Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1729
Peer-review started: October 15, 2021
First decision: December 1, 2021
Revised: December 5, 2021
Accepted: December 28, 2021
Article in press: December 28, 2021
Published online: February 16, 2022
Processing time: 118 Days and 20 Hours
Diffuse invasive signet ring cell carcinoma of the colorectum is extremely rare clinically. This type of colorectal cancer has certain clinical, pathological and biological characteristics that are different from ordinary colorectal cancer.
A 31-year-old young woman was admitted to the hospital for nearly 1 wk due to recurrent symptoms of mucopurulent bloody stools and abdominal distension. Preoperative colonoscopy showed a ring-shaped intestinal wall mass 10 cm from the rectum to the anus. Three pieces of tumor tissue were removed for examination. The pathological results showed rectal mucinous adenocarcinoma. The patient underwent laparoscopic exploration under general anesthesia, and then laparoscopic total colorectal resection, ileal pouch–anal anastomosis and ileostomy were performed. The patient was switched to a FOLFOX + cetuximab regimen. After the fifth cycle, the patient was unable to tolerate further treatment due to tumor progression and multiple organ dysfunction, and died at the end of May 2020. Overall survival was 7 mo.
Carcinogenesis of ulcerative colitis is different from sporadic colon cancer, and the overall prognosis is extremely poor.
Core tip: Primary signet-ring cell carcinoma (SRCC) of the colorectum is extremely rare clinically. This type of colorectal cancer has certain clinical, pathological and biological characteristics that are different from ordinary colorectal cancer. We report a rare case of ulcerative colitis leading to diffuse infiltrating SRCC of the colorectum, and review the relevant literature studying the disease.
- Citation: Zhang Z, Yu PF, Gu GL, Zhang YH, Wang YM, Dong ZW, Yang HR. Diffuse invasive signet ring cell carcinoma in total colorectum caused by ulcerative colitis: A case report and review of literature. World J Clin Cases 2022; 10(5): 1729-1737
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1729.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1729
Ulcerative colitis (UC) is a chronic nonspecific disease that is immune mediated and has multiple causes[1]. The disease was previously thought to be prevalent in western countries, with a prevalence of about 79–268/105 per year[2]; however, the number of cases reported in China has gradually increased in recent years, and it has become a more common gastrointestinal disease. The inflammation of the disease occurs mostly in the colonic mucosa and submucosa, usually involving the rectum first, then spreading to the entire colon. Typical clinical manifestations of the disease include mucopurulent bloody stools, abdominal pain, and diarrhea. A retrospective analysis of a large sample of cases of UC in China in 2007 found that extraintestinal manifestations were rare, causing only 0.4% of cases of colon cancer[3], and about 15% of patients with UC required colectomy[4]. In this paper, we report a rare case of UC leading to diffuse infiltrating signet ring cell carcinoma (SRCC) of the colorectum, and review the relevant literature.
A 31-year-old young woman presented with bloody stools.
The patient was admitted to the hospital for nearly 1 wk due to recurrent symptoms of mucopurulent bloody stools and abdominal distension.
The patient presented to the local hospital 8 years ago with symptoms of mucopurulent bloody stools and abdominal distension. After colonoscopy, she was diagnosed with UC. After taking prednisone acetate tablets orally for > 1 mo and sulfasalazine enteric coated tablets orally for about 1 year according to the doctor’s advice, the symptom of bloody stools was largely controlled, and no review or further treatment was performed.
The patient had no specific history of genetic diseases.
The whole abdomen had mild tenderness and no rebound pain. Digital rectal examination: No obvious mass was palpable on the fingertips, and the fingertips were stained with blood.
Tumor markers: carcinoembryonic antigen: 16.03 ng/mL↑, cancer antigen 72-4: 17.94 U/mL↑. The patient underwent genetic testing before surgery (gene capture hybridization combined with high-throughput sequencing technology). Reference genome: GRCH37/hg19. The number of target genes exceeded 20000. The results are shown in Tables 1 and 2. A test found that the patient’s tumor mutational burden (TMB) was 26.2/Mb. A high TMB type that suggested that the patient was more likely to benefit from PD-1 antibody monotherapy.
| Mutant gene | Abundance (%) | Exon | cDNA | Protein | Type |
| MTOR | 0.61 | 47 | c.6617A>G | p.N2206S | Non-synonymous |
| HRAS | 0.85 | 2 | c.81T>C | p.H27H | Synonymous |
| SLCO1B1 | 0.79 | 6 | c.597C>T | p.F199F | Synonymous |
| AKT1 | 0.57 | 3 | c.103T>C | p.F35L | Non-synonymous |
| TP53 | 0.5 | 8 | c.840A>G | p.R280R | Synonymous |
| STK11 | 0.51 | 4 | c.524A>T | p.K175M | Non-synonymous |
| 0.51 | 4 | c.530T>C | p.I177T | Non-synonymous | |
| XRCC1 | 0.66 | 10 | c.1196A>G | p.Q399R | Non-synonymous |
| VHL | 13.95 | 2 | c.T355C | p.F119L | Non-synonymous |
| XRCC1 | 10.34 | 6 | c.C580T | p.R194W | Non-synonymous |
| Gene | Mutation detection results |
| KRAS | Wild |
| NRAS | Wild |
| BRAF | Wild |
| NTRK | Not detected |
| BAT25 | Not detected |
| BAT26 | Not detected |
| NR21 | Not detected |
| NR24 | Not detected |
| NR27 | Not detected |
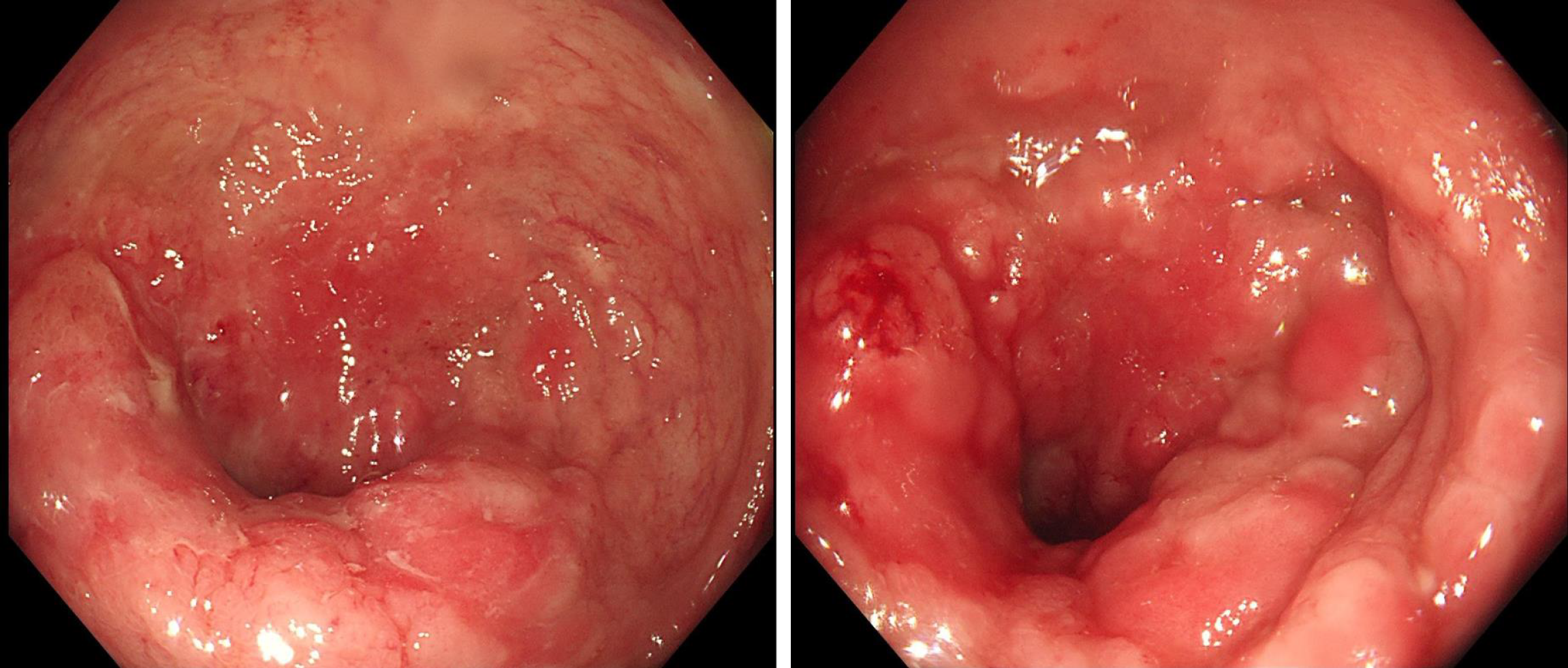
Preoperative colonoscopy: A ring-shaped intestinal wall mass was seen 10 cm from the rectum to the anus (Figure 1). Three pieces of tumor tissue were removed for examination. The pathological results showed rectal mucinous adenocarcinoma.
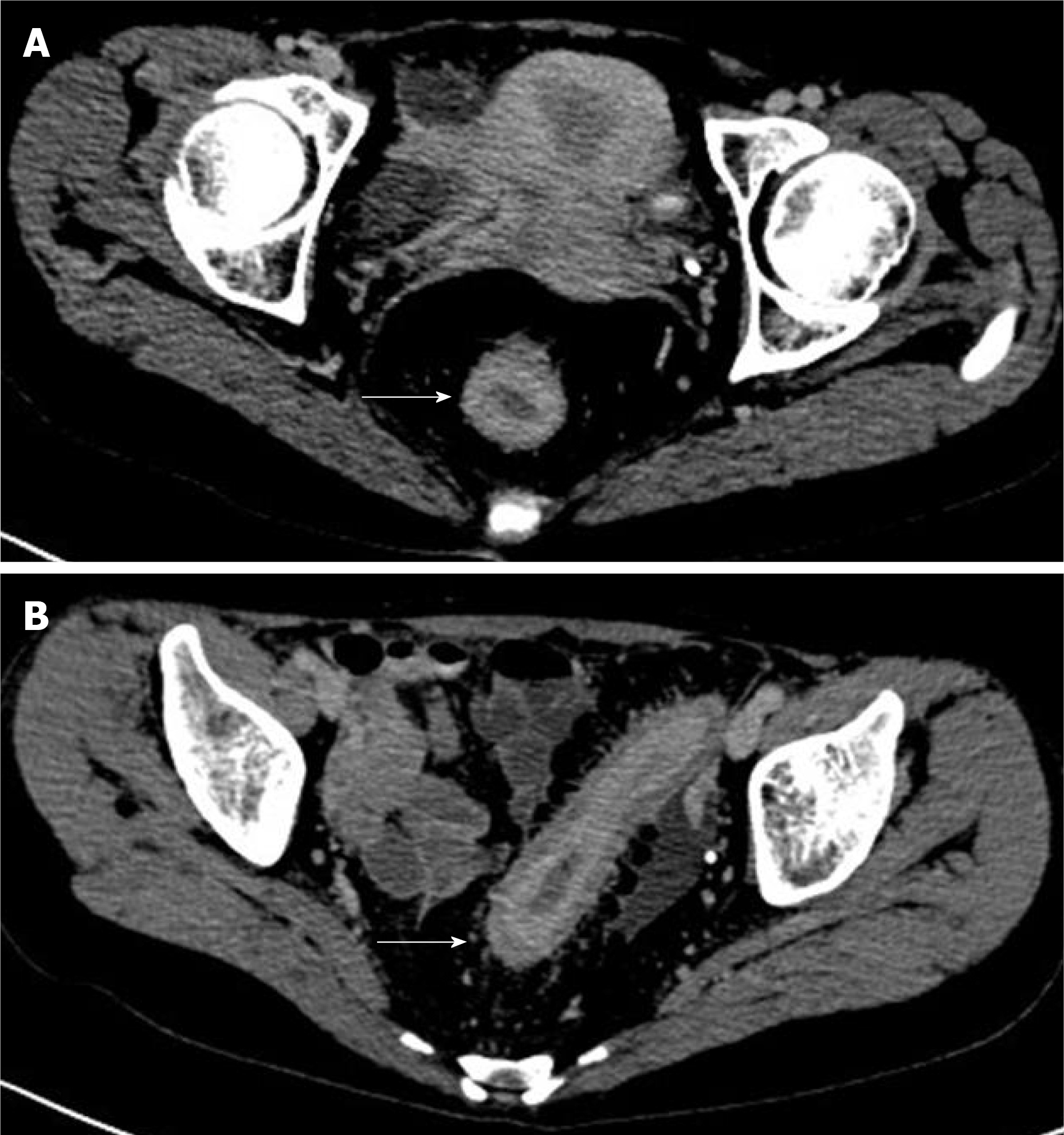
Preoperative computed tomography (CT): There were no obvious abnormalities in the scan of the chest and upper and lower abdomen. The enhanced CT scan of the pelvis showed that the rectal wall thickened uniformly in stages, visibly strengthened mucosal layer, blurred fat spaces around the intestines, and a small amount of effusion (Figure 2).
Rectal cancer caused by UC.
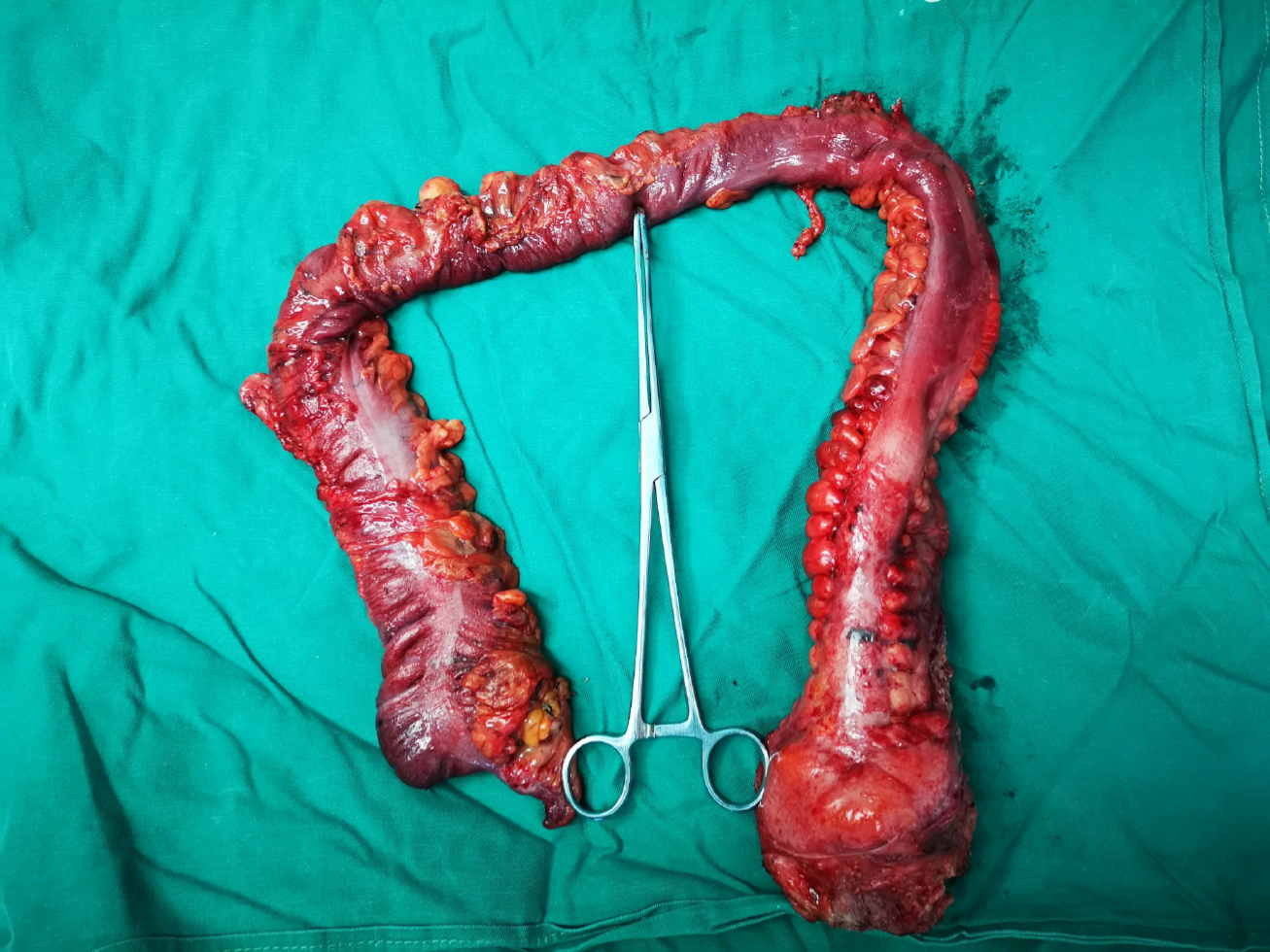
After discussion by the multidisciplinary team for gastrointestinal tumors in our hospital, the patient underwent laparoscopic exploration under general anesthesia on December 2, 2019. During the operation, there was inflammatory exudative ascites in the abdominal and pelvic cavity, obvious inflammatory hyperplasia and edema throughout the entire sigmoid colon and rectum, cancerous umbilical changes at the peritoneal reflection in the middle of the rectum, and obvious dilation and edema of part of the bowel. Scattered small patchy changes could be seen on the surface of the mesentery (Figure 3). Tissue was taken from the peritoneal reflex and sent for pathological examination, which showed SRCC. Laparoscopic total colorectal resection, ileal pouch–anal anastomosis (IPAA) and ileostomy were performed.
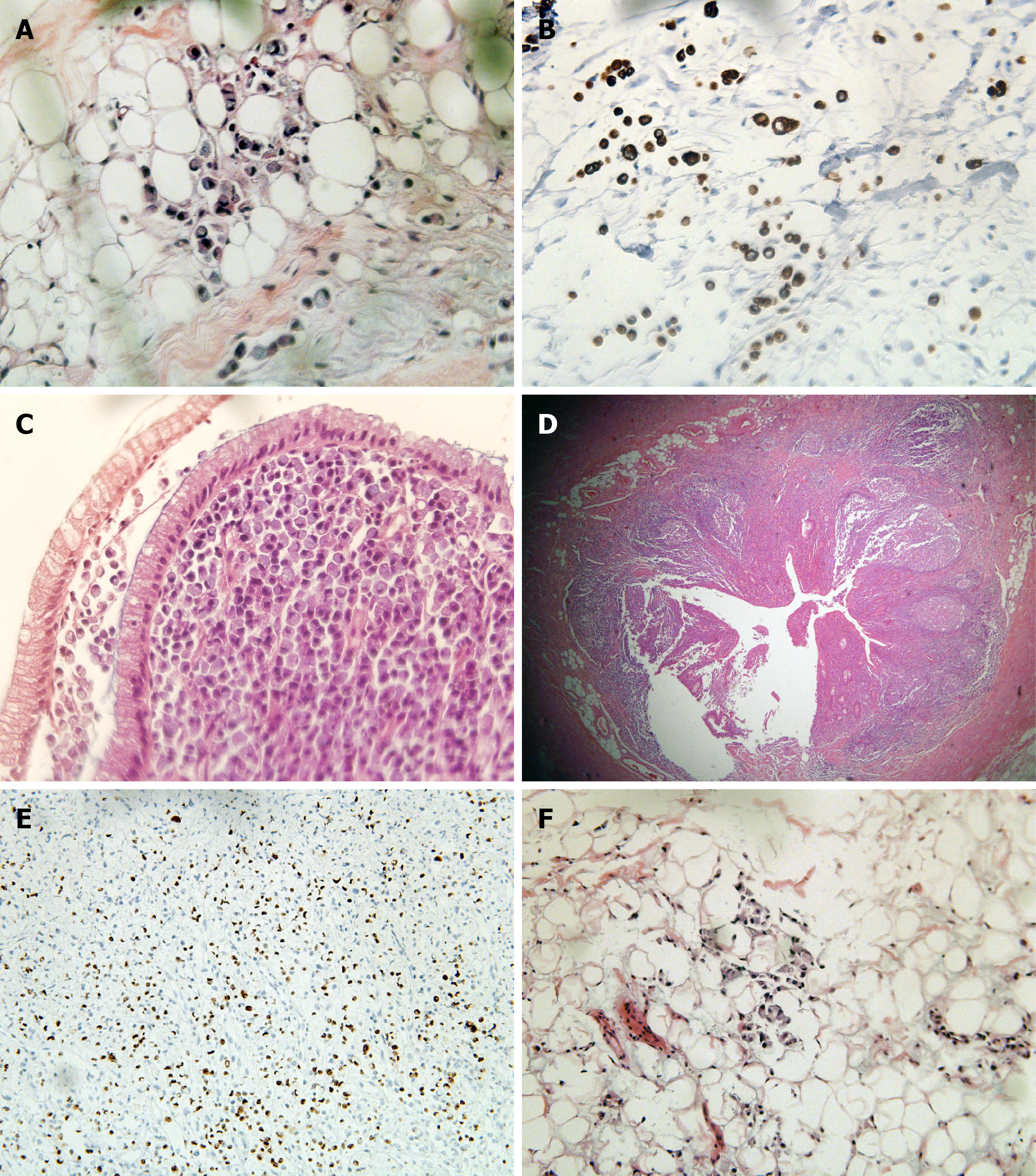
Postoperative pathological examination: The rectum and entire colon showed diffuse invasive SRCC. Rectal tumors invaded the submesangial adipose tissue, and colon tumors were confined to the mucosa and submucosa. Intravascular tumor thrombus and nerve invasion could be seen. The full thickness of the appendix showed SRCC, with visible metastasis to lymph nodes around the bowel (36/37) (Figure 4). The pathological stage was IVB (T4N2bM1c).
The patient started XELOX chemotherapy on the day 23 after surgery (oxaliplatin: Intravenous infusion for 3 h, day 1; capecitabine: Oral, 2 times/d, days 1-14). The first cycle of chemotherapy ended on January 7, 2020. Follow-up treatment was carried out at the local hospital.
Due to the impact of the COVID-19, the time for patients to receive follow-up chemotherapy was delayed by about 6 wk. After the end of the third cycle of chemotherapy, the patient was examined by imaging, the effects were evaluated as progressive disease, and the patient was switched to a FOLFOX + cetuximab regimen. After the fifth cycle, the patient was unable to tolerate further treatment due to tumor progression and multiple organ dysfunction, and died at the end of May 2020. Overall survival was 7 mo.
SRCC is a rare histological subtype of adenocarcinoma[5] that contains abundant intracytoplasmic mucin that displaces the nuclei to the periphery, thereby giving the characteristic appearance of an SRC[6]. Primary SRCC of the colon is extremely rare[7]. A study in the 20th century[8] found that only 11 cases of primary SRCC of the colon were found out of 12000 cases of primary colon cancer, with an incidence rate < 1/1000 cases of common colorectal adenocarcinoma. In about 80% of cases[9], the lesions are seen in the left colon at the distal end of the splenic flexure. This rare colorectal cancer has certain clinical, pathological and biological characteristics that are different from those of ordinary colorectal cancer, and which can be recognized as a stage-independent prognostic factor for adverse outcomes in colorectal cancer[10]. Following Laufman and Saphir[11] who first reported SRCC that occurred in the colon in 1951, new cases have been continuously reported[12-16]. Most cases are basically similar in general type, consisting of invasive tumors involving the entire thickness of the colon or rectal wall, leading to obvious thickening and induration. The lesions generally infiltrate the entire cecal wall and involve the proximal part of the appendix. Due to the infiltrative growth and highly aggressive nature of the tumor, most patients are found at an advanced stage, and the overall prognosis is extremely poor[13]. The difference between this patient and previous cases is that she had a clear history of UC, protracted course of disease and irregular follow-up treatment, providing a suitable environment for the later occurrence and development of tumors. Pontes et al[17] also reported a similar case to the present one: That patient had a 9-year history of UC, and although undergoing close endoscopic examination and treatment, he was eventually diagnosed with cancer. The pathological examination of the excised specimen showed SRCC of the sigmoid colon. Current research suggests that the occurrence of carcinoma in UC is positively correlated with the duration, degree of inflammation and extent of involvement of the patient[18]; and its occurrence and development experience the process of inflammation-low-grade intraepithelial neoplasia to high-grade intraepithelial neoplasia-carcinogenesis[19], which is different from the mode of gene mutation adenoma canceration of sporadic colorectal cancer[20]. In the same way, the mismatch repair proteins (MLH1, MSH2, MSH6 and PMS2) in this patient were all positive and microsatellite stable, and no mutations closely related to colorectal cancer were detected by genetic testing, yet advanced bowel cancer was detected at such a young age. In addition to the rarity of the case itself, it was more related to the history of UC.
As mentioned earlier, UC is a major type of inflammatory bowel disease (IBD). In the past two decades, the overall incidence of UC in China has been increasing year by year[3], but a consensus has not yet been reached on the exact pathogenesis. It is now believed that a combination of genetic, environmental, intestinal flora and host immune system factors contribute to the development of the disease[21-24]. Studies have shown[25,26] that 8%-14% of patients with UC have a family history of IBD, and the risk of first-degree relatives suffering from the disease is four times higher than that of the general population. To date, genome-wide association studies have confirmed the presence of nearly 200 disease risk genes for IBD; most of which can cause both UC and Crohn’s disease[27,28]. At present, research on IBD susceptibility genes is mainly focused on Crohn’s disease, and the internal molecular mechanism of the pathogenesis of UC has not been fully elucidated[29]. Compared with Crohn’s disease, UC has a weaker correlation with disease inheritance. Mantovani found that combination of CXC motif chemokine ligand (CXCL) 1 and CXC motif chemokine receptor (CXCR) 2 participates in the malignant behavior of solid and hematological tumors, and these two ligands indirectly act on tumor angiogenesis by regulating the transport of leukocytes that produce angiogenic factors and a variety of inflammatory cytokines[30]. The CXCL1/CXCR2 signaling pathway can regulate inflammation, promote tumor cell proliferation, invasion and transvascular metastasis, and play an important role in the progression of inflammation[31]. In animal experiments, Thaker et al[32] found that IDO1 indoleamine 2,3 dioxygenase (IDO)-1 metabolites activate β-catenin signaling to promote the proliferation of mouse cancer cells and induce colitis-related tumors in mice, indicating that IDO1 may play an important role in the progression of colon cancer caused by UC. In addition, the lack of cell regulatory factors, especially anti-inflammatory factors, plays an increasingly important role in the pathogenesis of UC[33-35]. In a study of nearly 2000 subjects, Franke tested > 400000 single nucleotide polymorphisms through genome-wide association and found that interleukin-10 dysfunction is the core cause of UC[36]. With the gradual deepening of research, new susceptibility genes are constantly being discovered, but genetic studies can only explain 7.5% of the disease differences, and the correlation between UC genotype and clinical phenotype is not clear[28,37], indicating that the disease has obvious genetic heterogeneity and a complex genetic background.
Primary SRCC of the colorectum is extremely rare clinically. This type of colorectal cancer has certain clinical, pathological and biological characteristics that are different from ordinary colorectal cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brochard C, Habeeb TAAM S-Editor: Wang JL L-Editor: Kerr C P-Editor: Wang JL
| 1. | Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L, Hibi T. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 984] [Article Influence: 196.8] [Reference Citation Analysis (0)] |
| 2. | Farrokhyar F, Swarbrick ET, Irvine EJ. A critical review of epidemiological studies in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:2-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Wang Y, Ouyang Q; APDW 2004 Chinese IBD working group. Ulcerative colitis in China: retrospective analysis of 3100 hospitalized patients. J Gastroenterol Hepatol. 2007;22:1450-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Magro F, Rodrigues A, Vieira AI, Portela F, Cremers I, Cotter J, Correia L, Duarte MA, Tavares ML, Lago P, Ministro P, Peixe P, Lopes S, Garcia EB. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012;18:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 6. | Chew MH, Yeo SA, Ng ZP, Lim KH, Koh PK, Ng KH, Eu KW. Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int J Colorectal Dis. 2010;25:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Song W, Wu SJ, He YL, Cai SR, Zhang CH, Zhang XH, Zhan WH. Clinicopathologic features and survival of patients with colorectal mucinous, signet-ring cell or non-mucinous adenocarcinoma: experience at an institution in southern China. Chin Med J (Engl). 2009;122:1486-1491. [PubMed] |
| 8. | Fahl JC, Dockerty MB, Judd ES. Scirrhous carcinoma of the colon and rectum. Surg Gynecol Obstet. 1960;111:759-766. [PubMed] |
| 9. | Amorn Y, Knight WA Jr. Primary linitis plastica of the colon: report of two cases and review of the literature. Cancer. 1978;41:2420-2425. [PubMed] |
| 10. | Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Laufman H, Saphir O. Primary linitis plastica type of carcinoma of the colon. AMA Arch Surg. 1951;62:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Bonello JC, Quan SH, Sternberg SS. Primary linitis plastica of the rectum. Dis Colon Rectum. 1980;23:337-42U. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Almagro UA. Primary signet-ring carcinoma of the colon. Cancer. 1983;52:1453-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Desai SR, Tata HR. Primary signet ring carcinoma of the large bowel--a case report. Indian J Pathol Microbiol. 2004;47:535-537. [PubMed] |
| 15. | Bianchi G, Terrinoni V, Lamazza A, Anselmi W, Abate O, Bellini N, Carbone G, Rengo M. [Signet ring cell adenocarcinoma of the colon (signet ring carcinoma): is it really a rare neoplasm? G Chir. 1997;18:283-285. [PubMed] |
| 16. | Giacchero A, Aste H, Baracchini P, Conio M, Fulcheri E, Lapertosa G, Tanzi R. Primary signet-ring carcinoma of the large bowel. Report of nine cases. Cancer. 1985;56:2723-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Pontes J, Fernandes C, Panao E, Castro L, Vicente L, Neto M, Campos M, Pontes F. Synchronic signet ring carcinoma and adenocarcinoma complicating extensive and long-standing ulcerative colitis. Hepatogastroenterology. 1999;46:236-239. [PubMed] |
| 18. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2077] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 19. | Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 21. | Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 904] [Article Influence: 113.0] [Reference Citation Analysis (3)] |
| 22. | Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014;20:1192-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 23. | Huang LJ, Mao XT, Li YY, Liu DD, Fan KQ, Liu RB, Wu TT, Wang HL, Zhang Y, Yang B, Ye CQ, Zhong JY, Chai RJ, Cao Q, Jin J. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn's disease. Immunity. 2021;54:1728-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 24. | Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20:64-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (3)] |
| 25. | Halme L, Paavola-Sakki P, Turunen U, Lappalainen M, Farkkila M, Kontula K. Family and twin studies in inflammatory bowel disease. World J Gastroenterol. 2006;12:3668-3672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 191] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 26. | Moller FT, Andersen V, Wohlfahrt J, Jess T. Familial risk of inflammatory bowel disease: a population-based cohort study 1977-2011. Am J Gastroenterol. 2015;110:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 27. | Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JY, Malekzadeh R, Westra HJ, Yamazaki K, Yang SK; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium, Barrett JC, Alizadeh BZ, Parkes M, Bk T, Daly MJ, Kubo M, Anderson CA, Weersma RK. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1898] [Cited by in RCA: 1861] [Article Influence: 186.1] [Reference Citation Analysis (0)] |
| 28. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H; International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3979] [Cited by in RCA: 3598] [Article Influence: 276.8] [Reference Citation Analysis (0)] |
| 29. | Chen ZA, Sun YF, Wang QX, Ma HH, Ma ZZ, Yang CJ. Integrated Analysis of Multiple Microarray Studies to Identify Novel Gene Signatures in Ulcerative Colitis. Front Genet. 2021;12:697514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 31. | Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massagué J. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 884] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 32. | Thaker AI, Rao MS, Bishnupuri KS, Kerr TA, Foster L, Marinshaw JM, Newberry RD, Stenson WF, Ciorba MA. IDO1 metabolites activate β-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. 2013;145:416-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 507] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 34. | Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 566] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 35. | Chen Y, Chen Y, Cao P, Su W, Zhan N, Dong W. Fusobacterium nucleatum facilitates ulcerative colitis through activating IL-17F signaling to NF-κB via the upregulation of CARD3 expression. J Pathol. 2020;250:170-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 36. | Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, Sina C, Onnie CM, Weersma RK, Stokkers PC, Wijmenga C, Gazouli M, Strachan D, McArdle WL, Vermeire S, Rutgeerts P, Rosenstiel P, Krawczak M, Vatn MH; IBSEN study group, Mathew CG, Schreiber S. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 37. | UK IBD Genetics Consortium; Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, Nimmo ER, Massey D, Blaszczyk K, Elliott T, Cotterill L, Dallal H, Lobo AJ, Mowat C, Sanderson JD, Jewell DP, Newman WG, Edwards C, Ahmad T, Mansfield JC, Satsangi J, Parkes M, Mathew CG; Wellcome Trust Case Control Consortium 2, Donnelly P, Peltonen L, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Craddock N, Deloukas P, Duncanson A, Jankowski J, Markus HS, Mathew CG, McCarthy MI, Palmer CN, Plomin R, Rautanen A, Sawcer SJ, Samani N, Trembath RC, Viswanathan AC, Wood N, Spencer CC, Barrett JC, Bellenguez C, Davison D, Freeman C, Strange A, Donnelly P, Langford C, Hunt SE, Edkins S, Gwilliam R, Blackburn H, Bumpstead SJ, Dronov S, Gillman M, Gray E, Hammond N, Jayakumar A, McCann OT, Liddle J, Perez ML, Potter SC, Ravindrarajah R, Ricketts M, Waller M, Weston P, Widaa S, Whittaker P, Deloukas P, Peltonen L, Mathew CG, Blackwell JM, Brown MA, Corvin A, McCarthy MI, Spencer CC, Attwood AP, Stephens J, Sambrook J, Ouwehand WH, McArdle WL, Ring SM, Strachan DP. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 417] [Article Influence: 26.1] [Reference Citation Analysis (0)] |