Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2443
Peer-review started: November 23, 2022
First decision: February 2, 2023
Revised: February 9, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: April 16, 2023
Processing time: 134 Days and 2.3 Hours
Neonatal hyperbilirubinemia is one of the common diseases of newborns that typically presents with yellow staining of skin, resulting in sequelaes such as hearing loss, motor and intellectual development disorders, and even death. The pathogenic factors of neonatal hyperbilirubinemia are complex. Different cases of hyperbilirubinemia may have a single or mixed etiology.
To explore the etiological characteristics of severe hyperbilirubinemia in term newborns of eastern Guangdong of China.
Term newborns with severe hyperbilirubinemia in one hospital from January 2012 to December 2021 were retrospectively analyzed. The etiology was determined according to the laboratory results and clinical manifestations.
Among 1602 term newborns with hyperbilirubinemia in eastern Guangdong of China, 32.20% (580/1602) was severe hyperbilirubinemia. Among the causes of severe hyperbilirubinemia, neonatal hemolysis accounted for 15.17%, breast milk jaundice accounted for 12.09%, infection accounted for 10.17%, glucose-6-phosphate dehydrogenase (G6PD) deficiency accounted for 9.14%, and the coexistence of multiple etiologies accounted for 6.55%, unknown etiology accounted for 41.72%. ABO hemolysis and G6PD deficiency were the most common causes in the 20 cases with bilirubin encephalopathy. 94 severe hyperbilirubinemia newborns were tested for uridine diphosphate glucuronosyl transferase 1A1 (UGT1A1)*6 variant (rs4148323, c.211G>A, p.Arg71Gly), 9 cases were 211 G to A homozygous variant, 37 cases were 211 G to A heterozygous variant, and 48 cases were wild genotypes.
The main cause for severe hyperbilirubinemia and bilirubin encephalopathy in eastern Guangdong of China were the hemolytic disease of the newborns, G6PD deficiency and infection. UGT1A1 gene variant was also a high-risk factor for neonatal hyperbilirubinemia. Targeted prevention and treatment according to the etiology may reduce the occurrence of bilirubin encephalopathy and kernicterus.
Core Tip: The main cause for severe hyperbilirubinemia and bilirubin encephalopathy in eastern Guangdong of China were the hemolytic disease of the newborns, glucose-6-phosphate dehydrogenase deficiency and infection. UGT1A1 gene variant was also a high-risk factor for neonatal hyperbilirubinemia.
- Citation: Xu JX, Lin F, Wu YH, Chen ZK, Ma YB, Yang LY. Etiology analysis for term newborns with severe hyperbilirubinemia in eastern Guangdong of China. World J Clin Cases 2023; 11(11): 2443-2451
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2443.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2443
Neonatal hyperbilirubinemia is one of the common diseases of newborns, which is mainly caused by the imbalance between the production and elimination of bilirubin in the body[1]. It can be manifested as yellow staining of skin mucosa and sclera and usually resolves within 1 wk after birth, and most of neonates with jaundice have a good prognosis. However, if the total bilirubin level exceeds a certain threshold, it can lead to bilirubin encephalopathy, resulting in sequelaes such as hearing loss, motor and intellectual development disorders, and even death, which will bring a heavy burden to the society and family.
The pathogenic factors of neonatal hyperbilirubinemia are complex. Different cases of hyperbilirubinemia may have a single or mixed etiology. The etiological composition and related genetic variants of hyperbilirubinemia in different regions may be different, resulting in different degrees of hyperbilirubinemia in clinic. The American Academy of Pediatrics published the percentile curve of hourly total serum bilirubin (TSB) of newborns in venous blood, which defined bilirubin more than 95% percentile as hyperbilirubinemia and should be intervened[2]. According to the level of bilirubin and clinical symptoms, hyperbilirubinemia can be divided into significant hyperbilirubinemia (TSB < 342 μmol/L), severe hyperbilirubinemia (TSB ≥ 342 μmol/L), extreme hyperbilirubinemia (TSB ≥ 428 μmol/L), hazardous or critical hyperbilirubinemia (TSB ≥ 513 μmol/L), acute bilirubin encephalopathy, and kernicterus[3,4].
Glucose 6-phosphate dehydrogenase (G6PD) deficiency is one of the commonest human enzymopathies, with an estimated global prevalence of 4.9% that affects more than 500 million individuals worldwide[5]. The prevalence of G6PD deficiency in Chaozhou was 3.36%[6]. To date, 12 mutations have been reported in Chaozhou population, Canton (c.1376 G>T) and Kaiping (c.1388 G>A) were the most common variants[6]. Uridine diphosphate glucuronosyl transferase 1A1 (UGT1A1) is a rate-limiting enzyme in the process of bilirubin metabolism[7]. It catalyzes the glucuronic acid binding reaction and converts unconjugated bilirubin into conjugated bilirubin[8]. UGT1A1 (UGT1A1*6, c.211G>A, p.Arg71Gly, rs4148323), one of the most common UGT1A1 gene polymorphisms in the coding regions, was reported to be a risk factor for neonatal hyperbilirubinema[9].
Early detection of high-risk factors for severe hyperbilirubinemia and prompt treatment are crucial for the prevention of bilirubin encephalopathy. This study analyzed the clinical data of term newborns with severe hyperbilirubinemia retrospectively, to explore the etiology and clinical characteristics of severe hyperbilirubinemia in term newborns in Chaozhou, eastern Guangdong province, and to provide basis for clinical prevention and early treatment of hyperbilirubinemia.
All of term newborns with hyperbilirubinemia (gestational age > 37 wk) admitted to neonatal intensive care unit of Chaozhou Central Hospital from January 2012 to December 2021 were selected. The age of admission was 1-28 d. Demographic and clinical data were collected from the medical records of the studied participants. Hyperbilirubinemia was defined for bilirubin more than 95% percentile and TSB ≥ 342 μmol/L was used as the diagnostic standard for severe hyperbilirubinemia[3,4].
This study was initially approved by the Ethics Committee of Chaozhou Central Hospital in 2011 (No. 2011021), and then the second ethical approval was obtained in 2015 (No. 2015001). The data was collected retrospectively, which did not require informed consent form.
General information including sex, gestational age, birth weight, admission age and weight, mode of delivery, feeding pattern, and treatment were collected. The major clinical etiologies included hemolytic disease of the newborn (HDN) (ABO hemolysis and Rh hemolysis), G6PD deficiency (G6PD enzyme activity ≤ 2500 U/L, Co-Heath Beijing Laboratories Co., Ltd.), infection (sepsis, pneumonia, urinary tract infection), extravascular hemorrhage (intracranial hematoma, scalp hematoma and other bleeding), polycythemia (venous red blood cells > 6 × 1012/L, hemoglobin > 220 g/L, and hematocrit > 65%), congenital hypothyroidism (diagnosed by neonatal screening), insufficient breastfeeding (breastfeeding newborns with 10% birth weight loss after birth), and breast milk jaundice. Breast milk jaundice was diagnosed by exclusion. Therefore, it is not included in the combined factors. Prolonged jaundice was defined as jaundice persisting for more than 2 wk, with bilirubin level > 150 μmol/L. Bilirubin encephalopathy (hyperbilirubinemia and clinical symptoms of nervous system for encephalopathy), and blood exchange indications (blood exchange reference standard recommended by American Academy of Pediatrics in 2004.) were also recorded[2].
The whole blood samples of ethylenediamine tetraacetic acid anticoagulants in newborns with severe hyperbilirubinemia were prospectively collected after clinical diagnosis and stored at -40 °C in a biobank. The whole blood DNA extraction kit (Chaozhou Hybribio Co., Ltd.) was used to extract DNA according to the instructions of the kit, and then the DNA quantity and purity were tested (NanoDrop One, Thermo Fisher Scientific Co., Ltd.). MJ Mini Personal Thermal Cycler (Bio-Rad Company) was used for polymerase chain reaction amplification of G6PD, and then, G6PD gene variant was detected by reverse dot blot method (Chaozhou Hybribio Co., Ltd.) for 13 common G6PD mutation types c.95A>G (G6PD Gaohe), c.392G>T (G6PD Qing Yan), c.487G>A (G6PD Mahidol), c.493A>G (G6PD Taipei), c.592G>T (G6PD Coimbra), c.871G>A (G6PD Viangchan), c.1004C>T (G6PD Fushan), c.1024C>T (G6PD Chinese-5), c.1360C>T (G6PD Union), c.1376G>T (G6PD Canton), c.1387C>T (G6PD Keelung), c.1388G>A (G6PD Kaiping), c.1381G>A (G6PD Yunan) and one polymorphism c.1311C>T[10]. UGT1A1 gene was amplified and sequenced (3500Dx, Life Technologies) to detect c.211G>A variant (UGT1A1*6, rs4148323), which was specifically described in our previous study[10].
A total of 94 severe hyperbilirubinemia newborns were tested for UGT1A1*6 variant (rs4148323). The control 65 newborns for UGT1A1*6 test were the term newborns without hyperbilirubinemia, and with a TSB not requiring phototherapy, their clinical characteristics were described previously[11].
SPSS 20.0 was used for data analysis. The continuous variables in the data group were expressed as mean ± SD. Count data was described by frequency and percentage. The Pearson chi-square test was used, and P < 0.05 was considered as statistically significant.
A total of 1602 newborns with hyperbilirubinemia (915 males and 687 females) were collected in this study, 580 newborns (346 males and 234 females) were severe hyperbilirubinemia (32.20%, 580/1602). In these 580 cases, birth weight was 2050-5500 g, with an average of 3211.6 ± 444.53 g, gestational age was 37-42 wk, with an average of 39.07 ± 1.10 wk; the age of the highest bilirubin value was 1-28 d, with an average of 8 ± 5 d; 315 cases were vaginal delivery, 265 cases were cesarean section; 174 cases were breastfeeding, 165 cases were formula feeding, 195 cases were mixed feeding and 46 cases were unknown. The peak value of TSB was 342.05-817.8 μmol/L, with an average of 398.07 ± 55.09 μmol/L. Among them, 213 cases met the standard of blood exchange, 52 cases were prolonged jaundice (22 cases were breast feeding), and 20 cases were complicated with bilirubin encephalopathy (Table 1).
| Basic data | Cases |
| Sex | |
| Male | 346 (59.66) |
| Female | 234 (40.34) |
| Delivery pattern | |
| Caesarean section | 265 (45.69) |
| Vaginal delivery | 315 (54.31) |
| Feeding pattern | |
| Breast feeding | 174 (29.90) |
| Mixed feeding | 195 (33.62) |
| Formula feeding | 165 (28.45) |
| Unknown | 46 (7.93) |
| Proportion of exchange transfusion level | 213 (36.72) |
| Prolonged jaundice | 52 (8.97) |
| Bilirubin encephalopathy | 20 (3.45) |
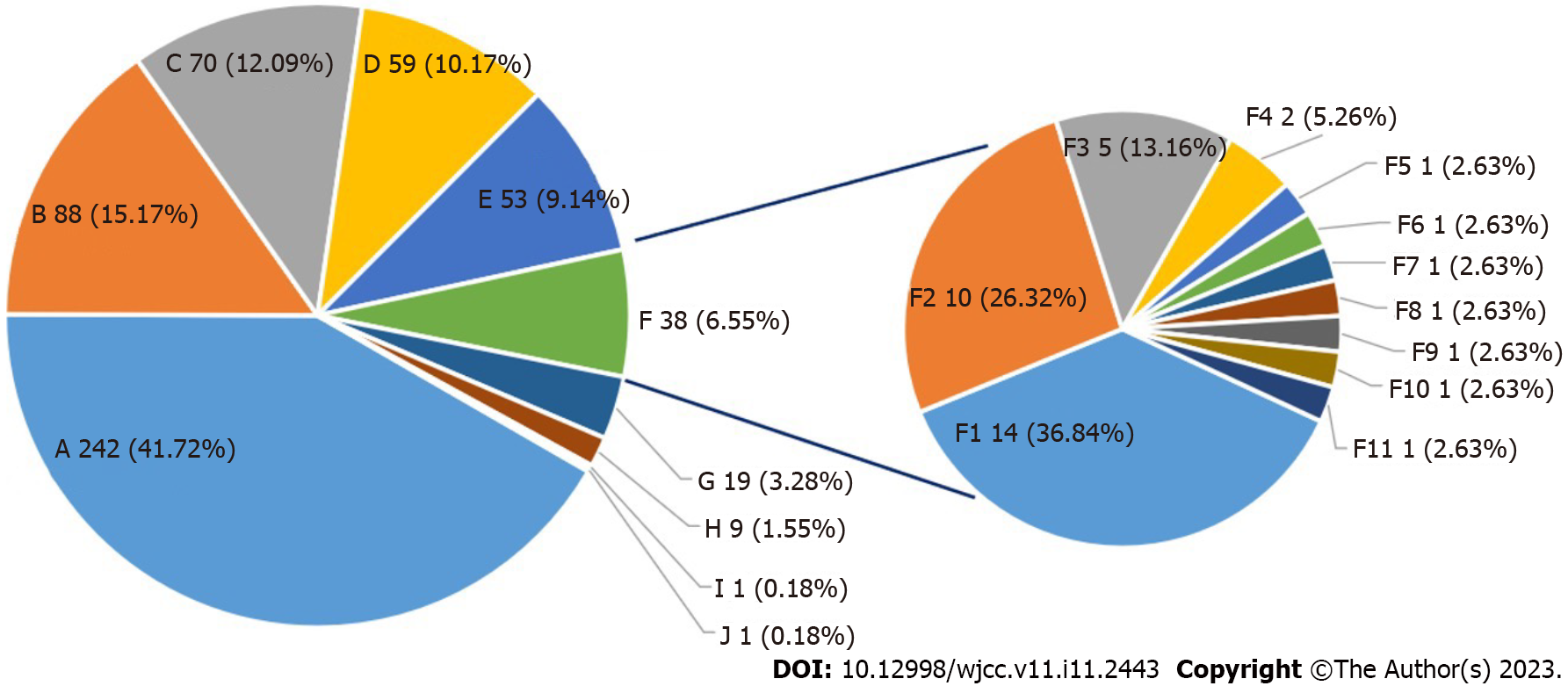
Among the cases of severe hyperbilirubinemia with clear etiology, the most common was neonatal hemolysis (15.17%), followed by breast milk jaundice (12.09%), infection (10.17%), G6PD deficiency (9.14%), and combined factors (6.55%), the etiology for the remaining 41.72% (242/580) cases was not clear. Among combined factors, neonatal hemolysis combined with infection (36.84%) was the most common, followed by G6PD deficiency combined with infection (26.32%) and infection combined with vascular bleeding (13.16%). In all cases of neonatal hemolysis, one case of Rh hemolysis was identified, the other 106 cases were ABO hemolysis (Figure 1).
Among 580 cases of severe hyperbilirubinemia, 68 cases were G6PD deficient by enzyme activity test, and 43 newborns were tested for 13 common G6PD gene mutations in Chinese[10]. Gene mutations were found in 40 cases, including 1 case c.95A>G (G6PD Gaohe), 1 case c.1024C>T (G6PD Chinese-5), 14 cases c.1376G>T (G6PD Canton), 23 cases c.1388G>A (G6PD Kaiping), and 1 case of composite mutation c.1311C>T combined with c.871G>A (G6PD Viangchan). No mutation was detected in the remaining 3 cases.
A total of 94 neonates with severe hyperbilirubinemia were selected to detect UGT1A1*6 variant (UGT1A1*6, c.211G>A, p.Arg71Gly, rs4148323). 48 cases were wild type, 37 cases were heterozygous variants, and 9 cases of homozygous variants were detected, with a gene variant frequency of 48.94 % (46/94). Among that 94 cases, a total of 18 cases of G6PD deficiency combined with UGT1A1 variants were found, including 2 cases of homozygous variants of UGT1A1*6 with c.1376G>T, 2 cases of homozygous variants of UGT1A1*6 with c.1388G>A, 4 cases of heterozygous variants of UGT1A1*6 with c.1376G>T, 10 cases of heterozygous variants of UGT1A1*6 with c.1388G>A (Table 2). Compared with our previous study on the UGT1A1 c.211G>A variant in 65 full-term newborns with normal bilirubin (Table 3)[11]. The proportion of c.211G>A variant in newborns with severe hyperbilirubinemia was higher than that in term neonates with normal bilirubin (P < 0.001). ABO hemolysis occurred in 34 of these 94 cases, and 10 cases (10.64%, 10/94) were ABO hemolysis combined with UGT1A1*6 variant.
| G6PD deficiency | G6PD normal | Total | P value | |
| G211A variation | ||||
| Wild type | 16 | 32 | 48 | 0.787 |
| c.211 hetereozygote | 14 | 23 | 37 | |
| c.211 homozygote | 4 | 5 | 9 | |
| Total | 34 | 60 | 94 |
Among 580 cases of severe hyperbilirubinemia, 20 cases were bilirubin encephalopathy, including 11 cases of male and 9 cases of female, and TSB bilirubin level was 359-817.8 μmol/L, with an average of 480.21 ± 64.19 μmol/ L. The main causes of bilirubin encephalopathy were ABO hemolysis (6 cases), followed by infection (sepsis in 1 case, pneumonia in 4 cases), G6PD deficiency (4 cases), unknown causes (3 cases), one case was ABO hemolysis combined with infection, and one case was ABO hemolysis combined with G6PD deficiency and infection.
Neonatal hyperbilirubinemia is one of the common diseases in neonatal period. The pathogenesis of severe neonatal hyperbilirubinemia is often multifactorial. In recent years, there have been many studies on the etiology and related risk factors of neonatal hyperbilirubinemia in China and abroad[12-14]. However, the etiology of hyperbilirubinemia is easily affected by environment and other relative factors. Therefore, the analysis of the etiology of neonates with severe hyperbilirubinemia in eastern Guangdong is of great significance to reduce the incidence of severe hyperbilirubinemia and its serious complications in this region.
Our study found that 32.20% of the 1602 hospitalized newborns with hyperbilirubinemia were severe hyperbilirubinemia. HDN was the most common cause of severe hyperbilirubinemia in term newborns. ABO blood group incompatibility is one of the most common causes of HDN which often occurs in infants of blood type A or B with mothers of blood type O. The blood type antibody in mother combines with the corresponding antigen on the surface of fetal red blood cells during pregnancy. Macrophages and natural killer cells in newborn destroy sensitized red blood cells, resulting in hemolysis, jaundice and anemia after birth[15].
A total of 68 cases of G6PD deficiency was identified (11.72%, 68/580) in this study, 53 cases were single G6PD deficiency and 15 cases were combined factor with other causes. The incidence of G6PD deficiency was relatively high, which might be related to the geographical location of Chaozhou. The prevalence of G6PD deficiency in Chaozhou was 3.36%[6]. The prevalence of G6PD deficiency in severe hyperbilirubinemia group was higher than that of the general population. G6PD deficiency causes increased susceptibility of erythrocytes to H2O2 and other reactive oxygen species that can lead to hemolytic anemia, and in neonatal hyperbilirubinemia cases, it even results in neonatal kernicterus. G6PD deficiency is an important reason for severe hyperbilirubinemia in term newborns. Of the 68 cases with G6PD deficiency, 11 cases were combined with infection. Infection was the main inducing cause of acute hemolysis in G6PD deficiency[16]. We performed gene analysis in 43 cases of G6PD deficiency and found that c.1388G>A and c.1376G>T were the most common variants in severe hyperbilirubinemia neonates. The bilirubin level in severe hyperbilirubinemia caused by hemolysis is high, and it occurs early and develops rapidly. In addition, neonatal blood-brain barrier is not fully matured, bilirubin encephalopathy and even death are prone to occur.
Infection was another cause of severe hyperbilirubinemia (10.17%). Hyperbilirubinemia caused by infection may be due to the imperfect immune function of newborns and lack of mature defense ability, and the neonate is easily to be infected by viruses and bacteria. The destruction of the erythrocyte membrane by the pathogen themselves or secreted toxins, results in hemolysis and increased bilirubin production. At the same time, infection can cause liver dysfunction, inhibit the activity of UGT1A1, and reduce the ability of liver to deal with bilirubin. Hyperbilirubinemia may be the only manifestation of infection[17]. Because of the low positive rate of blood culture, and late diagnosis for infection, the bilirubin may increase and progress to severe hyperbilirubinemia.
Breast milk jaundice is a clinical diagnosis made after excluding the known causes of hyperbilirubinemia. At present, there is still a lack of reliable laboratory testing methods to get this diagnosis. Breast milk jaundice can be divided into early breastfeeding jaundice and late breast milk jaundice[18]. In our study, 9 cases (1.55%) of early breastfeeding jaundice and 70 cases (12.07%) of late breast milk jaundice were identified. Early breast feeding deficiency leads to insufficient calorie intake, reduced intestinal peristalsis, and increased bilirubin enterohepatic circulation, resulting in early hyperbilirubinemia, which is manifested by a weight loss of 10% within one week after birth. Late breast milk jaundice occurs relatively late, and the bilirubin generally peaks on the 7th to 14th days of life. The jaundice can last for 2-3 wk or even 2-3 mo, which may be caused by many factors[19].
The variant of UGT1A1 c.211G>A was an important cause of breast milk jaundice in newborns, and the serum bilirubin level of breast milk jaundice newborns with this variant was significantly higher than that of breast milk jaundice newborns without this variant[20,21]. This indicated that the presence of UGT1A1 c.211G>A variant could promote breast milk jaundice. The product encoded by UGT1A1 gene is bilirubin uridine diphosphate glucuronyltransferase. UGT1A1 variant make the activity of this enzyme reduced, so that bilirubin cannot combine with glucuronide to form conjugated bilirubin. The unconjugated bilirubin accumulates in the body, and resulting in different degrees of hyperbilirubinemia[22,23].
The UGT1A1 c.211G>A was a risk factor for neonatal severe hyperbilirubinemia in eastern Guangdong, which might occur with other causes simultaneously. Among the 94 cases with UGT1A1 variant data, we found that 18 cases were UGT1A1 gene variant combined with G6PD deficiency, 10 cases were UGT1A1 gene variant combined with ABO hemolysis. Our previous study found that, in the newborns with hyperbilirubinemia which due to G6PD deficiency, bilirubin level showed an increasing trend with the accumulation of UGT1A1 c.211G>A variants[10]. Yang et al[24] reported that the risk of neonatal severe hyperbilirubinemia increased significantly when ABO hemolytic neonates were combined with UGT1A1 gene mutation (especially 211A>G homozygous mutation).
The causes for 41.2% of severe hyperbilirubinemia cases were unknown in our study. Previous studies have shown that mutations of other genes, such as heme oxygenase-1, biliverdin reductase A, and solute carrier organic anion transporter family member 1B1 could also affect the serum bilirubin[25]. For severe hyperbilirubinemia neonates with unknown etiology, due to limited conditions, we did not perform gene detection for all cases, resulting in some missing causes. For neonates with unknown etiology, except for UGT1A1 variants, other gene variants might contribute to increased bilirubin. Pathological jaundice, especially severe jaundice, is often the result of multiple influencing factors. Detection of bilirubin-related genes such as G6PD and UGT1A1 in neonates can be used as an auxiliary test for clinicians to assess the risk of neonatal severe hyperbilirubinemia, which will benefit for early prevention, etiological exploration and genetic counseling.
The main causes of severe hyperbilirubinemia in Chaozhou are neonatal hemolysis and infection. Genetic factors such as UGT1A1 variant and G6PD deficiency, were also the important risk factors for neonatal severe hyperbilirubinemia. It is very important to clarify the etiology and to treat neonatal severe hyperbilirubinemia timely, which can reduce the occurrence of acute bilirubin encephalopathy and kernicterus.
Neonatal hyperbilirubinemia is one of the common diseases of newborns, the pathogenic factors of neonatal hyperbilirubinemia in eastern Guangdong is not clear.
To understand the etiology for severe neonatal hyperbilirubinemia in Chaozhou.
To analyze the pathological factors contributing to severe hyperbilirubinemia in neonates.
The clinical data were retrospectively collected and genetic variants of glucose-6-phosphate dehydrogenase (G6PD) and UGT1A1 were determined by polymerase chain reaction and sequencing.
Among the causes of severe hyperbilirubinemia, neonatal hemolysis accounted for 15.17%, breast milk jaundice accounted for 12.09%, infection accounted for 10.17%, G6PD deficiency accounted for 9.14%, and the coexistence of multiple etiologies accounted for 6.55%, unknown etiology accounted for 41.72%. ABO hemolysis and G6PD deficiency were the most common causes in the 20 cases with bilirubin encephalopathy. 94 severe hyperbilirubinemia newborns were tested for UGT1A1*6 variant (rs4148323, c.211G>A, p.Arg71Gly), 9 cases were 211 G to A homozygous variant, 37 cases were 211 G to A heterozygous variant, and 48 cases were wild genotypes.
The main causes for severe hyperbilirubinemia and bilirubin encephalopathy in eastern Guangdong of China were the hemolytic disease of the newborns, G6PD deficiency and infection. UGT1A1 gene variant was also a high-risk factor for neonatal hyperbilirubinemia.
Multiple genetic variants may regulate the bilirubin levels in neonate, these gene analysis may contribute to the prognosis and management for neonatal jaundice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali A, Malaysia; Huang CS, Taiwan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Kaplan M, Muraca M, Hammerman C, Rubaltelli FF, Vilei MT, Vreman HJ, Stevenson DK. Imbalance between production and conjugation of bilirubin: a fundamental concept in the mechanism of neonatal jaundice. Pediatrics. 2002;110:e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1710] [Cited by in RCA: 1639] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 3. | Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Chinese Multicenter Study Coordination Group for Neonatal Hyperbilirubinemia. [Predictive value of hour-specific transcutaneous bilirubin nomogram for neonatal hyperbilirubinemia: a national multicenter study]. Zhonghua Er Ke Za Zhi. 2015;53:830-834. [PubMed] |
| 5. | Luzzatto L, Ally M, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Blood. 2020;136:1225-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 6. | Lin F, Wu J, Yang H, Lin M, Yang L. [Molecular epidemiology of G6PD deficiency in Chaozhou area of eastern Guangdong Province]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016;33:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Lin F, Lou ZY, Xing SY, Zhang L, Yang LY. The gene spectrum of glucose-6-phosphate dehydrogenase (G6PD) deficiency in Guangdong province, China. Gene. 2018;678:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Bentivegna A, Santambrogio J, Clerici M. UGT1A1 mutations and psychoses: towards understanding the relationship with unconjugated bilirubin. CNS Spectr. 2021;26:188-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Wang J, Yin J, Xue M, Lyu J, Wan Y. Roles of UGT1A1 Gly71Arg and TATA promoter polymorphisms in neonatal hyperbilirubinemia: A meta-analysis. Gene. 2020;736:144409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Xu JX, Lin F, Chen ZK, Luo ZY, Zhan XF, Wu JR, Ma YB, Li JD, Yang LY. Co-inheritance of G6PD deficiency and 211 G to a variation of UGT1A1 in neonates with hyperbilirubinemia in eastern Guangdong. BMC Pediatr. 2021;21:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Yang H, Wang Q, Zheng L, Zheng XB, Lin M, Zhan XF, Yang LY. Clinical Significance of UGT1A1 Genetic Analysis in Chinese Neonates with Severe Hyperbilirubinemia. Pediatr Neonatol. 2016;57:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Zhou J, Yang C, Zhu W, Chen S, Zeng Y, Wang J, Zhao H, Chen Y, Lin F. Identification of Genetic Risk Factors for Neonatal Hyperbilirubinemia in Fujian Province, Southeastern China: A Case-Control Study. Biomed Res Int. 2018;2018:7803175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Mei H, Dong X, Wu B, Wang H, Lu Y, Hu L, Wang J, Cao Y, Zhang R, Cheng G, Wang L, Li Z, Yang L, Zhou W. Clinical and Genetic Etiologies of Neonatal Unconjugated Hyperbilirubinemia in the China Neonatal Genomes Project. J Pediatr. 2022;243:53-60.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Osuorah CDI, Ekwochi U, Asinobi IN. Clinical evaluation of severe neonatal Hyperbilirubinaemia in a resource-limited setting: a 4-year longitudinal study in south-East Nigeria. BMC Pediatr. 2018;18:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Christensen RD, Baer VL, MacQueen BC, O'Brien EA, Ilstrup SJ. ABO hemolytic disease of the fetus and newborn: thirteen years of data after implementing a universal bilirubin screening and management program. J Perinatol. 2018;38:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Harcke SJ, Rizzolo D, Harcke HT. G6PD deficiency: An update. JAAPA. 2019;32:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Omar C, Hamza S, Bassem AM, Mariam R. Urinary tract infection and indirect hyperbilirubinemia in newborns. N Am J Med Sci. 2011;3:544-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Gartner LM. Breastfeeding and jaundice. J Perinatol. 2001;21 Suppl 1:S25-9; discussion S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Bratton S, Cantu RM, Stern M. Breast Milk Jaundice. 2022 Sep 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] |
| 20. | Maruo Y, Morioka Y, Fujito H, Nakahara S, Yanagi T, Matsui K, Mori A, Sato H, Tukey RH, Takeuchi Y. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Sato H, Uchida T, Toyota K, Kanno M, Hashimoto T, Watanabe M, Nakamura T, Tamiya G, Aoki K, Hayasaka K. Association of breast-fed neonatal hyperbilirubinemia with UGT1A1 polymorphisms: 211G>A (G71R) mutation becomes a risk factor under inadequate feeding. J Hum Genet. 2013;58:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Sarici SU, Saldir M. Genetic factors in neonatal hyperbilirubinemia and kernicterus. Turk J Pediatr. 2007;49:245-249. [PubMed] |
| 23. | Yang LY. Underestimation of the contribution of 211 G to A variation in UGT1A1 to neonatal hyperbilirubinemia in China. J Pediatr. 2022;245:251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 24. | Yang H, Lin F, Chen ZK, Zhang L, Xu JX, Wu YH, Gu JY, Ma YB, Li JD, Yang LY. UGT1A1 mutation association with increased bilirubin levels and severity of unconjugated hyperbilirubinemia in ABO incompatible newborns of China. BMC Pediatr. 2021;21:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Lin R, Wang Y, Fu W, Zhang D, Zheng H, Yu T, Shen M, Lei R, Wu H, Sun A, Zhang R, Wang X, Xiong M, Huang W, Jin L. Common variants of four bilirubin metabolism genes and their association with serum bilirubin and coronary artery disease in Chinese Han population. Pharmacogenet Genomics. 2009;19:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |