Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2650
Peer-review started: November 4, 2021
First decision: December 27, 2021
Revised: January 4, 2022
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 16, 2022
Processing time: 126 Days and 11.2 Hours
It now seems that all pulmonary hamartomas (PHs) are large cystic-solid lesions that are difficult to diagnose. However, few cases of large cystic-solid PHs have been reported. The present case report presents a large cystic-solid PH and provides a literature review of the imaging features, formation mechanism and histopathological basis of PHs.
A 53-year-old woman with no clinical symptoms underwent a chest computed tomography (CT) examination at our hospital. Nonenhanced CT images revealed a large, flat tumor with multiple air-containing cysts in the left thoracic cavity and a cystic part confined to the medial side of the tumor; the solid part of the tumor showed abundant fat and lamellar soft tissue components. Multiple small blood vessels were detected in the solid part of the tumor on contrast-enhanced CT images. Given the large size of the lesion, the patient elected to undergo surgery. Histological examination revealed PH. A detailed review of the patient’s CT imaging showed that the lesion had a small vascular pedicle to the left lower lobe, which was a clue to its lung tissue histological origin. According to immunohistochemical staining, the confined multiple air-containing cysts were caused by the entrapment of respiratory/alveolar epithelium.
This case shows the imaging manifestations of a large PH. Heightened awareness of its formation mechanism and histopathological basis may alert radiologists to consider this diagnosis in their daily workflow.
Core Tip: We describe a large pulmonary hamartoma (PH) and its preoperative computed tomography (CT) imaging features, including multiple air-containing cysts, a rich blood supply and a vascular pedicle. The CT imaging features, formation mechanism, and histopathological basis of a large PH are summarized in this case report.
- Citation: Guo XW, Jia XD, Ji AD, Zhang DQ, Jia DZ, Zhang Q, Shao Q, Liu Y. Large cystic-solid pulmonary hamartoma: A case report. World J Clin Cases 2022; 10(8): 2650-2656
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2650.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2650
Pulmonary hamartoma (PH) has been defined as a mesenchymal tumor consisting of varying combinations of cartilage, fibrous tissue, fat, smooth muscle, and respiratory epithelium derived from entrapped adjacent lung tissue[1]. It is the most common benign neoplasm and usually presents as solitary nodules in the lung. However, PH can show unusual characteristics and can be clinically and radiologically challenging to diagnose preoperatively. In addition, PHs larger than 10 cm and containing multiple air-containing cysts are rare. In this case report, we present a rare case of a large PH with multiple air-containing cysts. We aim to increase the awareness of its formation mechanism, histopathological basis, and computed tomography (CT) imaging features through a literature review. This diagnosis should be considered in the daily workflow to improve the accuracy of the preoperative diagnosis of this disease.
A 58-year-old woman who had never undergone a chest CT examination had a CT scan as part of a routine physical examination. Her medical history was negative for any symptoms of discomfort.
The patient’s history was unremarkable.
The patient underwent a hysterectomy for myoma 4 years prior. The patient had hypertension for 10 years, but her blood pressure was stable under drug control.
No personal and family history.
No abnormal positive indications were found in physical examination.
The blood biochemistry results were normal. Pulmonary function testing, arterial blood gas evaluation and electrocardiogram results were normal.
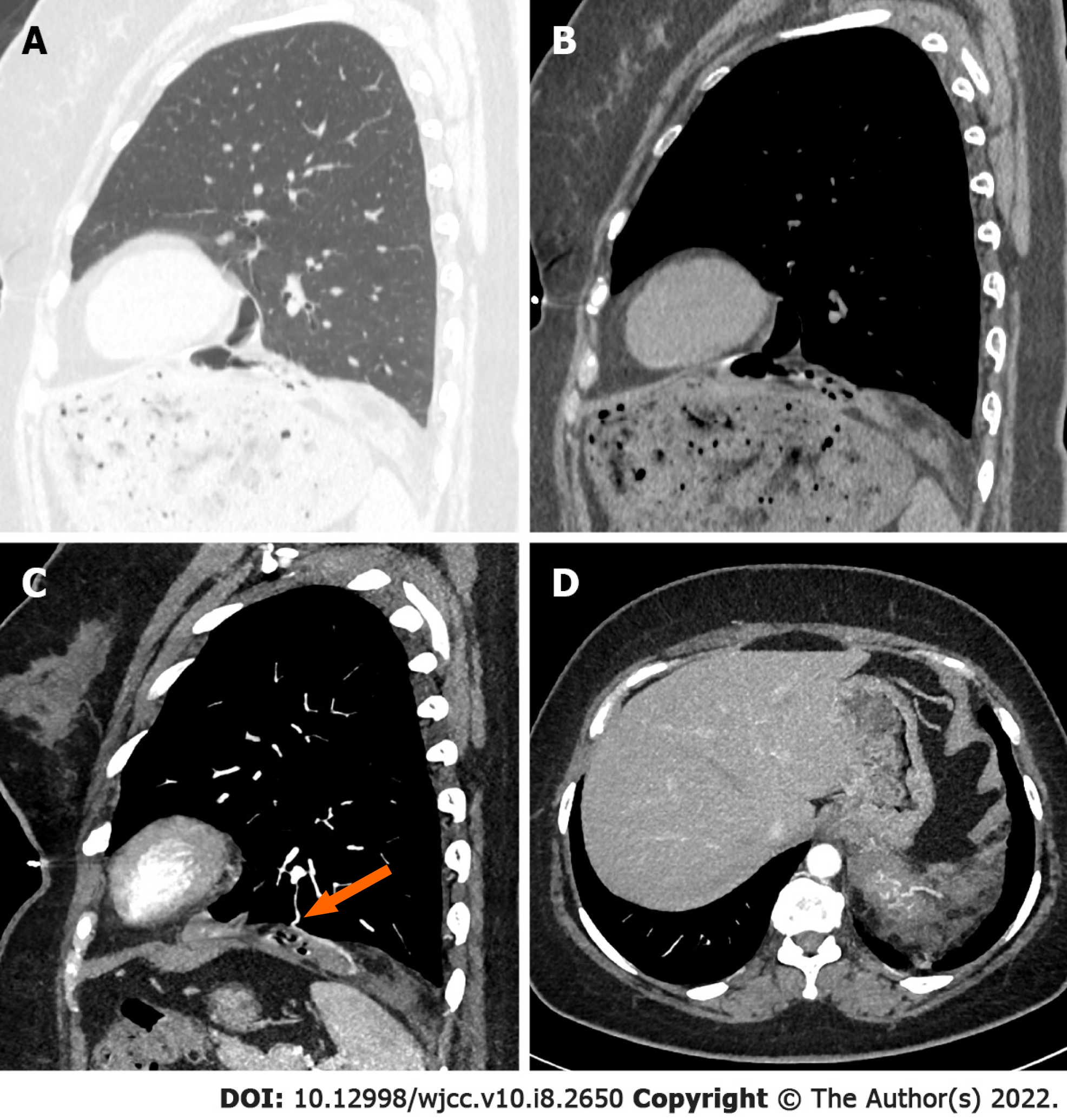
Initial nonenhanced chest CT images revealed a well-defined tumor with multiple air-containing cysts confined to the medial side of the tumor, and the solid part of the tumor showed abundant fat and lamellar soft tissue components. The tumor was well defined except for a locally unclear boundary with the left lower lung lobe (Figure 1A and B). Further contrast-enhanced chest CT examination showed multiple small blood vessels in the solid part of the tumor, and several blood supplies to the tumor were detected coming from the left lower lobe (Figure 1C and D).
The final diagnosis after histological confirmation was a large PH (Figure 2).
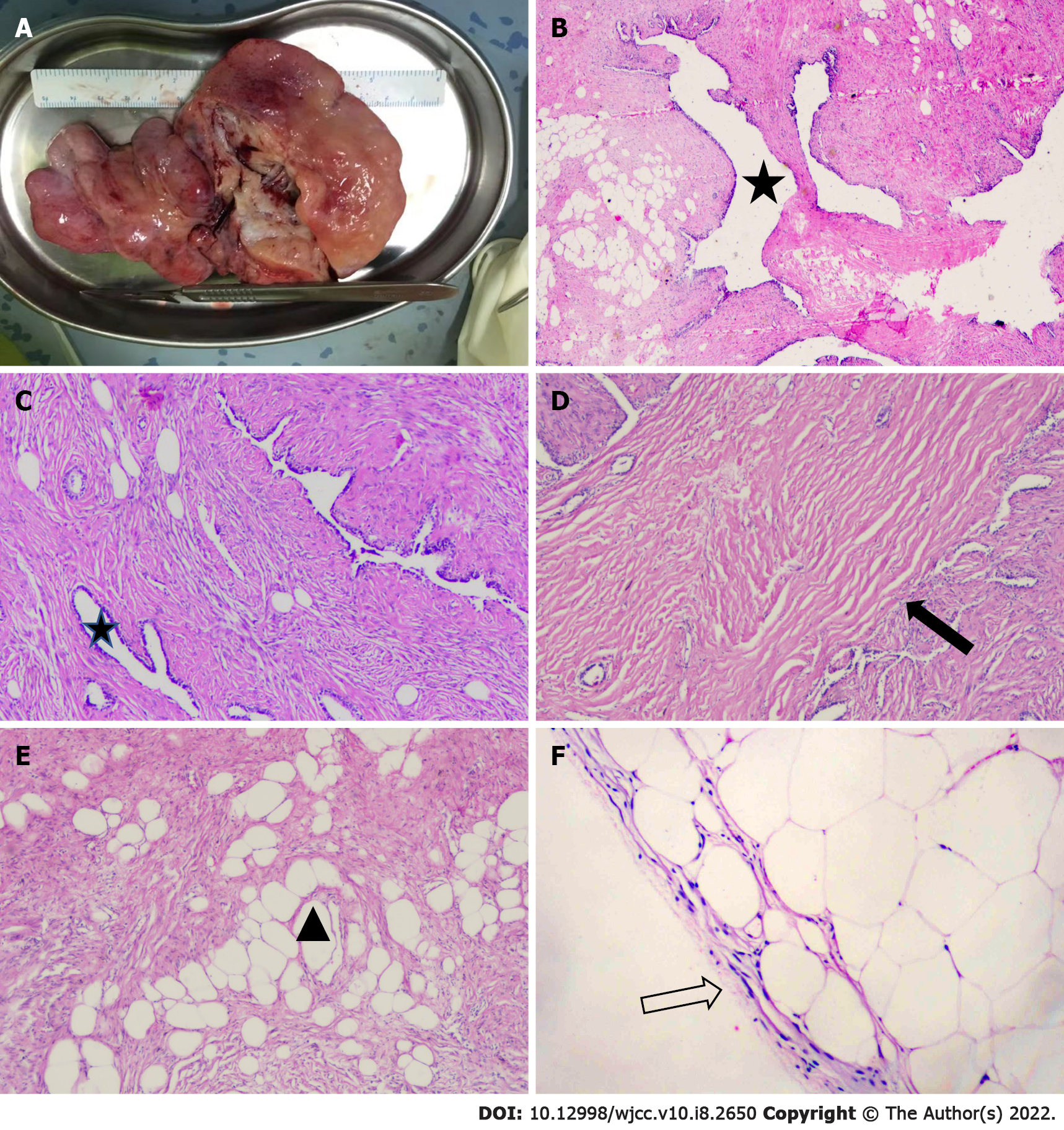
Single-hole exploratory video-assisted thoracoscope surgery was performed. There was no adhesion between the tumor and the lung tissue, except for a thin vascular pedicle connecting the tumor to the left lower lobe. The pedicle was dissected, and the tumor was completely removed. Gross examination showed a soft and flat-shaped tumor measuring 14.5 cm × 11.0 cm × 2.5 cm in size (Figure 2A). The multiple cystic components within the tumor were confined to one side, and the diameter of the cysts ranged from 1 cm to 3.5 cm.
Electron microscopy suggested that the well-developed epithelium lacked significant cytological atypia in the cystic part. Other parts had mesenchymal components, including fat, connective tissue and smooth muscle (Figure 2B-E). Immunohistochemical staining of the tumor was consistent with the components of normal lung tissue. Smooth muscle cells were observed in the tumor (SMA +) and were positive for desmin. Ciliated respiratory epithelium that lined clefts tested positive for thyroid transcription factor-1, napsin A and cytokeratin 7, and basal cells located within these epithelia tested positive for S-100, which indicated that these epithelia represented entrapped bronchioles and alveolar walls. Immunostaining with HMB45 was negative. The proliferation index Ki67 was low (< 5%). The patient recovered well after surgery, and no obvious abnormality has been found by chest CT examination at annual follow-ups thus far.
PH is the most common benign tumor of the lung. It is relatively easy to make a preoperative diagnosis of PH with typical CT imaging findings, such as a well-defined nodule with a size of less than 2 cm, popcorn-like calcification and a fat density component. Large PHs over 10 cm are unusual, and large cystic-solid PHs are even rarer. The final diagnosis of a large cystic-solid PH depends on postoperative pathology. The most common cause of these cysts is entrapped pulmonary epithelium. Although entrapment of the pulmonary epithelium by PH is well known, in our experience, the CT imaging features of this phenomenon have not received sufficient attention. We decided to review the literature on cystic-solid PHs, analyze their CT imaging features, formation mechanism and histopathological basis, and then discuss the sources of the challenges during preoperative diagnosis.
To our knowledge, only eleven cases of cystic-solid PHs have been reported thus far, of which 6 PHs were larger than 10 cm. The reason for the cyst formation is still unclear. Nevertheless, the literature focusing on this issue is sparse. According to the study of Erber et al[2], the entrapment of respiratory epithelium in primary and metastatic intrapulmonary nonepithelial neoplasms is a frequent morphological pattern but to variable extents. Their study involved 38 patients with pulmonary metastases (81%) and 8 patients with primary pulmonary nonepithelial lesions. There are two types of histological distribution of the entrapped pulmonary epithelium. In type one, the entrapped pulmonary epithelium is distributed mainly in the peripheral portion of the tumor, and in type two, the entrapped pulmonary epithelium is found throughout the tumor, albeit to a varying extent. Although the number of patients was limited, we thought this conclusion could be extrapolated to more primary and metastatic intrapulmonary nonepithelial neoplasms in the lungs. Because PH is the most common form of primary pulmonary nonepithelial lesions, the same applies to our case. Different types of histological distributions of entrapped pulmonary epithelium produce different CT images. Type one represents the histopathological basis of the cysts in the present case. The entrapped pulmonary epithelium was located at the margin of the tumor and connected to the adjacent lung tissue by a vascular pedicle. In this type, the cysts are dilated bronchioles lined by clear epithelial cells with adjacent spindle cell stroma. It has been speculated that a check-valve mechanism of the bronchioles of the entrapped pulmonary epithelium causes cysts to form[3]. A thin pedicle comprised of blood vessels and bronchioles between the tumor and the left lower lobe was found in the present case during surgery. The present case is the first large cystic-solid PH in which a vascular bronchial pedicle was found during the operation.
The abovementioned type two histological distribution represents the basis of cysts in cystic-solid PH cases where no clear pedicle between the tumor and the lung tissue is found during surgery. The cysts in such PHs are the result of growth coupled with degenerative changes, which ultimately lead to cleft-like spaces or ultimately expand into cysts[4]. Compared with type one lesions, type two lesions showed a mixed distribution of solid and cystic lesions without obvious boundaries on imaging. Such imaging findings of PH significantly increase the difficulty of preoperative diagnosis, and the final diagnosis depends on pathology and immunohistochemistry.
Notably, varying degrees of fluid are observed in the cysts of cystic-solid PHs. According to a previous study[2], glands in the entrapped pulmonary epithelium frequently show a reactive/ regenerative appearance. Furthermore, gland size and type vary greatly from small acinar-type glands or microcystic spaces lined by flattened epithelial cells and containing mucoid secretion to branching leaflet-like papillary spaces. All of the factors mentioned above result in differences in epithelial secretory function. Therefore, in previous case reports, various degrees of fluid were observed in the cysts of cystic-solid PHs: The cysts may be well inflated[5-9] or partially[3] or even completely filled with fluid[10-12].
In addition, through a literature review, we found that the CT image density of cystic-solid PHs can vary from ground glass density to solid density depending on the proportion of the solid part. In some cases, the proportion of solid components in cystic-solid PHs is very low, and cystic-solid PHs show extreme CT imaging, that is, a ground glass nodule appearance[12]. It is difficult to distinguish cystic-solid PHs from adenocarcinomas, which often present as ground glass nodules, and the final diagnosis depends on postoperative pathology. The other extreme case is that if the cystic-solid PH is dominated by the solid part, the cystic part may be too small to be observed on CT imaging[13].
Previous studies have demonstrated a high frequency of rearrangements involving 6p21 or 12q14-15 in PH[14] and HMGI-C and HMGI(Y) protein expression as a consequence of rearrangements involving 6p21 and 12q15[15]. These findings support the view that mesenchymal components of PHs represent neoplastic mesenchymal proliferation rather than neoplasms. Today, even with advancements in medical therapy, pulmonary resection remains the most important treatment measure for patients with PH[16,17]. However, controversy exists about the indication for surgery. For large cysts dominated by cystic-solid PHs, although malignant transformation of PHs is exceptional, prompt surgical resection is the recommended treatment. The main reasons are as follows. First, larger cystic-solid PHs are often located under the visceral pleura, similar to the present case, and separated from the thoracic cavity by only a thin layer of pleura (Figure 2F), so the cystic part is more vulnerable to rupture and can lead to secondary pneumothorax[3,18]. In addition, Secretions into the cysts of cystic-solid PHs are difficult to expel from the lungs and may lead to secondary infection. The patients involved in the present case and in the large cystic-solid PH cases discussed above had very good prognoses with uneventful outcomes after surgery.
Due to its epithelial involvement, clinicians and radiologists should be aware that cystic-solid PH is a diagnostic possibility in adults with large intrathoracic cystic-solid tumors. Cysts in PHs can show different features on CT images depending on the type of histological distribution of the entrapped pulmonary epithelium. If large cysts dominating cystic-solid PHs are treated in a timely manner after discovery, the patient will have a good prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bos S, Esteban-Zubero E, Roudi R S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Yamada R, Tonooka A, Horiguchi SI, Motoi T, Horio H, Hishima T. An unusual case of pulmonary hamartoma with predominant bronchial mucous glands in the peripheral lung. Pathol Int. 2018;68:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Erber R, Haller F, Hartmann A, Agaimy A. Prominent entrapment of respiratory epithelium in primary and metastatic intrapulmonary non-epithelial neoplasms: a frequent morphological pattern closely mimicking adenofibroma and other biphasic pulmonary lesions. Virchows Arch. 2020;477:195-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Saadi MM, Barakeh DH, Husain S, Hajjar WM. Large multicystic pulmonary chondroid hamartoma in a child presenting as pneumothorax. Saudi Med J. 2015;36:487-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Miura K, Hori T, Yoshizawa K, Hamaguchi N, Morita J. Cystic pulmonary hamartoma. Ann Thorac Surg. 1990;49:828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Greenspon A, Samuels L, Greenspon L. Extralobar Sequestration Complicated by a Cystic Hamartoma in an Adult. Ann Thorac Surg. 2019;108:e43-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Ozyurtkan MO, Dağli AF, Cakmak M, Balci AE. Multiple cystic pulmonary chondroid hamartomas colonized by Aspergillus species: report of a case. Surg Today. 2011;41:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Kim GY, Han J, Kim DH, Kim J, Lee KS. Giant cystic chondroid hamartoma. J Korean Med Sci. 2005;20:509-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Saxena P, Downie S, Amanuel B, Newman M, Konstantinov IE. Giant pulmonary hamartoma: an interesting clinico-pathologic entity. Heart Lung Circ. 2010;19:573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Shi H, Niu ZX, Peng J, Yang YS, Chen LQ. Successful removal of a giant pulmonary hamartoma coexisting with an anomalous common pulmonary venous trunk. J Thorac Dis. 2015;7:E23-E27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Borghesi A, Tironi A, Benvenuti MR, Bertagna F, De Leonardis MC, Pezzotti S, Bozzola G. Pulmonary hamartoma mimicking a mediastinal cyst-like lesion in a heavy smoker. Respir Med Case Rep. 2018;25:133-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Argemi X, Santelmo N, Matau C, Croce S, Weingertner N. Cystic Lung Lesions Mimicking Hydatidosis: Hamartoma or Benign Metastazing Leiomyoma? Ann Thorac Surg. 2019;108:e195-e198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Huang CC, Sheu CY, Tzen CY, Huang WC. Pulmonary hamartoma mimicking primary bronchoalveolar cell carcinoma. Thorax. 2012;67:187-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Ozbudak IH, Dertsiz L, Bassorgun CI, Ozbilim G. Giant cystic chondroid hamartoma of the lung. J Pediatr Surg. 2008;43:1909-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Fletcher JA, Pinkus GS, Donovan K, Naeem R, Sugarbaker DJ, Mentzer S, Pinkus JL, Longtine J. Clonal rearrangement of chromosome band 6p21 in the mesenchymal component of pulmonary chondroid hamartoma. Cancer Res. 1992;52:6224-6228. [PubMed] |
| 15. | Tallini G, Vanni R, Manfioletti G, Kazmierczak B, Faa G, Pauwels P, Bullerdiek J, Giancotti V, Van Den Berghe H, Dal Cin P. HMGI-C and HMGI(Y) immunoreactivity correlates with cytogenetic abnormalities in lipomas, pulmonary chondroid hamartomas, endometrial polyps, and uterine leiomyomas and is compatible with rearrangement of the HMGI-C and HMGI(Y) genes. Lab Invest. 2000;80:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Guo W, Zhao YP, Jiang YG, Wang RW, Ma Z. Surgical treatment and outcome of pulmonary hamartoma: a retrospective study of 20-year experience. J Exp Clin Cancer Res. 2008;27:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Esme H, Duran FM, Unlu Y. Surgical treatment and outcome of pulmonary hamartoma: a retrospective study of 10-year experience. Indian J Thorac Cardiovasc Surg. 2019;35:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Stella F, Davoli F, Brandolini J, Dolci G, Sellitri F, Fiorentino M, Bini A. A rare case of giant cystic chondroid hamartoma of the lung presenting with left side pneumothorax. Minerva Chir. 2009;64:117-119. [PubMed] |