Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1630
Peer-review started: August 1, 2021
First decision: November 7, 2021
Revised: November 14, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: February 16, 2022
Processing time: 193 Days and 21.3 Hours
Small-cell carcinoma of the prostate (SCCP) is a clinically rare malignant tumor, accounting for < 1% of all prostate tumors. However, negativity for all SCCP neuroendocrine markers is rare. Herein, we report a case of SCCP with completely negative neuroendocrine markers and explore its clinicopathologic features, thus improving the understanding of its clinical diagnosis and management.
We report the case of a 48-year-old patient with SCCP negative for common sensitive neuroendocrine-staining indicators. Dysuria was the first symptom, and rectal examination revealed a hard prostate, palpable nodules, diffuse prostate enlargement, no pressure pain, no blood staining in the finger sleeve, 1.33 ng/mL total prostate-specific antigen level, and a free-to-total prostate-specific antigen ratio of 0.21 ng/mL. Ultrasound suggested a prostate size of 5.3 cm × 5.8 cm × 5.6 cm, and magnetic resonance imaging suggested prostate cancer. The lower posterior bladder wall, rectal mesentery, and bilateral seminal vesicles were invaded, with multiple lymph node metastases in the pelvis. A whole-body bone scan suggested an abnormally active multiple bone metabolism and possible bone metastases. Head and lungs computed tomography revealed no significant nodal shadow. Following a pathological diagnosis of SCCP after a prostate puncture, with negative indicators of common sensitive neuroendocrine staining, chemotherapy was administered; the patient died 4-5 mo after SCCP diagnosis.
SCCP is a rare disease characterized by atypical clinical symptoms, limited treatment options, a short survival period, and a poor prognosis.
Core Tip: Small cell carcinoma of the prostate (SCCP) is a very rare type of prostate tumor, generally characterized by low differentiation, high malignancy, rapid growth, easy diffusion, and poor prognosis. Among SCCP types, mixed SCCP is relatively common, completely simple SCCP is rarely reported, and SCCP with negative neuroendocrine markers is even rarer. This study reports a rare case of SCCP with completely negative neuroendocrine markers, and it found SCCP to be characterized by atypical clinical symptoms, limited treatment options, a short survival period, and a poor prognosis, requiring pathological examination to confirm its diagnosis.
- Citation: Shi HJ, Fan ZN, Zhang JS, Xiong BB, Wang HF, Wang JS. Small-cell carcinoma of the prostate with negative CD56, NSE, Syn, and CgA indicators: A case report. World J Clin Cases 2022; 10(5): 1630-1638
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1630.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1630
Small-cell carcinoma of the prostate (SCCP) is one of the rarest types of prostate tumors. It is generally characterized by low differentiation, high malignancy, rapid growth, easy spread, and poor prognosis[1]. It has been reported on a case-by-case basis in both domestic and international literature, with more cases of mixed SCCP than those of completely simple SCCP being reported[2]. SCCP is generally characterized by neuroendocrine granules in the cytoplasm, and immunohistochemistry suggests at least one positive neuroendocrine marker, predominantly in small-cell neuroendocrine carcinoma of the prostate[3], whereas SCCP negativity for almost all neuroendocrine markers is even rarer. SCCP diagnosis and treatment have not yet been standardized internationally and are still being explored. Herein, we report the treatment of a patient with SCCP who was negative for almost all neuroendocrine markers. By reviewing the relevant literature from recent years, we analyzed and summarized SCCP diagnosis and treatment, aiming to deepen the understanding of this disease.
A 48-year-old man was admitted to our hospital on January 7, 2019, due to dysuria that lasted for 10 d.
A 48-year-old man was admitted to our hospital on January 7, 2019, due to dysuria that lasted for 10 d. No gross hematuria or acute urinary retention, and no treatment in other hospitals.
The patient had no history of surgery, trauma, or other diseases.
There was no history of hereditary diseases. No family members had similar symptoms.
On rectal examination, the prostate was hard and diffusely enlarged, the nodules were palpable, no pressure pain was experienced, and the finger sleeve was not stained with blood.
Laboratory tests revealed that the total prostate-specific antigen level and free-to-total prostate-specific antigen ratio were 1.33 ng/mL (reference range: 0-4 ng/mL) and 0.21 ng/mL (reference range: 0-0.944 ng/mL), respectively. Liver and kidney function was normal. Serum tumor markers were in the normal range.
B-mode ultrasound revealed a prostate size of 5.3 cm × 5.8 cm × 5.6 cm, echogenicity in the prostate was heterogeneous, and the envelope was not smooth.
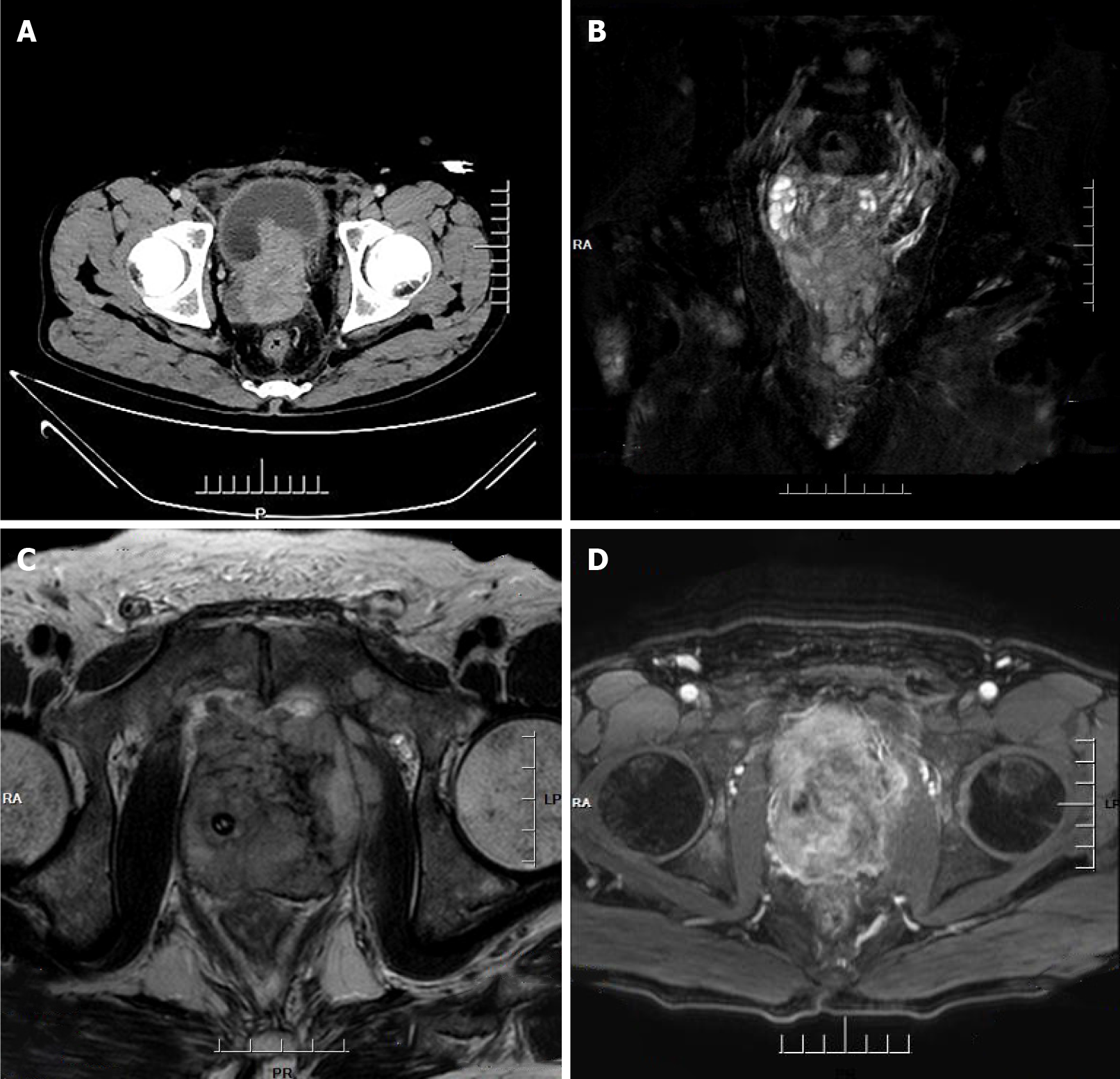
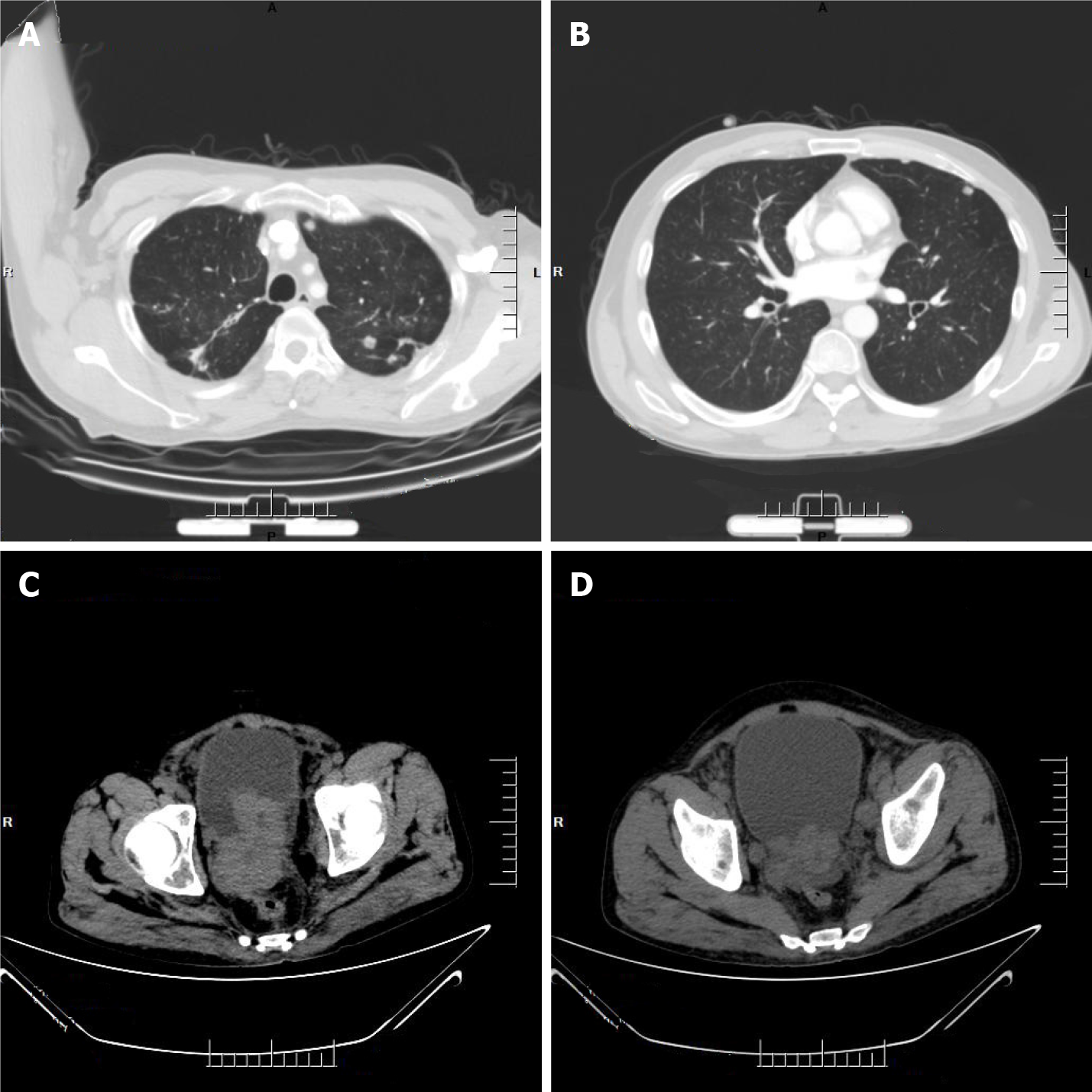
Computed tomography (CT) imaging of the abdomen exhibited heterogeneous density within the prostate and heterogeneous enhancement after enhancement, suggesting possible prostate cancer, possible pelvic lymph node metastasis, pelvic floor fascia, and rectal wall and seminal vesicle invasion (Figure 1A).
Prostate magnetic resonance imaging confirmed mixed signals in the prostate, with possible prostate tumor invasion of the lower posterior bladder wall, rectal mesentery and bilateral seminal vesicles, and multiple lymph node metastases in the pelvis (Figure 1B-D).
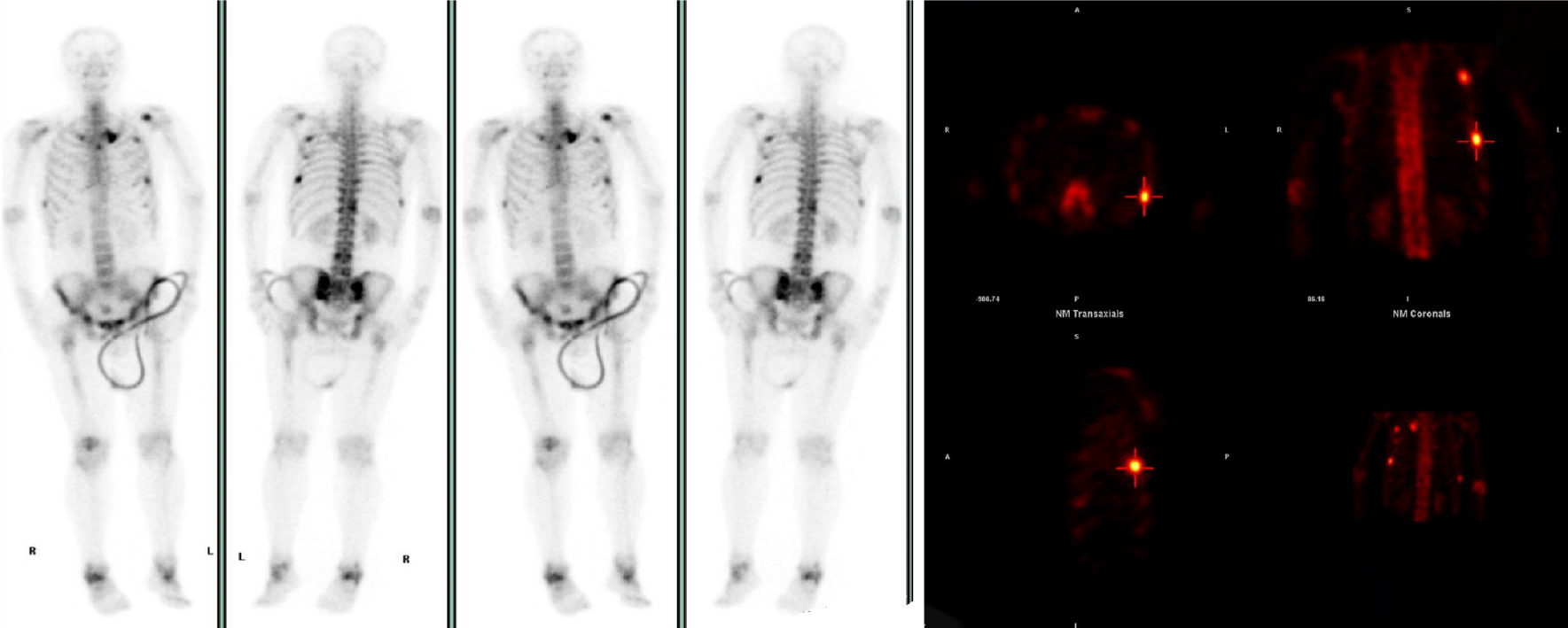
Whole-body bone scan revealed multiple abnormalities in bone metabolism, with possible bone metastases (Figure 2), and CT of the head and lung suggested no obvious nodal shadow.
We strongly recommend that the patient use 68Ga-PSMA PET/CT to look for small lesions around the prostate bed or extra-prostatic lymph node metastases. However, the patient and his family declined our offer due to the high cost.
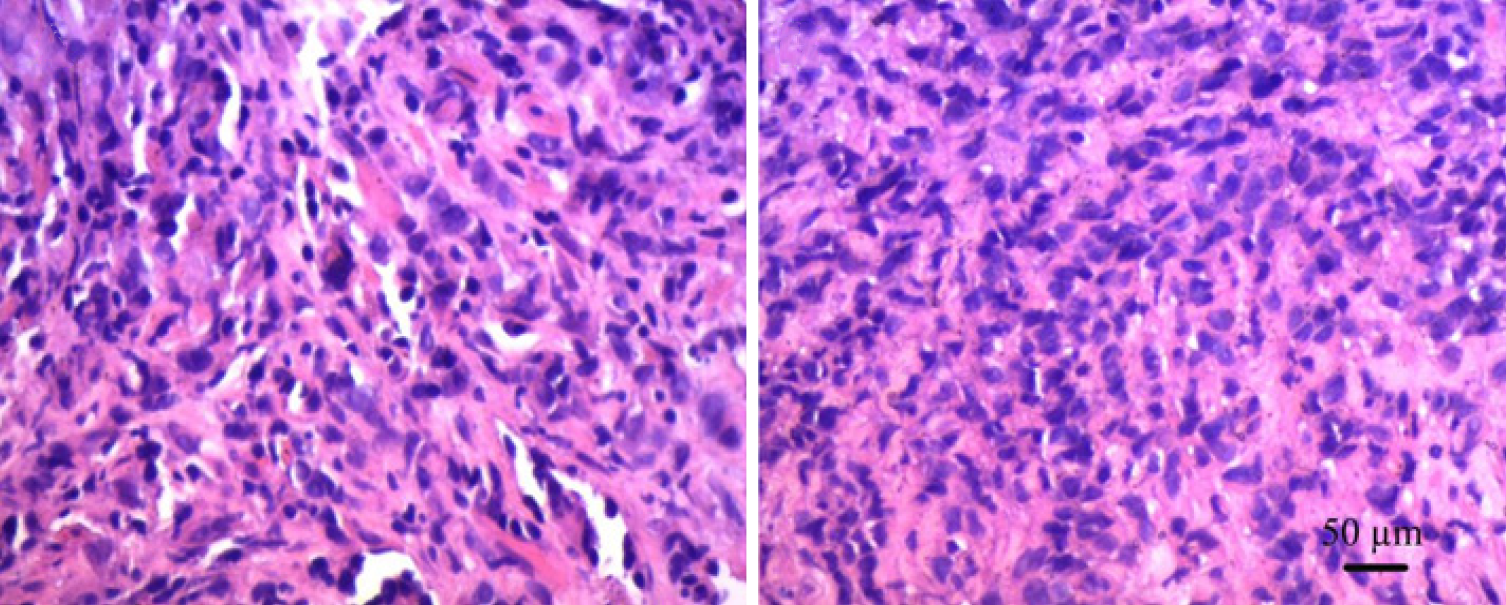
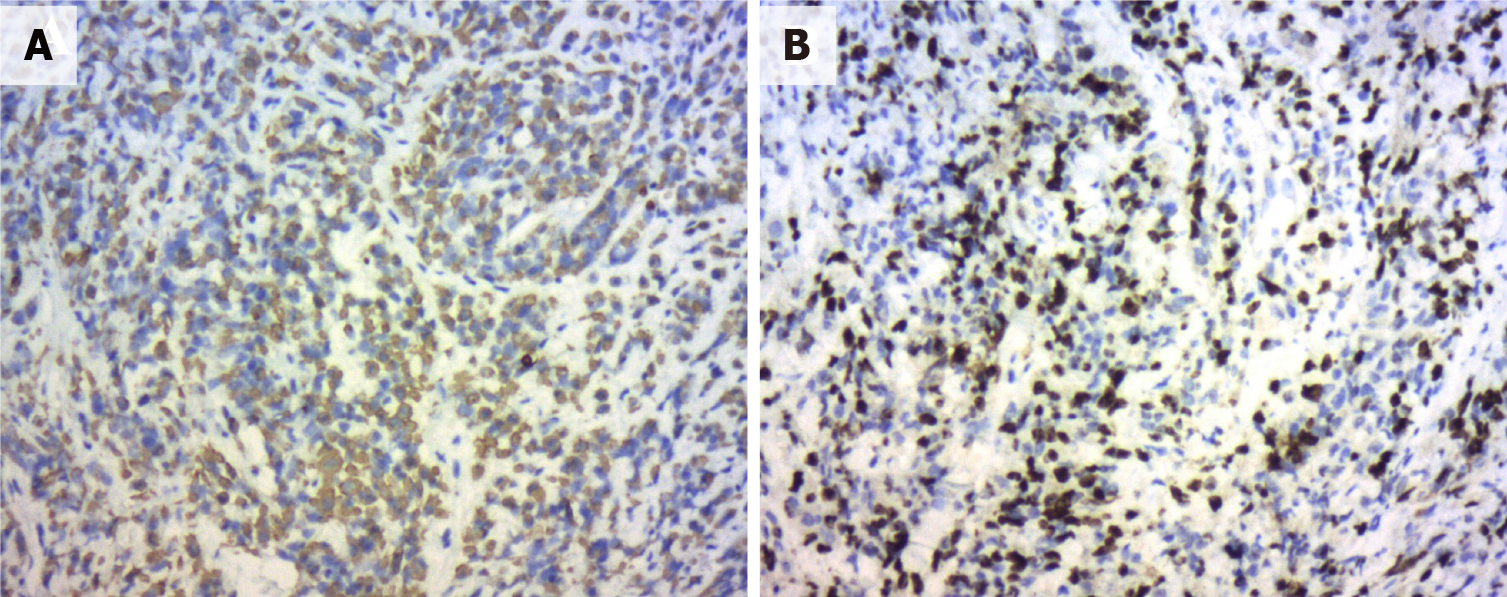
The patient was advised to initially undergo prostate puncture biopsy. The tumor cells appeared oval to spindle-shaped, with obvious heterogeneity and a mixture of oval and spindle-shaped cells (Figure 3). The immunophenotypes were as follows: CKPAN (foci +), KI67 (60%), AR (-), PSA (-), PSAP (-), P504S (-), CK5/6 (-), CKH (-), GATA3 (-), Vimentin (-), CEA (-), CK7 (-), CK20 (-), villin (-), NSE (-), Syn (-), CD56 (-), CgA (-), P40 (-), P63 (-), LCA (-), CD38 (-), CD138 (-), EMA (-), MUM1 (-), CD30 (-), Desmin (-), HMB45 (-), Melan-A (-), S100 (-), and MyoD1 (-) (Figure 4A and B). Hence, the initial diagnosis was small cell malignancy.
On combining the initial diagnosis with immunohistochemical markers, undifferentiated carcinoma was considered. On further combining with the adjuvant exami
The current treatment modality recommended radiotherapy; however, the patient and family declined radiotherapy and finally selected the etoposide combined with cisplatin (EP) chemotherapy regimen as follows: Cisplatin 80 mg on day 1 of chemo
A repeat of CT imaging of the lung on February 14, 2019, revealed the following: Multiple nodal shadows, suggesting possible SCCP metastasis (Figure 5A and B). Further CT imaging of the abdomen on March 17, 2019, suggested an irregular enlargement of SCCP, exhibiting a tendency to infiltrate, bilateral involvement of the inner segment of the ureteral bladder wall, dilatation and fluid retention in the urinary tract, multiple bone destruction in the pelvis (Figure 5C and D), and creatinine increase to 301 μmol/L, and the patient was treated with bilateral nephrostomy.
As the patient developed insensitivity to the EP regimen, EP was replaced with the gemcitabine combined with oxaliplatin (GEMOX) regimen, which was administered as follows: Gemcitabine 1 g on day 1 of chemotherapy as well as oxaliplatin 60 mg on days 1-5 and 21-28 in a course of 6 cycles. On April 25, 2019, the patient was admitted to the hospital for further chemotherapy, with routine blood work suggesting normal hemoglobin and central granulocytes, normal platelets, hemoglobin level of 48 g/L, and creatinine concentration of 156 μmol/L. After a blood transfusion, hemoglobin concentration rose to 110 g/L. The patient’s general condition was extremely poor (dyspnea, intolerable bone pain, and extreme wasting), and they could not tolerate further chemotherapy. The family decided to abandon the treatment, and the patient died of respiratory failure on May 20, 2019.
The lung is the most common site of small-cell carcinoma, and its most common site outside the lung is the prostate, accounting for approximately 3% of cases[4]. SCCP is a rare malignancy in clinical practice, accounting for less than 1% of all prostate tumors[4]. Despite having a low incidence, SCCP exhibits high malignancy and poor prognosis[5], and often metastasizes to tissues and organs, such as the brain, lung, liver, and bone via blood circulation[6]. At present, three hypotheses regarding the origin of SCCP exist: (1) The abnormal differentiation of prostatic adenocarcinoma into small-cell carcinoma after endocrine therapy[7]; (2) Derivation of the different types of adenocarcinoma and small-cell carcinoma of prostatic epithelium from pluripotent stem cells; and (3) Neuroendocrine cells in the prostatic epithelium. Prostatic neuroendocrine carcinoma includes prostate cancer with neuroendocrine differentiation, prostate carcinoid, prostatic small-cell neuroendocrine carcinoma, and prostatic large-cell neuroendocrine carcinoma, among others[1]. The above hypotheses have a certain degree of validity, and additional molecular genetic studies have identified multiple mechanisms involved in the pathogenesis of SCCP[8], including TMPRSS2-ERG gene rearrangement; RB1 gene deletion; MYCN and AURK4 gene overexpression and amplification; Akt, β-catenin, and P13k gene inactivation; P53 signaling pathway inactivation; upregulation of the EZH2 gene; and down-regulation of DUSP1 expression, among others[9,10].
The early stage of SCCP is typical without specific clinical symptoms. As the tumor progresses, it may invade the bladder and rectum, potentially causing difficult urination, hematuria, and perineal discomfort. In addition, signs of metastases may appear, involving the brain, lung, bone, and lymph nodes[11]. Very few patients produce ectopic endocrine hormones and exhibit paraneoplastic syndrome[2], including Cushing’s syndrome, neurological symptoms, and hypercalcemia, among others[12]. In this case, the patient was 48 years old and predominantly exhibited lower urinary tract obstruction symptoms; therefore, it is necessary to maintain a suspicious mindset and perform a rectal examination in middle-aged men with combined lower urinary tract obstruction symptoms.
SCCP diagnosis relies mainly on pathological examination. Microscopically, cells are observed as small round shapes, arranged in sheets or nests, with little cytoplasm, and the nucleoli are unclear with visible nuclear fission[13]. Immunohistochemistry is the main method of confirming SCCP diagnosis, and the typical sensitive neuroendocrine staining markers are NSE, Syn, CD56, and CgA[14], in addition to TTF-1, insulinoma-associated protein 1 (INSM1)[14], and FOXA2[15], among others. SCCP is a type of prostate neuroendocrine tumor that can be diagnosed provided one or more of the following indicators are positive: NSE, Syn, CD56, CgA, TTF-1, INSM1, and FOXA2. AR, PSA, PSAP, P504S, and other classical immune indicators of prostate adenocarcinoma are also widely expressed in poorly differentiated adenocarcinoma, whereas in SCCP, they are almost not expressed; therefore, they can be used to distinguish SCCP from poorly differentiated adenocarcinoma[16]. The patient described in this paper exhibited negative NSE, Syn, CD56, CgA, AR, PSA, PSAP, and P504S indexes, and due to equipment limitations in the hospital pathology department, TTF-1, INSM1, and FOXA2 immune indexes were not assessed, rendering the final diagnosis rather limited. While the final diagnosis was SCCP (undifferentiated carcinoma), the author still considered neuroendocrine small-cell carcinoma based on current relevant tests and related examinations. Overall, the probability that the most sensitive immune indicators, such as NSE, Syn, CD56, and CgA, would be simultaneously negative in prostate neuroendocrine small-cell carcinoma was considerably low.
Currently, there is no unified standard treatment for SCCP. Clinically, the treatment plan of lung small-cell carcinoma is generally referred to, which is mainly combined with chemotherapy, supplemented by surgery and radiotherapy[3]. Surgery is suitable for early-stage SCCP without metastasis or local progression. Relevant studies have concluded that for early-stage or locally progressive SCCP, early surgery and postoperative combined chemotherapy can effectively improve patient survival or even cure[17]. However, SCCP progresses rapidly, and most patients are at an advanced stage, losing the opportunity for surgery, and palliative surgery potentially improves patients’ quality of life. Chemotherapy is based on the EP regimen, with an efficiency of up to 61.0%[18]. If ineffective, other regimens can alternatively be used. In this case, the patient was not sensitive to the EP regimen; thus, it was subsequently replaced with the GEMOX regimen, which was unsatisfactory. Hence, the chemotherapy regimen for SCCP is still currently being explored. Radiotherapy combined with chemotherapy may improve the patient’s prognosis. Radiotherapy alone is ineffective and is generally used in patients with bone metastases to control pain symptoms caused by these metastases[19]. In this case, the patient declined radiotherapy, and the patient and family accepted chemotherapy in consideration of the toxic reactions in the urinary tract and rectum caused by radiotherapy.
The application of 68Ga-PSMA PET/CT in the whole diagnosis of prostate cancer has developed rapidly, which plays a vital role in assisting clinical tumor staging. It can accurately detect local lesions, lymph node metastasis, and distant metastasis in prostate cancer, with high sensitivity and specificity. It can be found that the advantage is especially significant at a low PSA value (< 0.5 ng/mL)[20]. The patient described in this article refuses to undergo 68Ga-PSMA PET/CT examination, when the patient's CT scan finds the lesions in the lung as a metastatic site, but we do not know about the function of the lesion. Parghane and Basu[21] used Dual-tracer (68Ga-PSMA and 18F-FDG) PET/CT in the case of metastatic SCCP, Interestingly, whereas metastatic SCCP transformed pelvic and penile lesions were nonavid with 68Ga-PSMA but avid with 18F-FDG. Therefore, the role of new tracer 18F-FDG in metastatic SCCP should not be underestimated. The bone and pelvic lesions demonstrated a favorable response to a multimodal therapeutic approach (177Lu-PSMA radioligand therapy and radiotherapy), a trend toward a decrease in PSA level[21]. 177Lu-PSMA radioligand therapy shows promising prospects for metastatic SCCP patients who progress after conventional therapy. For patients with metastatic SCCP patients that progress after chemotherapy, 177Lu-PSMA radioligand therapy is expected to change the status quo of patients with short survival and poor quality of life[22]. 68GA-PSMA PET/CT is used to screen patients suitable for 177Lu-PSMA radioligand therapy, and then 177Lu-PSMA radioligand therapy for the suitable patients for targeted therapy, can intuitively and visually dynamic evaluation of efficacy. Tumor staging is carried out to realize the integration of diagnosis and treatment, which embodies the precision and personalized diagnosis and treatment concept of nuclear medicine. Despite urgent clinical needs, 177Lu-PSMA radioligand therapy for metastatic SCCP has yet to be approved by FDA and the European Medicines Agency. However, with the accumulation of global research data, it is expected to become an extension and complement to the clinical routine treatment of metastatic SCCP.
In recent years, targeted therapies have emerged through continued research into the molecular mechanisms of SCCP. AURKA plays an important role in the treatment development of SCCP, and AURKA inhibitors (danusertib, CD532, and MLN8237) improve patient outcomes[6]. Fifty percent of SCCP samples have been found to have fusion rearrangements of TMPRSS2-ERG. PARP1 inhibitor (olaparib) potentially improves the sensitivity of tumor cells to radiotherapy, thus improving the effect of chemoradiotherapy on SCCP[23]. Researchers have continued to explore the molecular mechanisms of SCCP to establish a pathway for precision therapy.
The current patient was 48 years old and was admitted to the hospital with symptoms of dysuria. On combining the initial diagnosis with the medical history and relevant investigations, the patient was considered to have advanced primary SCCP, with negative CD56, NSE, Syn, and CgA indicators, which is a rare phenomenon. The treatment strategy was to consider the patient’s young age and use preoperative chemotherapy, await tumor shrinkage before surgery, and subsequently combine radiotherapy after surgery to improve the patient’s survival; however, the patient’s preoperative chemotherapy was not effective and failed to control tumor progression. A few studies have investigated SCCP with negative CD56, NSE, Syn, and CgA indicators, and whether it is insensitive to EP chemotherapy and effective with targeted therapy warrants full elucidation.
In summary, SCCP is a rare malignant tumor, and SCCP with negative CD56, NSE, Syn, and CgA indexes is even rarer, with no specific symptoms in the early stage. Further, it is often detected in its advanced stage, with diagnosis relying on pathological examination. An in-depth study of the molecular mechanism of SCCP may provide a new basis for the diagnosis and treatment of SCCP.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dadgar H S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Nadal R, Schweizer M, Kryvenko ON, Epstein JI, Eisenberger MA. Small cell carcinoma of the prostate. Nat Rev Urol. 2014;11:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 2. | Rueda-Camino JA, Losada-Vila B, De Ancos-Aracil CL, Rodríguez-Lajusticia L, Tardío JC, Zapatero-Gaviria A. Small cell carcinoma of the prostate presenting with Cushing Syndrome. A narrative review of an uncommon condition. Ann Med. 2016;48:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Puca L, Vlachostergios PJ, Beltran H. Neuroendocrine Differentiation in Prostate Cancer: Emerging Biology, Models, and Therapies. Cold Spring Harb Perspect Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Hingorani R, Young J, Alweis R. Mixed adenocarcinoma and neuroendocrine prostate cancer: a case report. J Community Hosp Intern Med Perspect. 2014;4:25176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Lopez-Barcons LA. Small-cell neuroendocrine carcinoma of the prostate: are heterotransplants a better experimental model? Asian J Androl. 2010;12:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Monn MF, Cheng L. Emerging trends in the evaluation and management of small cell prostate cancer: a clinical and molecular perspective. Expert Rev Anticancer Ther. 2016;16:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Lotan TL, Gupta NS, Wang W, Toubaji A, Haffner MC, Chaux A, Hicks JL, Meeker AK, Bieberich CJ, De Marzo AM, Epstein JI, Netto GJ. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod Pathol. 2011;24:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Carneiro BA, Pamarthy S, Shah AN, Sagar V, Unno K, Han H, Yang XJ, Costa RB, Nagy RJ, Lanman RB, Kuzel TM, Ross JS, Gay L, Elvin JA, Ali SM, Cristofanilli M, Chae YK, Giles FJ, Abdulkadir SA. Anaplastic Lymphoma Kinase Mutation (ALK F1174C) in Small Cell Carcinoma of the Prostate and Molecular Response to Alectinib. Clin Cancer Res. 2018;24:2732-2739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Kumar K, Ahmed R, Chukwunonso C, Tariq H, Niazi M, Makker J, Ihimoyan A. Poorly Differentiated Small-Cell-Type Neuroendocrine Carcinoma of the Prostate: A Case Report and Literature Review. Case Rep Oncol. 2018;11:676-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Zhang Y, Zhang Y, Chen M, Liu C, Xiang C. DUSP1 is involved in the progression of small cell carcinoma of the prostate. Saudi J Biol Sci. 2018;25:858-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Priemer DS, Montironi R, Wang L, Williamson SR, Lopez-Beltran A, Cheng L. Neuroendocrine Tumors of the Prostate: Emerging Insights from Molecular Data and Updates to the 2016 World Health Organization Classification. Endocr Pathol. 2016;27:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Elston MS, Crawford VB, Swarbrick M, Dray MS, Head M, Conaglen JV. Severe Cushing's syndrome due to small cell prostate carcinoma: a case and review of literature. Endocr Connect. 2017;6:R80-R86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Weprin S, Yonover P. Small Cell Carcinoma of the Prostate: A Case Report and Brief Review of the Literature. Urol Case Rep. 2017;13:61-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Xin Z, Zhang Y, Jiang Z, Zhao L, Fan L, Wang Y, Xie S, Shangguan X, Zhu Y, Pan J, Liu Q, Huang Y, Dong B, Xue W. Insulinoma-associated protein 1 is a novel sensitive and specific marker for small cell carcinoma of the prostate. Hum Pathol. 2018;79:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Park JW, Lee JK, Witte ON, Huang J. FOXA2 is a sensitive and specific marker for small cell neuroendocrine carcinoma of the prostate. Mod Pathol. 2017;30:1262-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 17. | Guo A, Wen S, Ma Y, Wei L, Liu A. Clinicopathological analysis on small cell carcinoma of the prostate in chinese patients. J Cancer. 2014;5:797-803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Papandreou CN, Daliani DD, Thall PF, Tu SM, Wang X, Reyes A, Troncoso P, Logothetis CJ. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol. 2002;20:3072-3080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 197] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Palmgren JS, Karavadia SS, Wakefield MR. Unusual and underappreciated: small cell carcinoma of the prostate. Semin Oncol. 2007;34:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Treglia G, Pereira Mestre R, Ferrari M, Bosetti DG, Pascale M, Oikonomou E, De Dosso S, Jermini F, Prior JO, Roggero E, Giovanella L. Radiolabelled choline versus PSMA PET/CT in prostate cancer restaging: a meta-analysis. Am J Nucl Med Mol Imaging. 2019;9:127-139. [PubMed] |
| 21. | Parghane R, Basu S. Small Cell Transformation of Metastatic Prostate Adenocarcinoma Diagnosed by Dual-Tracer PET/CT (68Ga-PSMA and 18F-FDG): Potential Clinical Utility in Therapeutic Decision Making and Treatment Monitoring. J Nucl Med Technol. 2019;47:85-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, Gärtner F, Rogenhofer S, Schäfers M, Essler M. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 23. | Chedgy EC, Vandekerkhove G, Herberts C, Annala M, Donoghue AJ, Sigouros M, Ritch E, Struss W, Konomura S, Liew J, Parimi S, Vergidis J, Hurtado-Coll A, Sboner A, Fazli L, Beltran H, Chi KN, Wyatt AW. Biallelic tumour suppressor loss and DNA repair defects in de novo small-cell prostate carcinoma. J Pathol. 2018;246:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |