Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1738
Peer-review started: October 16, 2021
First decision: November 17, 2021
Revised: November 29, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: February 16, 2022
Processing time: 118 Days and 2.2 Hours
Neurothekeomas (NTKs) are rare benign soft tissue tumours that typically occur in the head, trunk, and upper limbs and are rare in other parts of the body.
Herein, we present two rare cases in which primary NTKs were located in the hallux and axilla. A 47-year-old woman complained of a verrucous bulge on the plantar side of the left hallux. The surface skin of the tumour was abraded due to poor wound healing. A 6-year-old boy complained of a gradually growing subcutaneous mass in the axilla. The tumours of both patients were completely resected, and the diagnosis of NTK was confirmed by histopathology. At the one-year follow-up, both patients had a good prognosis without local recurrence.
To date, NTKs located in the hallux and axilla have rarely been reported in the literature. We describe NTKs that occurred in unconventional areas and summa
Core Tip: In these patients, the lack of specificity of clinical symptoms and imaging examination findings as well as the unusual location of neurothekeomas increased the difficulty in diagnosis and treatment. Histopathological examination and immunohistochemical staining may help confirm the diagnosis, but there are still many challenges in the identification of similar diseases.
- Citation: Huang WY, Zhang YQ, Yang XH. Neurothekeoma located in the hallux and axilla: Two case reports. World J Clin Cases 2022; 10(5): 1738-1746
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1738.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1738
Neurothekeomas (NTKs) are rare, benign, superficial soft tissue tumours that typically present as solitary nodules with a predilection for the head, neck, and upper limbs of females[1,2]. Due to the low prevalence and undefined clinical symptoms of NTKs, it is difficult to accurately distinguish them from other skin tumours. NTKs rarely occur in the lower limbs or axillae and have been reported only once in the areas of the toes and axillae[3,4]. In this report, we describe two different types of NTKs arising in the hallux of a 47-year-old female and the axilla of a 6-year-old boy. Both patients underwent surgical resection, and the final diagnosis was confirmed through histopathological examination.
Case 1: A 47-year-old woman complained of a painless, verrucous bulge on the plantar side of the left hallux for 3 years.
Case 2: A 6-year-old boy visited our hospital and complained of a gradually increasing subcutaneous mass in the axilla for 2 years.
Case 1: The verrucous mass appeared on the plantar side of the left hallux three years previously, and the surface skin of the tumour was abraded due to poor wound healing. Inflammatory granulation tissue formation was observed in the wound. The patient intermittently adhered to conservative treatment, but her condition was not relieved.
Case 2: The subcutaneous mass was found in the axilla two years previously, and the colour of the mass was the same as that of the normal skin. The mass was only 1 cm in diameter when it was first discovered but gradually grew to 2 cm within two years.
Case 1: The patient was diagnosed with tuberculous pleurisy 20 years previously and was cured, and she underwent uterine fibroid surgery 1 year previously.
Case 2: The patient did not complain of any prior specific symptoms.
Cases 1 and 2: Both patients denied any history of smoking, drinking, or drug abuse. Underlying systemic disease and family genetic history were denied.
Case 1: The patient's general condition was stable with normal vital signs (body temperature 36.8 °C, blood pressure 140/80 mmHg, pulse 110 bpm). A red solid mass with a diameter of 0.8 cm was found on the plantar side of the left hallux with a tough texture, normal skin temperature, and good dorsal artery pulsation.
Case 2: The patient's general condition was stable with normal vital signs (body temperature 36.7 °C, blood pressure 95/65 mmHg, pulse 103 bpm). A subcutaneous mass with a diameter of 2 cm was observed in the left armpit with good mobility, normal surface skin colour and temperature, and mild palpable pain. The superficial lymph nodes were not enlarged.
Case 1: Laboratory tests revealed signs of inflammation in the urinary system, and the percentage of neutrophils (73.9%) in blood and white blood cell (99.6/μL) and bacterial (1014.5/μL) counts in urine were slightly elevated.
Case 2: No obvious abnormality was noted in the laboratory examination results.
Case 1: An ultrasound from the local hospital showed a solid nodule on the plantar side of the left hallux with abundant blood supply.
Case 2: An ultrasound from the local hospital showed a round, well-demarcated soft tissue mass in the left armpit with nosignificant alterations in the surrounding tissue.
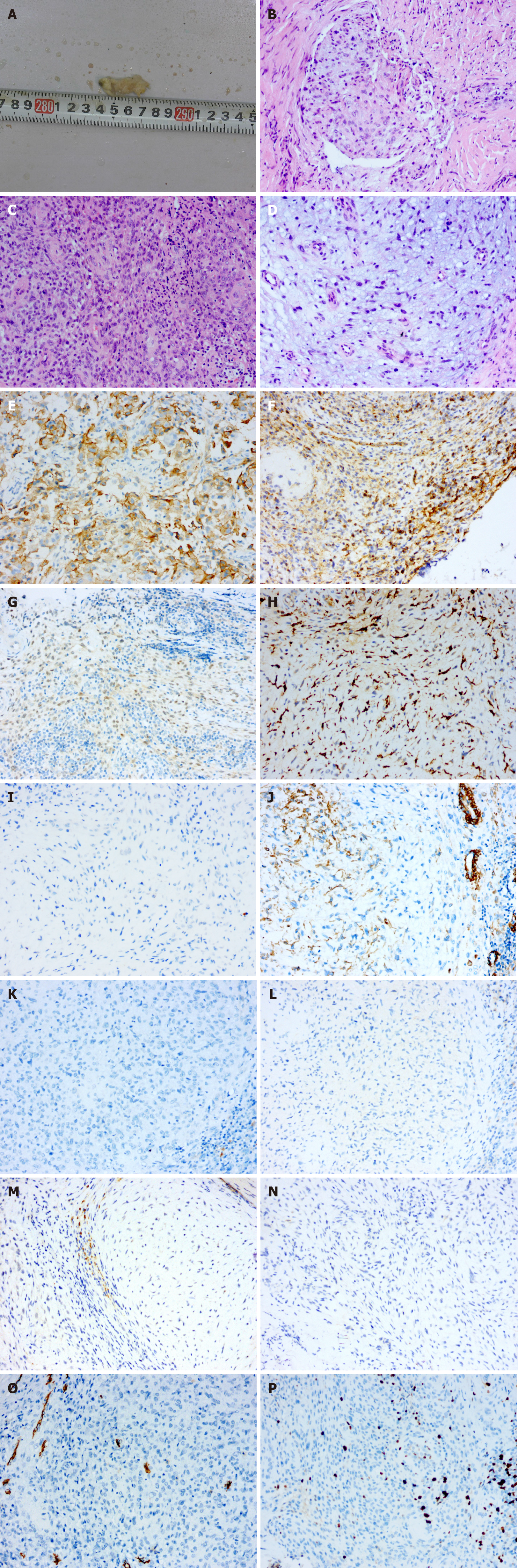
Case 1: In general, the red, solid, verrucous mass was approximately 0.8 cm in diameter and had a tough texture (Figure 1A). Histopathological examination of the specimen showed that the tumour tissue was in the form of multiple small nodules or clusters. The nodules, which were composed of oval and spindle tumour cells, were abundant in some areas and sparse in other areas. In the cellular area, oval cells were relatively uniform in size with a rich and eosinophilic cytoplasm, a visible nucleolus, and a mild to moderate degree of mitotic activity. In the intermediate area, spindle cells were arranged in bundles and exhibited a benign morphology. Myxoid matrix could be observed in the nodules or interstitium (approximately 40%) (Figure 1B-D). Immunohistochemical examination revealed positive staining for CD10, CD99, transcription factor binding to IGHM enhancer-3 (TFE3) and CD163, indicating NTK (Figure 1E-H). Negative staining for S-100, cytokeratin (CK), epithelial membrane antigen, smooth muscle actin (SMA), desmin, Stat6, anaplastic lymphoma kinase (ALK), and neuron-specific enolase (NSE) can be helpful in differential diagnosis, as this profile distinguishes NTKs from other soft tissue tumours such as dermal nerve sheath myxomas (DNSMs), smooth muscle cell-derived tumours, solitary fibrous tumours, epithelioid fibrous histiocytomas (EFHs), and neuroblastomas (Figure 1I-N). CD34 staining suggested vascular hyperplasia, and the Ki-67 proliferation index was approximately 20% (Figure 1O and P).
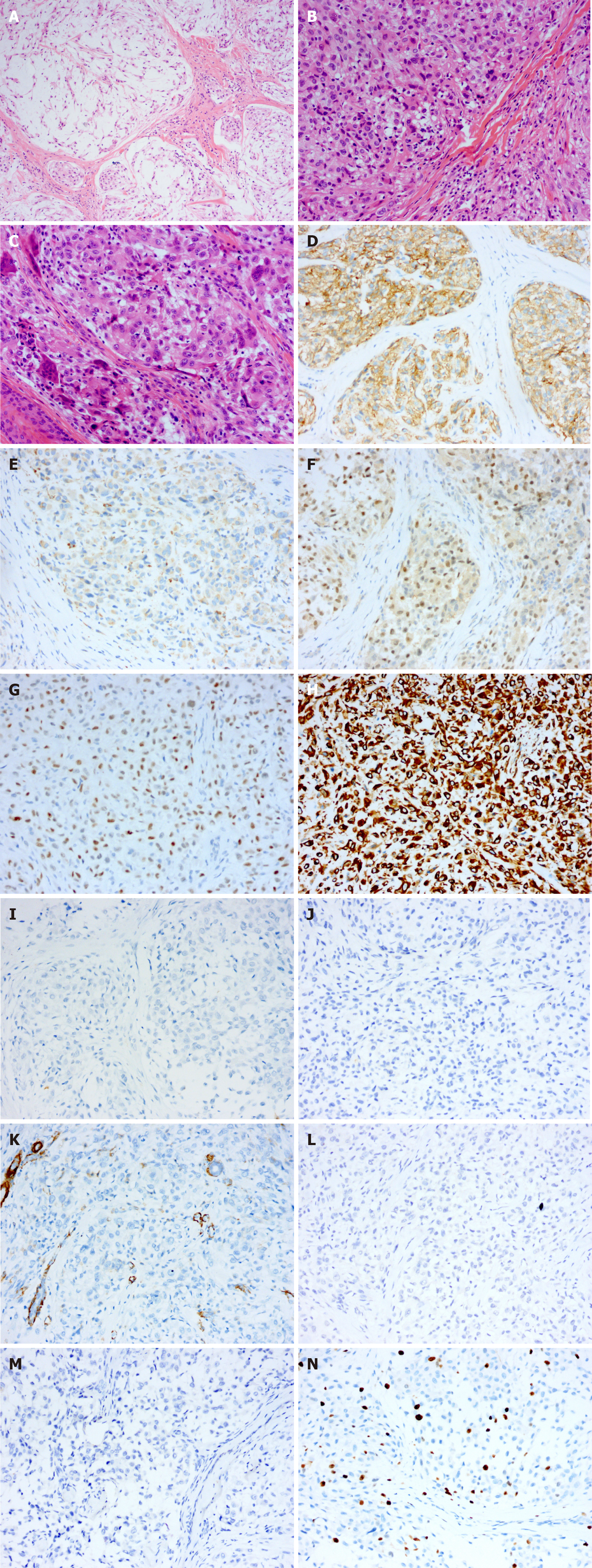
Case 2: Histopathological examination of the specimen showed that the tumour tissue was composed of multiple small nodules, and the nodules were separated by hyalinized collagen fibres (Figure 2A). The nodules were composed mainly of uniformly sized eosinophilic oval cells, in which nucleoli and mitosis were observed. A small number of multinucleated giant cells infiltrated the nodules, and no myxoid matrix was observed in the interstitium (Figure 2B and C). Immunohistochemical examination revealed positive staining for CD10, CD68, TFE3, p63 and vimentin (Figure 2D-H) and negative staining for S-100, CK, SMA, glial fibrillary acidic protein (GFAP) and CD1a (Figure 2I-M). The Ki-67proliferation index was approximately 15% (Figure 2N).
NTK, mixed subtype (left hallux).
Cellular NTK (left axilla).
Surgical treatment was performed with local infiltration anaesthesia. The 0.8-cm tumour was located in the superficial layer of the flexor tendon and had an incomplete capsule. The tumour was completely resected and submitted for pathological examination.
Surgical treatment was performed with local infiltration anaesthesia. The solid, well-defined, 2-cm tumour was located in the subcutaneous soft tissue of the armpit and seemed to lack a defined capsule. After complete removal of the tumour and complete haemostasis, the incision was sutured.
At the 12-mo follow-up, both patients had maintained a favourable postoperative clinical evolution without local pain or motion limitation. The surgical incisions had healed well, and neither patient showed signs of recurrence or metastasis.
NTKs are rare, benign soft tissue tumours that were first described by Gallager and Helwig in 1980[5]. NTKs were initially considered to be neurogenic tumours originating from Schwann cells, and NTKs were diagnosed and reported for many years as one of the subtypes of dermal nerve sheath myxomas[6]. Recently, studies have shown that, unlike DNSMs, NTKs do not express the S-100 protein[7]. Further analysis of gene expression profiles shows that DNSMs are similar to schwannomas, while NTKs show evidence of myofibroblastic differentiation and possible relation to dermatofibromas[8]. Therefore, NTK was classified as an independent disease for diagnosis. NTKs clinically manifest primarily as painless, slow-growing subcutaneous nodules with good mobility. Clinical diagnosis of these rare neoplasms is challenging because NTKs are not distinctive in physical examinations and imaging examinations. NTK is often mistaken for a sebaceous cyst, a Spitz naevus, a fibrous histiocytoma, a basal cell carcinoma, or a skin adnexal tumour (mainly pilomatricoma)[9,10], and an accurate diagnosis depends on histopathological and immunohistochemical examination.
Histopathologically, NTK is a poorly circumscribed nodule typically composed of fascicles of spindle-shaped and epithelioid tumour cells with a sparse or no mucinous matrix[10]. Epithelioid cells, which present as oval or polygonal eosinophilic cells, are rich in the cytoplasm and arranged in a nodular or plexiform pattern; spindle-shaped cells are arranged in bunches or swirls and have a benign morphology. The stroma is composed of hyalinized collagen fibres often accompanied by mucoid degeneration. Depending on the amount of myxoid matrix, NTKs have been subclassified into three types: myxoid (myxoid matrix > 50%), mixed (10% < myxoid matrix ≤ 50%) and cellular (myxoid matrix < 10%). The myxoid matrix can occasionally superficially infiltrate skin or muscle tissue. The nuclei can show mild to moderate atypia, and approximately 0-25 mitotic nuclei per high power field can be observed.
Immunohistochemical examination demonstrates that tumour cells express CD10, CD63 (NKI/C3), and mitf. Most tumours also express CD99. A few tumours express SMA and are negative for S-100, cytokeratin, Melan-A, and SOX-10 staining[11,12], indicating that NTKs are associated with scattered histiocytes.
Accurate diagnosis of NTKs is essential given that these lesions can be mistaken for malignancies, leading to unnecessary treatment. The differential diagnosis includes mainly various types of cysts, DNSMs, plexiform fibrous histiocytomas (PFHs), EFHs, Spitz nevi, etc. The clinical manifestations of these diseases are similar, but the pathological characteristics are different: subcutaneous cysts contain keratinized or necrotic material without tumour cells; DNSMs express S-100, GFAP and SOX10, as assessed by immunohistochemistry[13]; PFHs have spindle-shaped fibroblasts around the nodular-arranged tumour cells[14]; EFH tumour cells are mostly diffusely arranged and do not express CD63[15]; and Spitz nevi are composed of epithelioid or spindle-shaped pigment cells and express S-100[16]. The differential diagnosis of these diseases is shown in Table 1.
| NTK | DNSM | PFH | EFH | Spitz nevus | |
| Average age | Adult/teenager | Adult | Teenager | Adult/teenager | Teenager/adult under 35 |
| Sex | Female | Both | Female | Female | Female |
| Predilection site | Head/upper limbs/trunk | Finger/lower limbs | Upper limbs/trunk | Lower limbs | Face/head/lower limbs |
| Tumour boundary | Blurred | Clear | Clear | Clear | Clear |
| Arrangement of tumour cells | Lobular/nodular/clump/whirlpool like | Lobular/nodular | Nodular/clump | Mosaic or whirlpool-like | Nested |
| Tumour cell morphology | Round/oval/spindle | Round/oval/spindle | Round/oval/spindle | Round/oval | Polygon/spindle |
| Atypia of tumour cells | Mild-moderate | Rare | Rare | Rare | Rare |
| Mitosis of nucleus | 0-25/WHPF | - | Rare | Rare | Rare |
| Immunohistochemical phenotype | CD10(+); CD63(+); mitf(+) | S-100(+); GFAP(+); SOX10(+) | S-100(+); vimentin(+); lysozyme(+) | ALK(+); TFE3(+); S-100(-) | S-100(+); HMB-45(+) |
Herein, we report two rare NTKs; both were completely resected after clinical evaluation, and an accurate diagnosis was obtained after the histopathological and immunohistochemical examination. In addition, we provide a summary of the differential diagnosis and the possible diagnostic pitfalls. The diagnostic and therapeutic experience reported here can be used as a reference for other surgeons and patholo
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME, Vij M S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Lau SK, Cassarino DS, Koh SS. Multiple myxoid cellular neurothekeomas in a patient with systemic lupus erythematosus. J Cutan Pathol. 2021;48:980-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Massimo JA, Gasibe M, Massimo I, Damilano CP, De Matteo E, Fiorentino J. Neurothekeoma: Report of two cases in children and review of the literature. Pediatr Dermatol. 2020;37:187-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Wiemeyer S, Hafer G. Neurothekeoma of the toe. Foot Ankle Spec. 2013;6:479-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Silva CMM, Fontenele JPU, Lopes JR, de Brito GCC, Teixeira MJD, Rocha FAC. Neurothekeoma in the Axilla Causing Persistent Shoulder Pain: Case Report. Rev Bras Ortop (Sao Paulo). 2020;55:804-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Cavicchini S, Guanziroli E, Del Gobbo A, Scaparro M, Gianotti R. Neurothekeoma, a hard to diagnose neoplasm among red nodules. Australas J Dermatol. 2018;59:e280-e282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Vetrano IG, Levi V, Pollo B, Chiapparini L, Messina G, Nazzi V. Sleeve-Shaped Neurothekeoma of the Ulnar Nerve: A Unique Case of a Still Unclear Pathological Entity. Hand (N Y). 2020;15:NP7-NP10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Abuawad YG, Saraiva MI, Westin AT, Valente NY. S-100 negative myxoid neurothekeoma: a new type of neurothekeoma? An Bras Dermatol. 2017;92:153-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Abdaljaleel M, North JP. Positive MITF and NKI/C3 Expression in Cellular Neurothekeoma and Dermatofibroma. Appl Immunohistochem Mol Morphol. 2021;29:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Fetsch JF, Laskin WB, Hallman JR, Lupton GP, Miettinen M. Neurothekeoma: an analysis of 178 tumors with detailed immunohistochemical data and long-term patient follow-up information. Am J Surg Pathol. 2007;31:1103-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Tran P, Mclemore M. Atypical cellular neurothekeoma: A potential diagnostic pitfall for benign and malignant spindle cell lesions in skin. J Cutan Pathol. 2018;45:619-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Murphrey M, Huy Nguyen A, White KP, Krol A, Bernert R, Yarbrough K. Pediatric cellular neurothekeoma: Seven cases and systematic review of the literature. Pediatr Dermatol. 2020;37:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | See TRO, Stålhammar G, Grossniklaus HE. Neurothekeoma of the eye, conjunctiva, and periorbital adnexa: A report of two cases and brief review. Surv Ophthalmol. 2019;64:852-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Khashaba H, Hafez E, Burezq H. Nerve Sheath Myxoma: A rare tumor, a case report and literature review. Int J Surg Case Rep. 2020;73:183-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Ghuman M, Hwang S, Antonescu CR, Panicek DM. Plexiform fibrohistiocytic tumor: imaging features and clinical findings. Skeletal Radiol. 2019;48:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Dickson BC, Swanson D, Charames GS, Fletcher CD, Hornick JL. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol. 2018;31:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Harms KL, Lowe L, Fullen DR, Harms PW. Atypical Spitz Tumors: A Diagnostic Challenge. Arch Pathol Lab Med. 2015;139:1263-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |