterium tuberculosis is found in intraocular fluid is clinically rare. A case was recently discovered in our hospital, and a summary report and literature review were carried out to analyze the results of tuberculosis-related testing, in order to improve clinicians’ awareness and standardize treatment of the disease.
A 24-year-old woman who was 31-wk pregnant visited Hebei Chest Hospital due to intermittent chest pain, fever, decreased vision for 3 mo, temperature up to 39.4°C, with chills, dizziness, headache, fatigue and other symptoms, occasional cough, dry cough, and shortness of breath, aggravated after activity, The local fever clinic considered that the patient had “pneumonia”, and she was given “cephalosporin” for 10 d, but the symptoms were not significantly relieved. Further examination of the thoracic cavity revealed “left pleural effusion”, a pleural tube was placed and yellow effusion was extracted twice, with a total volume of approximately 800 mL. The pleural effusion test showed “tuberculosis pleurisy”, and her temperature was better than before, fluctuating at around 37.5°C. As the patient chose to continue the pregnancy without treatment, she was again admitted to our hospital with high fever.
The patient developed pain at the left costal margin without obvious inducement 3 mo previously, which was prick-like pain, aggravated by deep inspiratory coughing and vomiting. There was no posterior sternal pressing sensation and radiating pain in the left shoulder. Electrocardiogram examination in the local hospital showed no abnormalities.
The patient had no previous history of disease.
No family history of disease.
Body temperature was 38.9°C, the superficial lymph nodes were small, the left lower lung had percussion dullness, breath sounds were decreased on auscultation, and no wet or dry rales were heard. Ophthalmic examination showed that corrected visual acuity was 1.0 in the right eye, 0.08 in the left eye, no hyperemia in bulbar conjunctiva of the right eye, transparent cornea, a little keratic precipitate (KP) in the posterior cornea, good depth in the anterior chamber, normal pupil size, sensitive light reflex, clear lens, clear edge of the optic disc in the fundus, yellow and white exudation and linear bleeding were seen below the center of the macula. Left eye bulbar conjunctival hyperemia, corneal transparency, KP (+++), aqueous humor opacification, drug-induced pupil dilation, clear lens, vitreous opacification, and invisible fundus were observed. Intraocular pressure was 16 mmHg in the right eye and 17 mmHg in the left eye.
Routine blood tests revealed the following: White blood cells: 8.93 × 109/L, neutrophils 85.7%; C-reactive protein: 51.7 ng/mL; T-spot: 277; Mycobacterium tuberculosis 31.52; Procalcitonin: 0.370 ng/mL; erythrocyte sedimentation rate: 87 mm/h; metagenomic pathogen detection (metagenomic next-generation sequencing, mNGS) was sent for Xpert examination. The result of Mycobacterium tuberculosis detection by Xpert was positive. The PPT test was 10 mm × 10 mm positive.
Chest computed tomography (CT) showed hematogenous disseminated pulmonary tuberculosis.
A healthy baby boy (37 wk of intrauterine gestation) was delivered by cesarean section in the Department of Obstetrics and Gynecology in our hospital. Placenta Xpert examination showed that Mycobacterium tuberculosis was detected, with very low number of bacteria. At this time, the patient had received anti-tuberculosis therapy for 2 mo, and her general condition had improved, and the ocular aqueous humor turbidity was aggravated. Considering that the drug could not pass through the blood-eye barrier, the second-generation gene test of the left ocular aqueous humor was performed to determine intraocular infection, and four sequences of “Mycobacterium tuberculosis” were found in the aqueous humor test.
Destruction of the blood-eye barrier is often accompanied by destruction of the blood-brain barrier. Furthermore, brain magnetic resonance imaging (MRI) examination suggested multiple abnormal signal shadows in the brain parenchyma which were enhanced punctate and nodular, and miliary tuberculosis was considered.
Systemic diagnosis included: Acute hematogenous disseminated pulmonary tuberculosis; tuberculous pleurisy; tuberculous encephalitis; left tuberculous meningitis. Ophthalmologic diagnosis included: Tuberculous uveitis in both eyes; retinal vasculitis in both eyes. A variety of molecular biology detection methods were performed to determine the diagnosis.
With the cooperation of the Department of Tuberculosis, Obstetrics and Ophthalmology, the patient was given isoniazid 0.3 g orally 1/d, rifampicin 0.45 g orally 1/d, pyrazinamide capsule 0.5 g orally 3/d, and ethambutol 0.75 g orally 1/d. The Ophthalmology Department provided local anti-inflammatory mydriasis, and dexamethasone sodium phosphate 5 mg by peribulbar injection, twice a week. Tobramycin dexamethasone eye drops were administered to the left eye 6/d, pranoprofen eye drops to the left eye 4/d, and compound tropicamide eye drops to the left eye three times before bed.
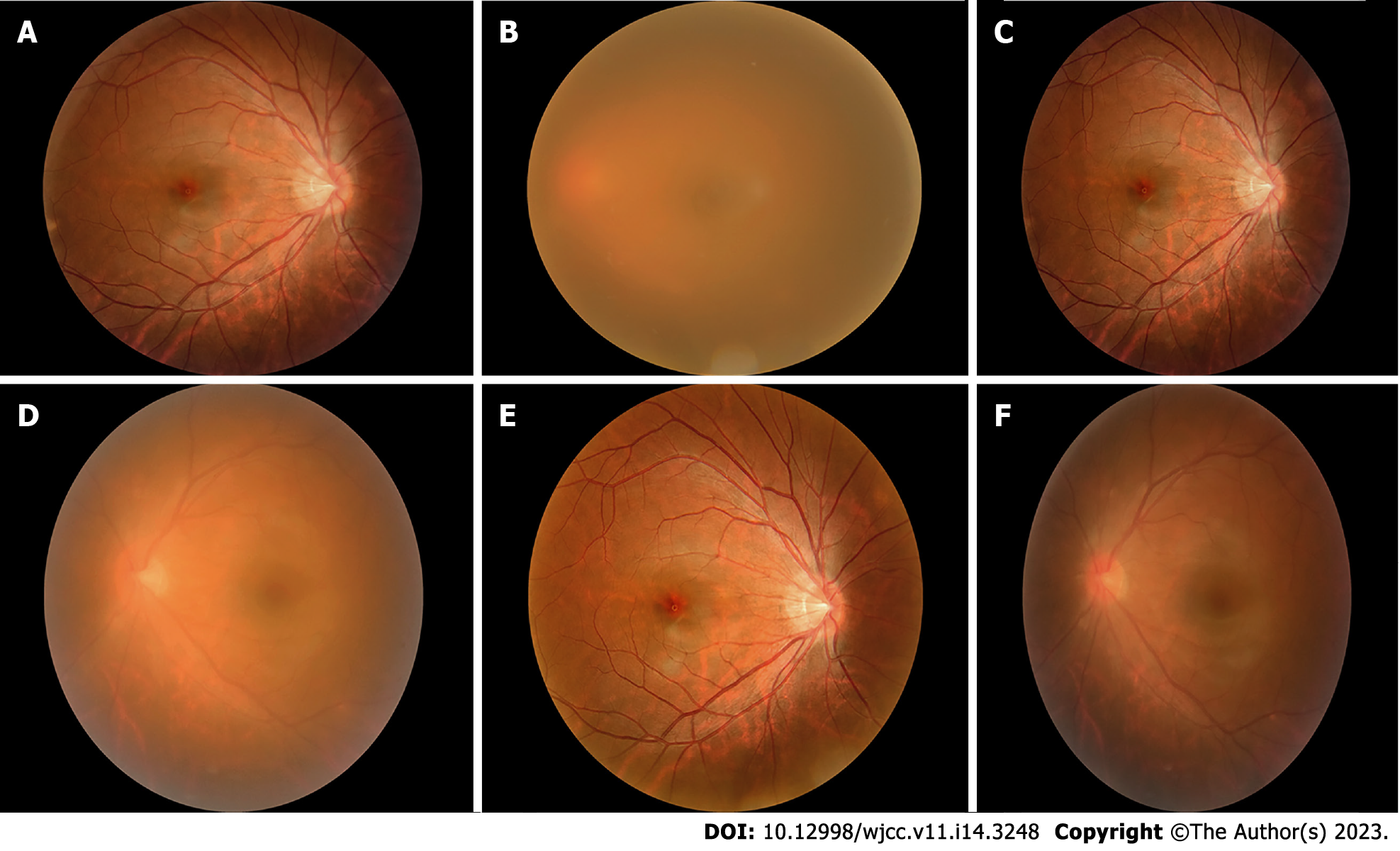
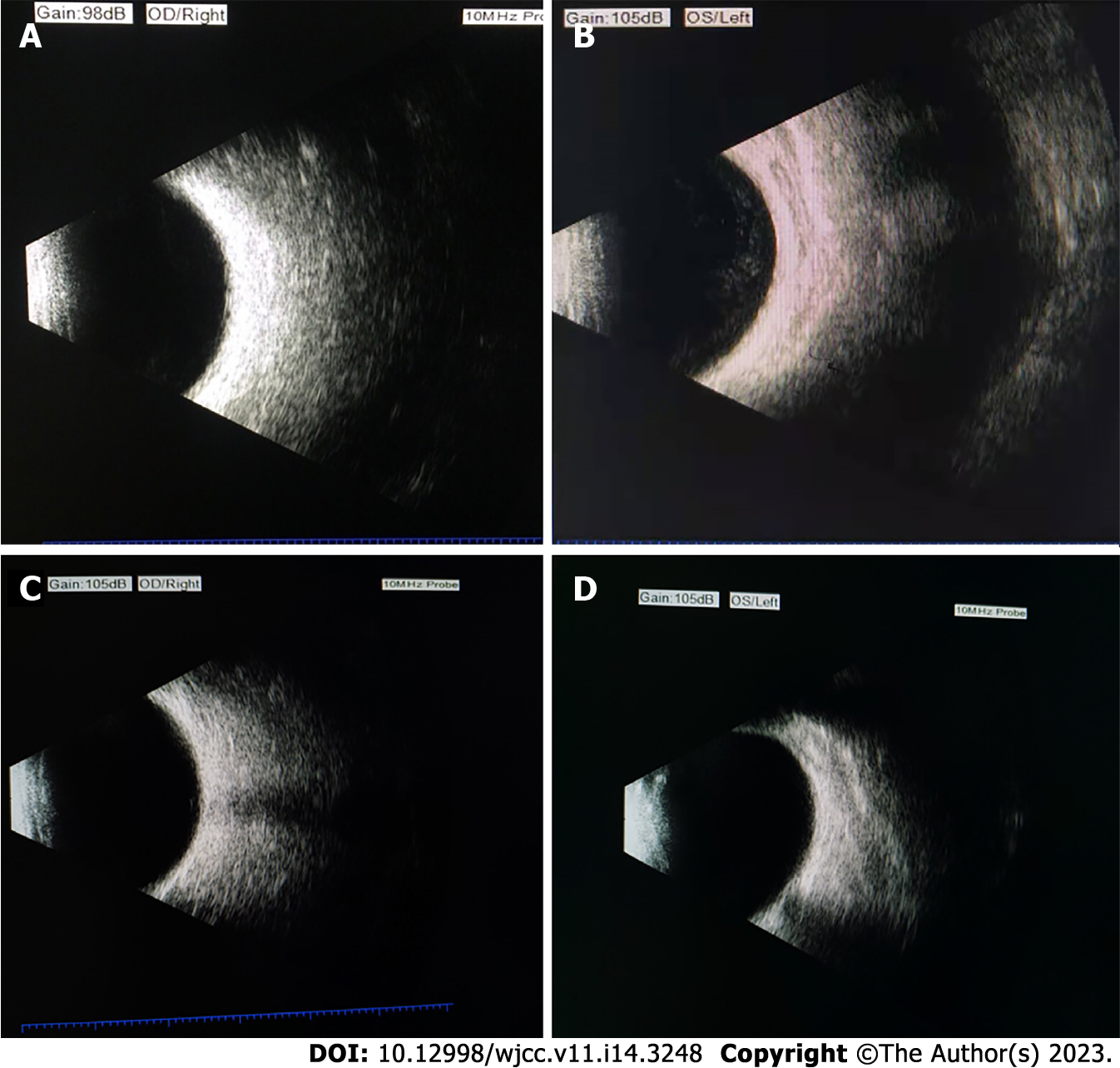
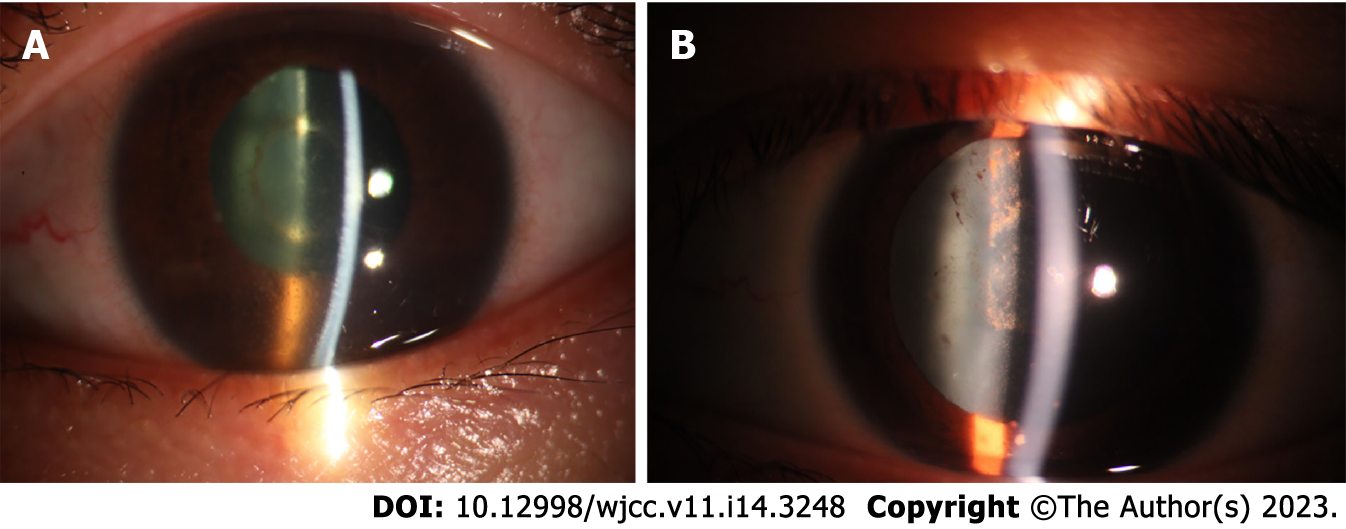
After systemic treatment, the patient’s general condition gradually improved, and corrected vision in the left eye gradually increased from 0.08 to 1.0. According to the uveitis standard working group (Standardization of Uveitis Nomenclature, SUN) standard assessment, the grade of anterior chamber cells and vitreous opacity was evaluated. The patient’s anterior chamber aqueous humor was considered to be grade three: the anterior chamber had 21 to 50 cells/field of vision, and the iris and lens were difficult to recognize and classified as grade 1: The aqueous humor had no anterior chamber flash or weak anterior chamber flash, and no inflammatory cells. Vitreous opacity gradually changed from 4 + to 0.5 +. The results of fundus photography are shown in Figures 1A-F, and the changes in ocular B-ultrasound are shown in Figures 2A-D. The changes in anterior segment photography are shown in Figures 3A-B.
About one-third of the world’s population is infected with Mycobacterium tuberculosis[3], but only 10% of those infected have clinical manifestations. Tuberculosis is most common in the lungs, but it can actually affect other organs, of which 16%-27% are extrapulmonary infections. Extrapulmonary tuberculosis can involve multiple systems and organs such as skin, eyes, cardiovascular system, digestive system, bones and joints, urinary system and the central nervous system. Intraocular tuberculosis is a unique form of extrapulmonary tuberculosis. All eye tissues except the lens can be infected with Mycobacterium tuberculosis[4]. The uvea is rich in blood vessels, containing 96% of the blood flow of the eyeball, and the flow rate in the eye is slow. Previous studies have shown that ocular tuberculosis is relatively rare, mostly secondary to tuberculosis foci in other parts of the body[5]. With the deepening of clinicians’ understanding of the disease and improvements in imaging and laboratory testing methods, the detection rate of ocular tuberculosis is increasing. Tuberculous uveitis accounts for 6.9%-10.5% of unexplained uveitis, and 1.4%-6.8% of active tuberculosis patients are complicated by ocular tuberculosis[6-9]. Eye tuberculosis pathophysiology mechanisms include: (1) Active Mycobacterium tuberculosis infection - blood system spread of Mycobacterium tuberculosis directly into local eye tissue, such as choroid granuloma; and (2) The immune response, has nothing to do with copying an infection, and is related to extrapulmonary organs (eye) of Mycobacterium tuberculosis late-onset allergic reactions, such as stomach morphic choroiditis.
Ocular tuberculosis is usually monocular, but can be binocular. The left eye has been shown to have a higher incidence than the right eye. This is due to anatomical position, as the left common carotid artery emerges directly from the aortic arch, the tuberculous bacterium present in the blood flow via the aortic arch enters directly into the left ocular artery, and on the right side needs to pass the innominate artery.
In this case, the tuberculin skin test [postpartum depression (PPD)] was strongly positive, tuberculosis infection T-cell positive T-spot: 277 increased, and three positive findings were found. Brain MRI showed multiple intracranial nodules and diffuse miliary nodules, and chest CT showed miliary nodules in both lungs, which supported the diagnosis of hematogenous disseminated pulmonary tuberculosis. Diagnostic criteria for tuberculous uveitis are as follows: (1) History of systemic tuberculosis or previous history of tuberculosis; (2) Detection of tuberculous bacilli in body fluids or tissues; (3) Ocular lesions consistent with tuberculosis manifestations; (4) Strong PPD positive tuberculin skin test; (5) Effective anti-tuberculosis therapy; and (6) Differential diagnosis: Choroidal inflammation caused by syphilis, toxoplasmosis and other systemic diseases was excluded by laboratory examination. At present, in terms of diagnosis, aqueous humor or vitreous fluid sampling from intraocular fluid is performed under topical anesthesia, which is easier to obtain than other tissue fluid such as lumbar puncture for cerebrospinal fluid and thoracic puncture for pleural effusion. The incidence of intraocular tuberculosis in patients with uveitis has been reported in the literature, including 6.9% in Japan, 4% in China, 10.5% in Saudi Arabia and 20% in India[7,8,10]. The detection method used in this report was molecular biology technology, and the proportion of intraocular tuberculosis diagnosed by polymerase chain reaction (PCR) detection of intraocular fluid is up to 20%. This indicates that the proportion of intraocular tuberculosis in uveitis infection increases with the improvement of examination methods.
Previous studies have found that the positive rate of the tuberculin skin test and chest X-ray in patients with confirmed ocular tuberculosis is only 40% and 57%, respectively[11]. The positive detection rate of chest CT was higher at 68.6%. Therefore, it is necessary to consider the general condition of suspected patients. In the general population, the infection rate of latent tuberculosis is very high. Under the existing conditions, the correct interpretation of tuberculate-related test results can improve the correct diagnostic rate of systemic tuberculosis and reduce the chance of missing the cause of tuberculosis in “idiopathic uveitis”[12]. PPD rhizomorph skin test and tuberculosis infected T cells are the two most basic methods to confirm previous tuberculosis[13]. The tuberculin test is a type of cellular immune response, low immunity will result in false positives, tuberculosis infected T cells is an immunology examination, it is not affected by immunity, and a positive result shows that the patient had a BCG vaccine or a previous tuberculosis infection. During tuberculosis infection, this test if positive is not significant, and should be based on the size of the value obtained, combined with the patient’s own and other imaging examination indicators following a comprehensive analysis.
Intraocular fluid Xpert and metagenomic sequencing, as emerging detection methods, can also help in the diagnosis of tuberculous uveitis. These detection techniques based on molecular biology and polymerase reaction technology, are able to quickly detect Mycobacterium tuberculosis and rifampicin resistance, they can trace the tissue fluid in patients following tuberculosis DNA extraction, amplification of ropB genes, and more than 95% rifampicin resistant strains with ropB gene mutations. Most rifampicin resistant strains are also resistant to isoniazid at the same time. Therefore, this test can not only detect rifampicin resistant strains, but also, to a certain extent, indicate whether there are multiple drug-resistant strains. In this case, tuberculosis bacillus DNA was detected in sputum and placenta using this method. mNGS of the intraocular fluid in this case, and Xpert was not only used to evaluate sputum and placental tissue, but also used to extract intraocular fluid from the anterior chamber aqueous humor. As there were only a few samples, only mNGS samples were sent for examination, which is a next-generation sequencing technology based on metagenomics and directly extracts the DNA or RNA of all microorganisms from clinical samples, and studies the genetic composition and community functions of all microorganisms contained in the samples using genomic research strategies. The positive rate of Mycobacterium tuberculosis in patients with systemic active tuberculosis complicated by uveitis is relatively high. Mycobacterium tuberculosis is an intracellular bacterium with a thick cell wall, which is difficult to detect using conventional detection methods. In this case, the cell-free DNA extraction and library construction process was used to reduce the loss and contamination during the process of wall breaking genome extraction and enzyme digestion interruption, and to reduce contamination of the human sequence, effectively improving the detection rate of difficult-to-detect pathogens[14]. In this case, four sequences of Mycobacterium tuberculosis were detected in aqueous humor. Zhou et al[15] reported that the sensitivity of mNGS for the diagnosis of active tuberculosis was 44%. They proposed that intracellular bacteria release less extracellular nucleic acids, resulting in a high false-negative rate of mNGS results. Biswas et al[16] reported that the sensitivity of intraocular fluid PCR detection was 33.33% in tuberculous retinal vasculitis and 66.67% in granulomatous uveitis. In this case, sputum and placental tissue were detected by Xpert, and intraocular fluid was detected by metagenomic sequencing. At present, the sensitivity of Xpert and metagenomic sequencing for intraocular fluid samples is unclear. The study showed that the detection sensitivity of metagenomic sequencing for all active tuberculosis cases was 44%, which was similar to that of Xpert (42%). The sensitivity can be increased to 60%[15].
For the purpose of intraocular fluid detection in this patient, on the one hand, it was clear that there were pathogens in the eye, and on the other hand, the turbid inflammatory cells were directly removed during the extraction of aqueous humor, and new aqueous humor was generated to replace the aqueous humor lost. The positive rate of mNGS tuberculosis is high in patients with ocular manifestations of vitreous haze and endophthalmitis, and extraocular manifestations of hematogenous disseminated tuberculosis. The positive rate of mNGS tuberculosis was relatively low in patients with ischemic retinal vasculitis, choroidal tuberculoma, and choroiditis. Possible reasons for this are as follows: (1) There is no active replication of Mycobacterium tuberculosis in the eye, and the disease manifestation is caused by a delayed hypersensitivity reaction to Mycobacterium tuberculosis; and (2) The pathogen is located at the chorioretinal level and not released into the vitreous body[17]. The aqueous humor or vitreous fluid with planktonic cells should be selected to improve the positive rate of intraocular fluid detection.
This article should remind tuberculous physicians that when systemic problems such as hematogenous disseminated tuberculous lesions and tuberculous meningitis are found, attention should be paid to the diagnosis of possible tuberculous eye diseases, in order to avoid missing the diagnosis and delayed treatment, resulting in blindness. In addition, infection in other parts of the body should be considered in the diagnosis and treatment of tuberculous eye disease, and molecular biology detection methods can be used to improve the detection rate, providing patients with an early diagnosis and standardized treatment.
This article introduces the use of intraocular fluid in the diagnosis of tuberculous uveitis, the application of molecular biology methods for diagnosis, and the recovery of visual acuity following treatment. These findings illustrate the importance of intraocular fluid in the diagnosis of tuberculous uveitis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Das Mohapatra SS, India S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Li X
| 1. | Krishna U, Ajanaku D, Denniston AK, Gkika T. Uveitis: a sight-threatening disease which can impact all systems. Postgrad Med J. 2017;93:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Zhang MX, Zhang JJ. [Diagnosis and treatment of choroidal tuberculosis]. Chin J Ophthalmol. 2012;48:4. [DOI] [Full Text] |
| 3. | Eurosurveillance editorial team. WHO publishes Global tuberculosis report 2013. Euro Surveill. 2013;18. [PubMed] |
| 4. | Wang JB, Zhang Q, Zhao N, Yu HL, Guan J. [A case of choroidal tuberculoma]. J Clin Ophthalmol. 2012;20:2. [DOI] [Full Text] |
| 5. | Liao MB, Shen JK. [Clinicopathological analysis of ocular tuberculosis (report of 6 cases)]. J Pract Ophthalmol. 1990;8:3. |
| 6. | Mercanti A, Parolini B, Bonora A, Lequaglie Q, Tomazzoli L. Epidemiology of endogenous uveitis in north-eastern Italy. Analysis of 655 new cases. Acta Ophthalmol Scand. 2001;79:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Wakabayashi T, Morimura Y, Miyamoto Y, Okada AA. Changing patterns of intraocular inflammatory disease in Japan. Ocul Immunol Inflamm. 2003;11:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Islam SM, Tabbara KF. Causes of uveitis at The Eye Center in Saudi Arabia: a retrospective review. Ophthalmic Epidemiol. 2002;9:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Lara LP, Ocampo V Jr. Prevalence of presumed ocular tuberculosis among pulmonary tuberculosis patients in a tertiary hospital in the Philippines. J Ophthalmic Inflamm Infect. 2013;3:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Abrahams IW, Jiang YQ. Ophthalmology in China. Endogenous uveitis in a Chinese ophthalmological clinic. Arch Ophthalmol. 1986;104:444-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Wroblewski KJ, Hidayat AA, Neafie RC, Rao NA, Zapor M. Ocular tuberculosis: a clinicopathologic and molecular study. Ophthalmology. 2011;118:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Peng XY, Mao Y. [The dilemma and countermeasure of diagnosis and treatment in tuberculous uveitis]. Ophthalmol China. 2019;28:325-327. |
| 13. | Trad S, Bodaghi B, Saadoun D. Update on Immunological Test (Quantiferon-TB Gold) Contribution in the Management of Tuberculosis-Related Ocular Inflammation. Ocul Immunol Inflamm. 2018;26:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Zeitz O, Keserü M. Kallikrein-kinin activation by altered vitreous pH: New perspectives for treatment and pathogenesis of diabetic macular edema? Comment on: Gao BB et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007 Feb; 13(2): 181-188. Graefes Arch Clin Exp Ophthalmol. 2007;245:1745-1747. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Zhou X, Wu H, Ruan Q, Jiang N, Chen X, Shen Y, Zhu YM, Ying Y, Qian YY, Wang X, Ai JW, Zhang WH. Clinical Evaluation of Diagnosis Efficacy of Active Mycobacterium tuberculosis Complex Infection via Metagenomic Next-Generation Sequencing of Direct Clinical Samples. Front Cell Infect Microbiol. 2019;9:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Biswas J, Madhavan HN, Gopal L, Badrinath SS. Intraocular tuberculosis. Clinicopathologic study of five cases. Retina. 1995;15:461-468. [PubMed] |
| 17. | Ramanjulu R, Dubey D, Shanmugam MP. Simultaneous mutually exclusive active tubercular posterior uveitis. Indian J Ophthalmol. 2020;68:2049-2050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |