Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1572
Peer-review started: November 30, 2020
First decision: September 28, 2021
Revised: October 9, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: February 16, 2022
Processing time: 441 Days and 4.6 Hours
Nodular fasciitis (NF) is a self-limiting tumor that mostly occurs in the subcutaneous superficial fascia. NF originating from the appendicular periosteum is extremely rare. A large NF lesion of periosteal origin can be misdiagnosed as a malignant bone tumor and may cause overtreatment.
A right axillary mass was found in a 46-year-old man and was initially diagnosed intraoperatively as low-grade sarcoma, but later diagnosed as NF after post-resection histopathological evaluation. Furthermore, fluorescence in situ hybridization analysis revealed a USP6 gene rearrangement that confirmed the diagnosis. To the best of our knowledge, this is the first case of NF in the humeral periosteum.
NF poses a diagnostic challenge as it is often mistaken for sarcoma. Postoperative histopathological examination of whole sections can be combined with immunohistochemical staining and, if necessary, the diagnosis can be confirmed by molecular detection, and thus help avoid overtreatment.
Core Tip: This article provides a comprehensive overview of the clinicopathological, immunohistochemical, and molecular features of nodular fasciitis originating from the humeral periosteum. To date, this is the first report of nodular fasciitis originating from the humeral periosteum and this type of research is critical to further our understanding of these lesions and advance pathological diagnoses.
- Citation: Yu SL, Sun PL, Li J, Jia M, Gao HW. Giant nodular fasciitis originating from the humeral periosteum: A case report. World J Clin Cases 2022; 10(5): 1572-1579
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1572.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1572
Nodular fasciitis (NF) was first described as a pseudosarcomatous fasciitis by Konwaler et al[1] in 1955. Similar to other soft-tissue sarcomas, NF is a rapidly growing, benign proliferation of fibroblasts and myofibroblasts displaying abundant, spindle-shaped cells and high mitotic activity. NF presents most typically in the upper extremities (46%), trunk (20%), and head and neck (18%)[2]. The peak incidences of NF are seen at ages 20 and 40, often presenting with tenderness, and it is a rare disease in children[3]. Most NF lesions are small, measuring less than 2 cm in diameter[2,4]. Periosteal fasciitis is considered a rare subtype of NF, with some case reports in the published literature and most of those were published over 20 years ago; only one case of periosteal fasciitis has been published recently, in 2017. The frequently reported sites of periosteal fasciitis are the maxilla and the hand; however, there are no reports of periosteal fasciitis in the limbs, and all reported cases described tumors that were smaller than 5 cm.
As NF has a nonspecific immunohistochemical profile[4], its histomorphological characteristics are the primary diagnostic criteria. Therefore, it remains a challenge to distinguish NF from other spindle cell lesions, particularly those of the myofibroblastic lineage.
In 2011, Erickson-Johnson et al[5] reported the rearrangement of the USP6 gene on chromosome 17p13 as a recurrent and specific finding in NF. Subsequently in 2013, Amary et al[6] found USP6 gene rearrangements in 91% of the 34 NF cases in their study, thereby making USP6 fluorescence in situ hybridization (FISH) analysis a reliable and useful ancillary diagnostic test for NF.
This report presents findings from the first case of large-sized NF originating from the humeral periosteum. We emphasize the importance of highlighting this rare clinical entity, which usually represents a diagnostic dilemma.
Intermittent pain in the right axilla for 1 mo.
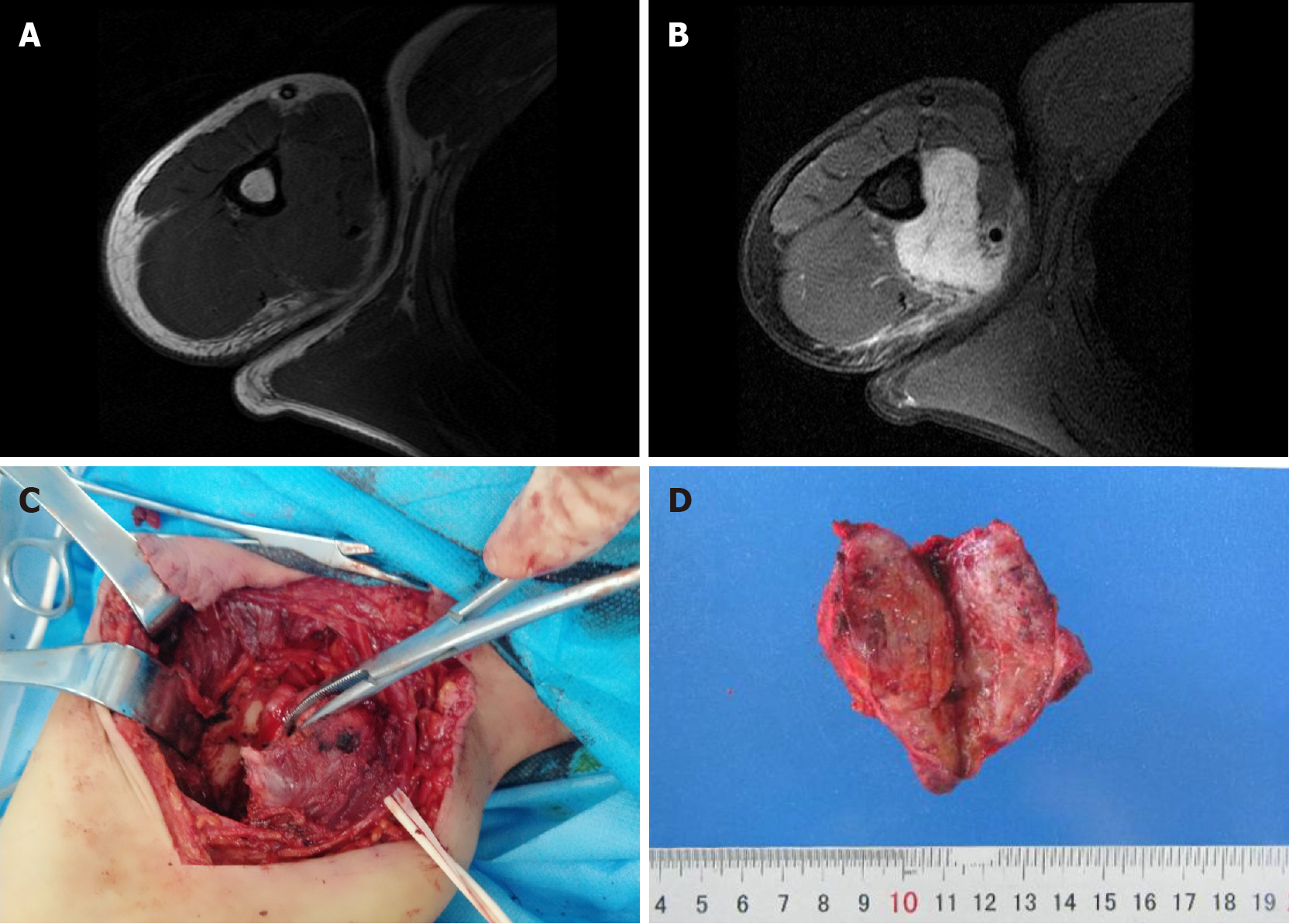
The patient had intermittent right axillary pain with no obvious cause of for 1 mo. And he found a lump under his axilla. Magnetic resonance imaging (MRI) showed a lesion measuring 62 mm × 58 mm × 44 mm, with relatively well-demarcated margins, and the lesion encircled the humerus, with localized thinning of the humeral cortex, and was closely related to the radial artery. The clinician recommended surgical treatment.
There was no history of past illness.
There was no personal and family history.
A tough mass was locally palpable on the medial side of the upper right arm and was approximately 7 cm in size.
No abnormalities were found in routine laboratory tests.
An MRI scan showed a high signal intensity in the agglomerated pressure-fat phase near the right axillary region. The MRI images showed a lesion measuring 62 mm × 58 mm × 44 mm, with relatively well-demarcated margins. The lesion encircled the humerus, with localized thinning of the humeral cortex, and was closely related to the radial artery.
NF.
Surgical tumor resection.
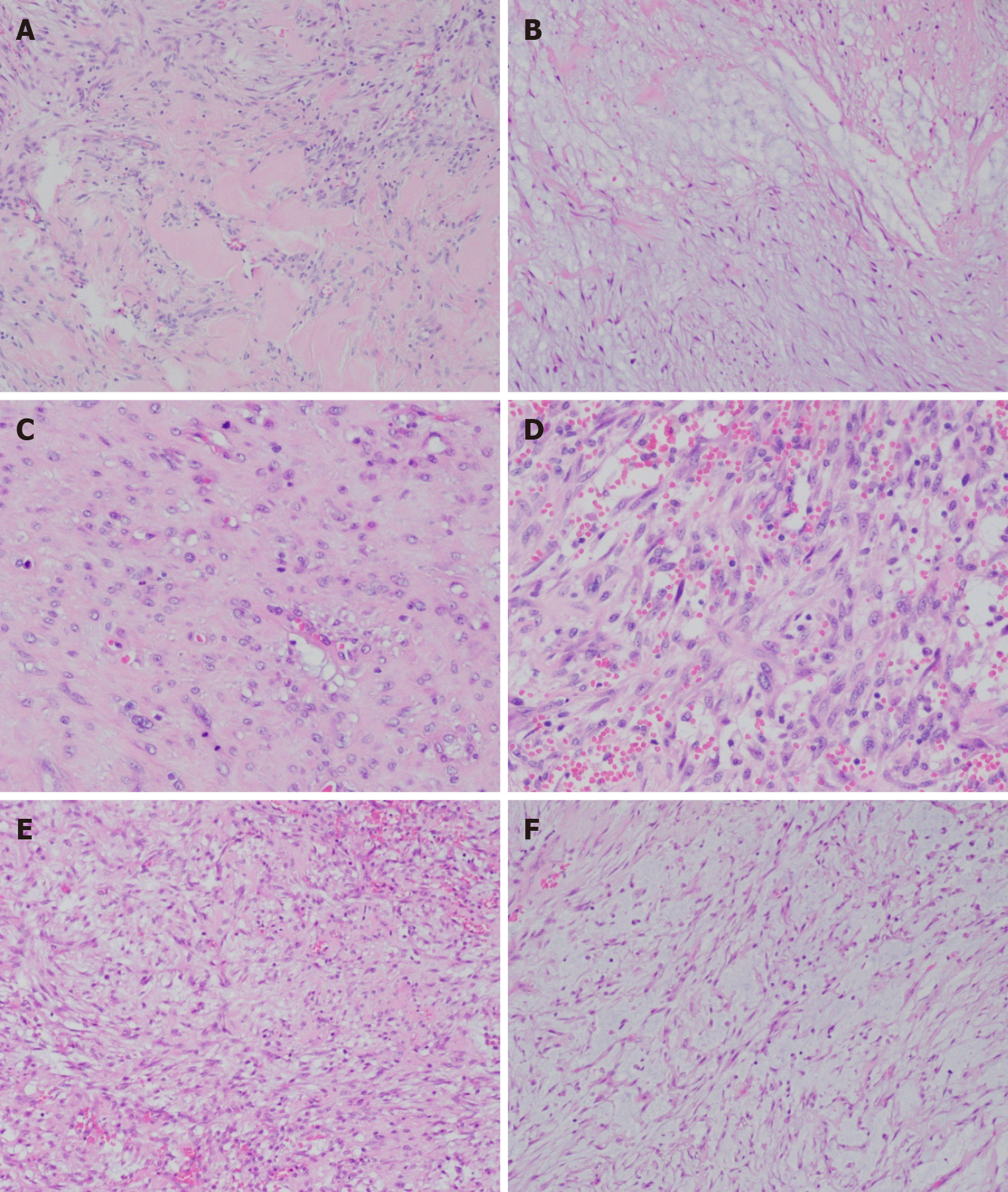
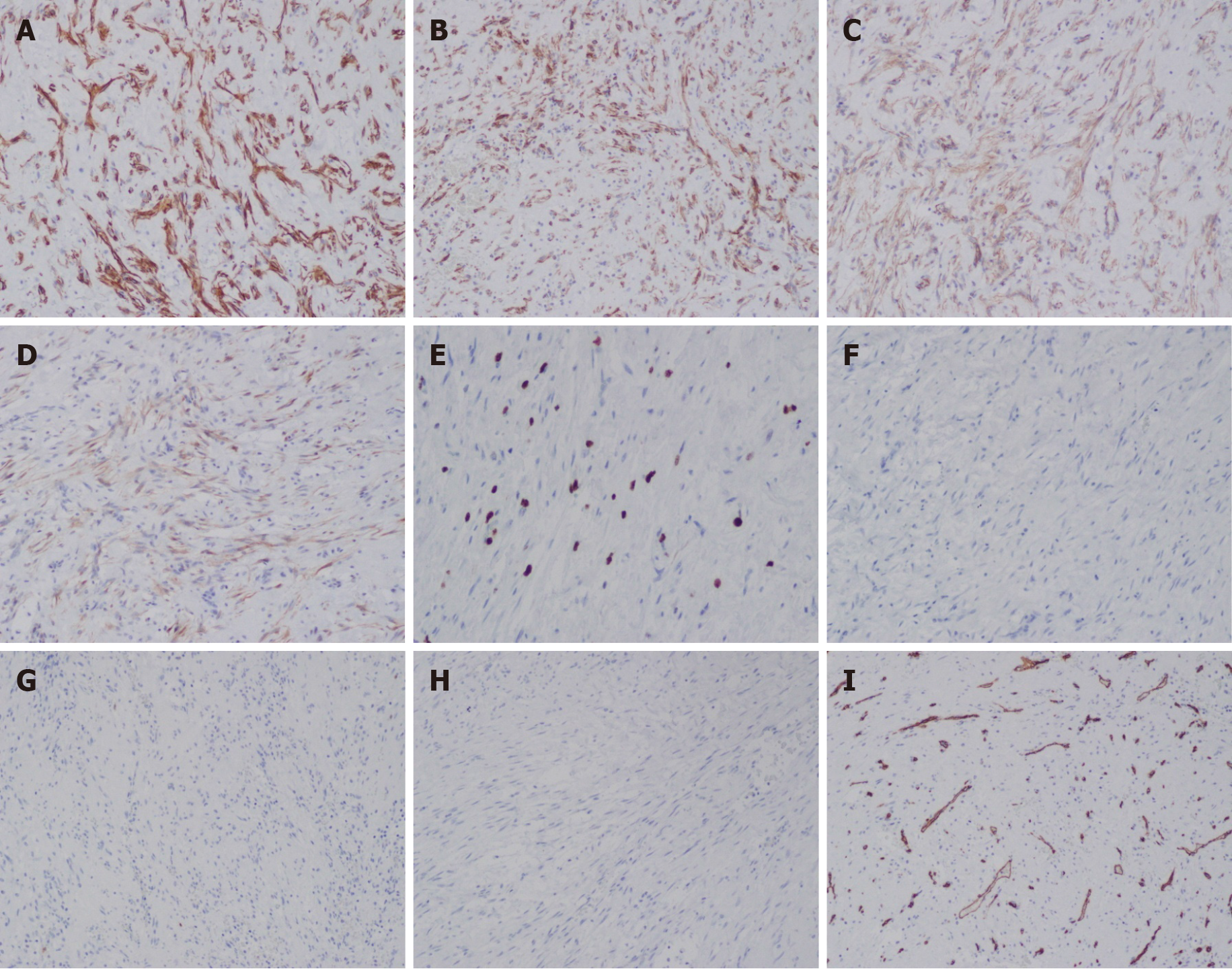
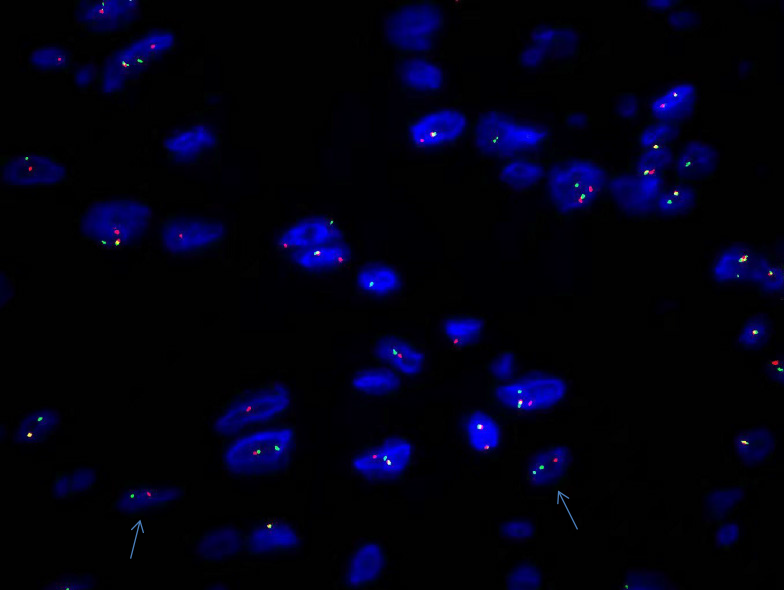
The differential diagnosis of sarcoma was made, and the patient underwent surgical tumor resection. Intraoperatively, we identified a mass with an approximate diameter of 7 cm that was closely related to the humerus, with a relatively clear boundary that separated it from the surrounding tissue. The tumor was completely separated from the periosteum. The surgical specimen was intraoperatively subjected to rapid histopathological examination. Gross examination revealed a gray nodule measuring 7.5 cm × 4 cm × 4 cm that had a reddish gray surface appearance on cross section and relatively tough texture (Figure 1). Microscopically, the lesion mainly comprised spindle-shaped fibroblast-like cells, with mucinous degeneration, mild atypia of some cells, and 3-4 mitotic figures per 10 high power fields. The intraoperative provisional pathological diagnosis was a mesenchymal neoplasm; the final diagnosis would be definitively based on the postoperative pathology. The postoperative histopathology of the lesions revealed spindle-shaped tumor cells with abundant extracellular mucoid matrix (Figure 2B and F); similarly, on examination of the frozen sections, some areas showed fibrous hyperplasia and hyaline degeneration (Figure 1A), whereas other areas had extravasation of red blood cells (Figure 2D). Tumor cells in areas with relatively high cellularity showed mild atypia (Figure 2C and D) and mitotic figures (Figure 2C). Immunohistochemistry showed that the specimen stained negative for CD34, S100, and β-catenin and positive for CD10 and SMA (Figure 3). FISH analysis revealed a USP6 gene fracture rearrangement (Figure 4) with signal patterns as follows: 1G1R1F 16.5%, 1G1R 8.5%, 2F 35.5%, 1F 25.0%, 1G1F 7.0%, and 1R1F 7.5%.
The patient had an uneventful recovery after surgery and no further treatment was given. There was no recurrence during the 20-mo follow-up period.
The published literature describes NF as a benign myofibroblastic proliferation, which was initially reported in 1955 as a pseudosarcomatous fibromatosis or fasciitis[1]. The NF lesion typically develops in the subcutaneous superficial fascia of the upper limbs (46%), especially over the volar aspect of the forearm, followed by the head and neck (20%), trunk (18%), and lower extremities (16%). There are no gender differences in NF incidence, and all reported lesions measure less than 5 cm in diameter.
Periosteal fasciitis, a subtype of NF, is characterized by periosteal overgrowth and reactive new bone formation. There are only a few case reports (10 cases) of periosteal fasciitis in the literature, most of which were reported in the 1970s and 1980s, although one case was recently reported in 2017. Among those ten cases (four males; six females), four occurred in the jaw (one in the maxilla, three in the mandible) and six in the hand. The largest reported tumor diameter was approximately 5 cm. Most of the cases were diagnosed by histomorphological features, and FISH was undertaken in only one case in the recent literature and showed USP6 gene-related heterotopia. All patients were followed, and there are no reports of recurrence (Table 1). In our case, NF was initially diagnosed by histomorphology and immunohistochemistry; however, because of the unusually large tumor and its periosteal origin, we undertook a USP6 FISH examination. The results showed USP6-related ectopia, which further confirmed a diagnosis of NF. The patient has shown no recurrence on follow-up for 10 mo. This report presents a rare case of clinical NF of the humeral periosteum with a tumor diameter of 7.5 cm.
| Ref. | Number of cases | Sex | Age (yr) | Symptom presence and duration | Location | Treatment | Size (cm) | USP6 gene | Follow-up (mo) | Recurrence | Injury |
| Lääveri et al[11], 2017 | 1 | Female | 7 | No | Mandible | Local resection | 3 | Yes | 36 | No | No |
| Rankin et al[12], 1991 | 1 | Female | 39 | No | Hand | Local resection | 5 | NA | 10 | No | No |
| Mostofi et al[13], 1987 | 1 | Male | 46 | No | Mandible | Local resection | 3 | NA | 30 | No | No |
| Sato et al[14], 1981 | 1 | Male | 31 | Pain for 2 mo | Maxillary | Local resection | 4 | NA | 8 | No | No |
| McCarthy et al[15], 1976 | 1 | Male | 40 | No | Ring finger | Amputation | NA | NA | 12 | No | No |
| Johnson and Lawrence[16], 1975 | 1 | Male | 38 | Pain and swelling for 3 mo | Metacarpal and ring finger | Local resection | NA | NA | 12 | No | No |
| Goncalves[17], 1974 | 1 | Female | 23 | Pain and swelling for 2 wk | Index finger | Amputation | NA | NA | 60 | No | No |
| Lumerman et al[18], 1972 | 1 | Female | 31 | Pain for 3 d | Mandible | Local resection | 2 | NA | 30 | No | No |
| Carpenter and Lublin[19], 1967 | 1 | Female | 32 | Pain and swelling for 7 mo | Proximal and middle phalanges, ring finger | Amputation | NA | NA | 12 | No | No |
| Mallory[20], 1933 | 1 | Female | 28 | Pain, swelling for 4 wk | 4th and 5th metacarpals | Incomplete local resection | NA | NA | 12 | No | No |
Due to its fast and infiltrative growth pattern, NF remains one of the most commonly misdiagnosed benign spindle cell neoplasms. A common differential diagnosis of NF is low-grade malignant myofibroblastic tumors because, despite their large size, the tumor cells are characterized by mild atypia; positive staining for actin, desmin, calponin, and CD34 (focal), and negative staining for S100 and nuclear β-catenin[7-9]. However, FISH shows no USP6 gene-related ectopia, and myofibroblastic tumors have a high recurrence after surgical resection.
Sometimes, it may be difficult to distinguish low-grade myxofibrosarcoma from NF, especially in cases with small tumor volume and without specific immunohistochemical markers. Nonetheless, curvilinear thin-walled blood vessels and pseudolipoblasts suggest the possibility of a myxofibrosarcoma, and FISH examination shows no USP6 gene-related ectopia.
Low-grade malignant fibromyxoid sarcoma is another differential diagnosis of NF. The identification can be comprehensively evaluated by immunohistochemical staining and molecular detection. Immunohistochemistry shows EMA positivity from focally to 80%, and MUC4 positivity has high sensitivity and specificity for the detection of fibromyxoid sarcoma[10]. Molecular genetics show FUS-CREB3L2 or FUS-CREB3L1 gene fusion (Table 2).
| Tumor type | Epidemiology | Clinical features | Size | Histopathology | Immunophenotype | Genetics |
| Nodular fasciitis | Young adults, no gender difference | Grows rapidly, painless, recurrence is rare | Median size, ≤ 2 cm (always < 5 cm) | Spindle-shaped fibroblasts, growth in S- or C- shaped, interstitium is loose and myxoid, visible exosmosis of erythrocytes | Positive: SMA, Calponin, CD10; negative: S100, CD34, nuclear β-catenin | MYH9–USP6 gene fusion |
| Low-grade fibromyxoid sarcoma | Typically affect young adults, no gender difference | Slow growth, no pain, easy recurrence | Median size, 5 cm (1-20 cm) | Original glue and myxoid region are mixed, spindle cell, small blood vessels, early formation of collagen rosettes | EMA positive from focally to 80%, MUC4 positive has high sensitivity and specificity | FUS-CREB3L2 or FUS-CREB3L1 gene fusion |
| Low-grade myofibroblastic sarcoma | Predominantly in adults, 40-50 yr see more, slight predominance in males | Enlarging mass, painless, easy recurrence | Median size, 4 cm (1.4-17 cm) | Diffusely infiltrative growth, spindle cells arranged in a storiform pattern or fascicles | Positive: actin, desmin, calponin, CD34 (focal); negative: S100, nuclear β-catenin | Only one showed a circular chromosome |
| Low-grade myxofibrosarcoma | Elderly patients, over 60 yr, slight predominance in males | Slowly enlarging, painless, easy recurrence | Larger volume (range variable) | Spindle cells, mild atypia, curvilinear thin-walled blood vessels, pseudolipoblasts | Positive: SMA, negative: Desmin and histiocyte-specific markers | No specific aberration |
Immunohistochemical staining has no specific significance in the identification of NF; however, it can be used as an auxiliary and differential diagnostic tool because spindle cells in NF often diffusely express SMA, and are negative for desmin. Recent studies have shown that USP6 in situ hybridization has higher specificity and sensitivity in the diagnosis of NF[6], particularly in cases with uncharacteristic morphology.
Furthermore, NF can be accurately diagnosed by combining tumor morphological characteristics, immunohistochemical findings, and USP6 detection, thereby avoiding misdiagnosis and overtreatment of patients.
NF poses a diagnostic challenge as it is often mistaken for a sarcoma, or easily misdiagnosed as a sarcomatous lesion such as malignant fibrous histiocytoma or fibrosarcoma, because of its rapid growth, rich cellularity, and poorly circumscribed nature. NF is a tumor with rapid growth and relatively clear boundary, but it is sometimes difficult to distinguish from low-grade sarcoma under the microscope. When the tumor location is atypical and volume is large, the possibility of the disease should also be considered, especially during the operation, which can avoid excessive treatment. Postoperative histopathological examination of whole sections can be combined with immunohistochemical staining and, if necessary, the diagnosis can be confirmed by molecular detection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martínez-Pérez A S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Gao CC
| 1. | Konwaler BE, Keasbey L, Kaplan L. Subcutaneous pseudosarcomatous fibromatosis (fasciitis). Am J Clin Pathol. 1955;25:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 287] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Meister P, Bückmann FW, Konrad E. Nodular fasciitis (analysis of 100 cases and review of the literature). Pathol Res Pract. 1978;162:133-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Kayaselçuk F, Demirhan B, Kayaselçuk U, Ozerdem OR, Tuncer I. Vimentin, smooth muscle actin, desmin, S-100 protein, p53, and estrogen receptor expression in elastofibroma and nodular fasciitis. Ann Diagn Pathol. 2002;6:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 147] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Sinhasan SP, K V B, Bhat RV, Hartimath BC. Intra-muscular Nodular Fasciitis Presenting as Swelling in Neck: Challenging Entity for Diagnosis. J Clin Diagn Res. 2014;8:155-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Mentzel T, Dry S, Katenkamp D, Fletcher CD. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol. 1998;22:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 192] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 9. | Qiu X, Montgomery E, Sun B. Inflammatory myofibroblastic tumor and low-grade myofibroblastic sarcoma: a comparative study of clinicopathologic features and further observations on the immunohistochemical profile of myofibroblasts. Hum Pathol. 2008;39:846-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Doyle LA, Wang WL, Dal Cin P, Lopez-Terrada D, Mertens F, Lazar AJ, Fletcher CD, Hornick JL. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol. 2012;36:1444-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Lääveri M, Heikinheimo K, Baumhoer D, Slootweg PJ, Happonen RP. Periosteal fasciitis in a 7-year old girl: a diagnostic dilemma. Int J Oral Maxillofac Surg. 2017;46:883-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Rankin G, Kuschner SH, Gellman H. Nodular fasciitis: a rapidly growing tumor of the hand. J Hand Surg Am. 1991;16:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Mostofi RS, Soltani K, Beste L, Polak E, Benca P. Intraoral periosteal nodular fasciitis. Int J Oral Maxillofac Surg. 1987;16:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Sato M, Yanagawa T, Yoshida H, Yura Y, Shirasuna K, Miyazaki T. Submucosal nodular fasciitis arising within the buccal area. Report of case. Int J Oral Surg. 1981;10:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | McCarthy EF, Ireland DC, Sprague BL, Bonfiglio M. Parosteal (nodular) fasciitis of the hand. A case report. J Bone Joint Surg Am. 1976;58:714-716. [PubMed] |
| 16. | Johnson MK, Lawrence JF. Metaplastic bone formation (myositis ossificans) in the soft tissues of the hand. Case report. J Bone Joint Surg Am. 1975;57:999-1000. [PubMed] |
| 17. | Goncalves D. Fast growing non-malignant tumour of a finger. Hand. 1974;6:95-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Lumerman H, Bodner B, Zambito R. Intraoral (submucosal) pseudosarcomatous nodular fasciitis. Report of a case. Oral Surg Oral Med Oral Pathol. 1972;34:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Carpenter EB, Lublin B. An unusual osteogenic lesion of a finger. J Bone Joint Surg Am. 1967;49:527-531. [PubMed] |
| 20. | Mallory TB. A Group of Metaplastic and Neoplastic Bone- and Cartilage-Containing Tumors of Soft Parts. Am J Pathol. 1933;9:765-776.3. [PubMed] |