Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1527
Peer-review started: November 14, 2021
First decision: December 9, 2021
Revised: December 27, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 16, 2022
Processing time: 88 Days and 21.4 Hours
Osteoporosis is a systemic bone disease characterized by decreased bone mass, impaired bone mass, and reduced bone strength that leads to increased bone fragility and fracture. Type 2 diabetes mellitus (T2DM) complicated with osteoporosis is a common systemic metabolic bone disease, and reduced bone mass and bone strength are considered the main clinical features; however, the pathogenesis of this disease has not been fully clarified. Its occurrence is considered related to sex, age, and genetic factors. There are many risk factors for diabetes complicated with osteoporosis. Therefore, exploring these risk factors will help prevent it.
To investigate the relationships among serum glucagon-like peptide-1 (GLP-1) levels, matrix Gla protein (MGP) levels, and diabetes with osteoporosis.
Sixty patients with T2DM complicated with osteoporosis confirmed by the endocrinology department of our hospital were selected as the case group. Sixty T2DM patients with bone loss were selected as the control group. Sixty healthy participants were selected as the healthy group. The general data, bone mineral density index, and bone metabolic markers of the three groups were compared. The relationships among GLP-1 levels, MGP levels, and the bone mineral density index of the case group were analyzed using linear correlation analysis and a logistic regression model.
Differences in sex, smoking, and drinking among the case group, control group, and healthy group were not statistically significant (P > 0.05). The mean age of the case group was older than those of the control and healthy groups (P < 0.05). The body mass index, fasting plasma glucose level, HbA1c level, hypertension rate, and coronary heart disease rate of the case and control groups were higher than those of the healthy group (P < 0.05). The serum GLP-1 and MGP levels of the case group were lower than those of the control and healthy groups; these differences were statistically significant (P < 0.05). The serum GLP-1 and MGP levels of the control group were lower than those of the healthy group; these differences were statistically significant (P < 0.05). The serum GLP-1 and MGP levels of the case group were significantly positively correlated with the bone mineral density values of the hip and lumbar spine (P < 0.05). The results of the logistic regression model showed that age and duration of diabetes were independent risk factors for osteoporosis in diabetic patients (P < 0.05) and that increased GLP-1 and MGP values were protective factors against osteoporosis in diabetic patients (P < 0.05).
Serum GLP-1 and MGP levels of diabetic patients with osteoporosis were significantly decreased and positively correlated with bone mineral density and were independent risk factors for osteoporosis in diabetic patients.
Core Tip: Serum glucagon-like peptide-1 (GLP-1) and matrix Gla protein (MGP)levels were significantly positively correlated with bone mineral density values of the hip joint and lumbar vertebrae. They were significantly negatively correlated with type 1 procollagen amino-terminal propeptide, osteocalcin, and special sequence of carboxy-terminal peptide β of type 1 collagen. Older age and duration of diabetes were independent risk factors for osteoporosis for diabetic patients. Increased GLP-1 and MGP levels were protective factors against osteoporosis for diabetic patients. GLP-1 and MGP levels should be used as auxiliary evaluation indexes to evaluate the risk of osteoporosis for patients with diabetes to enable early detection of and intervention for diabetes with osteoporosis and improve its prognosis.
- Citation: Xie FF, Zhang YF, Hu YF, Xie YY, Wang XY, Wang SZ, Xie BQ. Significance of serum glucagon-like peptide-1 and matrix Gla protein levels in patients with diabetes and osteoporosis. World J Clin Cases 2022; 10(5): 1527-1535
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1527.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1527
Type 2 diabetes mellitus (T2DM) is a systemic metabolic disorder that can cause metabolic abnormalities of sugar, lipids, and proteins. It can also cause an imbalance of water and electrolytes and abnormal metabolism of bone minerals, resulting in massive losses of calcium, magnesium, phosphorus, and other trace elements, resulting in osteopenia. Additionally, the relative lack of insulin leads to decreased bone matrix synthesis, thereby causing decreased bone mineral density. Therefore, patients with T2DM are more likely to have osteoporosis[1]. When fracture healing occurs slowly in patients with T2DM, they are prone to infectious complications that have adverse effects on their physical and mental health. Therefore, early detection of and interventions for osteoporosis are particularly essential for patients with T2DM[2].
Glucagon-like peptide-1 (GLP-1) is an endocrine hormone that can stimulate insulin secretion in a glucose-dependent manner and protect islet β cells through various mechanisms. It has been widely used for the diagnosis and treatment of T2DM. Recent studies have suggested that GLP-1 can inhibit bone resorption by promoting the secretion of calcitonin[3]. Matrix Gla protein (MGP) is a circulating protein related to vitamin K that can inhibit calcium and phosphorus deposition and play a role in the regulation of bone metabolism[4]. However, there are few studies of its effect on diabetic patients. This study explored the relationships among serum GLP-1 Levels, MGP levels, and diabetes with osteoporosis.
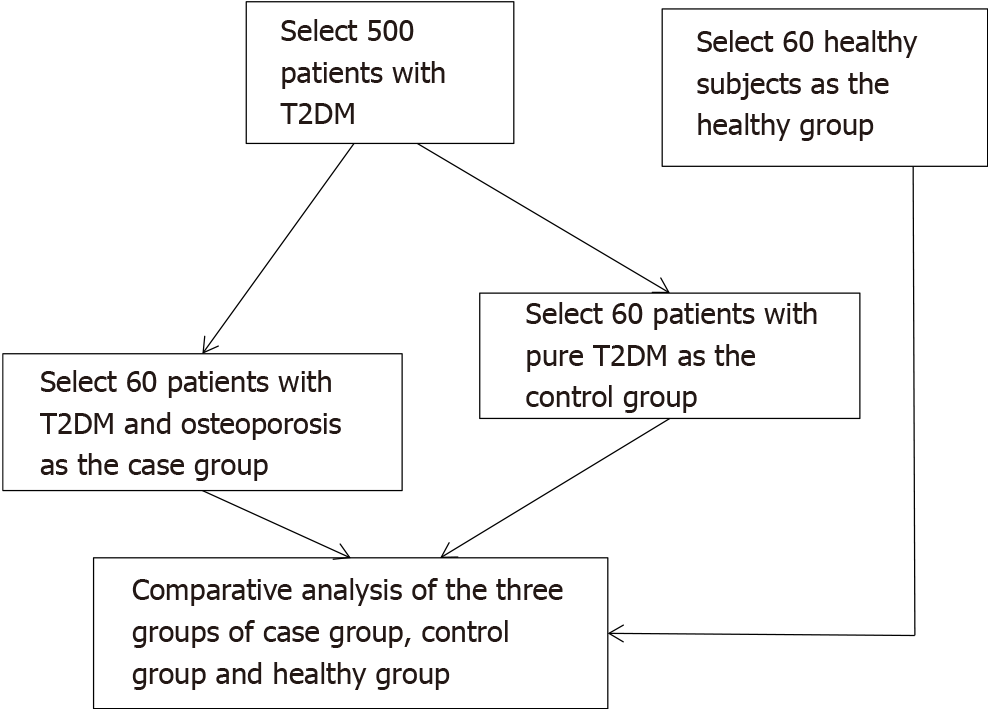
Sixty patients with T2DM complicated with osteoporosis diagnosed by the staff of the endocrinology department of our hospital from April 2019 to January 2020 were selected as the case group. Sixty patients with T2DM complicated with osteopenia were selected as the control group. Sixty healthy participants were selected as the healthy group. The flow chart of the selection of the three groups of study participants is presented in Figure 1. Inclusion criteria were as follows: T2DM diagnosed according to the criteria of the Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2017 edition)[5]; age 52 to 81 years; and bone mass reduction indicated by a bone mineral density T value of 1 to -2.5 (the diagnostic criteria for osteoporosis were a bone mineral density T value < -2.5 and/or one or more fractures)[6]. The healthy group included volunteers who underwent a physical examination. Exclusion criteria were as follows: complicated infectious diseases; thyroid disease; long-term use of hormone drugs; malignant tumors and tuberculosis; and use of drugs related to bone metabolism within 6 mo.
Patients and their families were advised about this study before its implementation and signed informed consent forms. This study was performed after approval was obtained from the medical ethics committee of our hospital. The baseline data of the participants in the three groups are shown in Table 1. The case group and control group had good equilibrium and comparability.
| Factors | Case group (n = 60) | Control group (n = 60) | Healthy group (n = 60) | F/χ2/t | P value |
| Age (yr) | 66.78 ± 5.85 | 61.83 ± 5.36 | 61.92 ± 5.48 | 15.563 | 0.000 |
| Course of disease (yr) | 12.99 ± 4.01 | 8.98 ± 3.83 | - | 5.601 | 0.000 |
| BMI (kg/m2) | 24.52 ± 2.32 | 24.73 ± 2.49 | 23.68 ± 2.05 | 3.510 | 0.032 |
| FPG (mmol/L) | 8.50 ± 1.50 | 8.27 ± 1.24 | 5.77 ± 0.61 | 99.314 | 0.000 |
| HbA1c (%) | 9.18 ± 1.12 | 8.76 ± 1.26 | 5.89 ± 0.70 | 184.683 | 0.000 |
| Sex | 5.896 | 0.052 | |||
| Male | 15 (25.00) | 25 (41.67) | 27 (45.00) | ||
| Female | 45 (75.00) | 35 (58.33) | 33 (55.00) | ||
| Smoking | 2.553 | 0.279 | |||
| Yes | 9 (15.00) | 16 (26.67) | 14 (23.33) | ||
| No | 51 (85.00) | 44 (73.33) | 46 (76.67) | ||
| Drinking | 2.182 | 0.336 | |||
| Yes | 7 (11.67) | 11 (18.33) | 13 (21.67) | ||
| No | 53 (88.33) | 49 (81.67) | 47 (78.33) | ||
| Hypertension | 23.054 | 0.000 | |||
| Yes | 15 (25.00) | 20 (33.33) | 0 (0.00) | ||
| No | 45 (75.00) | 40 (66.67) | 60 (100.00) | ||
| Coronary heart disease | 9.449 | 0.009 | |||
| Yes | 5 (8.33) | 9 (15.00) | 0 (0.00) | ||
| No | 55 (91.67) | 51 (85.00) | 60 (100.00) |
Venous blood samples (2 mL) were extracted from all participants during the morning after fasting for more than 8 h; they were kept at room temperature for 2 h after centrifugation. Serum was obtained after centrifugation at 3000 rpm for 10 min, and GLP-1 and MGP were detected using an enzyme-linked immunoassay. Serum samples were encapsulated, sealed, combined, incubated with the primary antibody, washed, incubated with the secondary antibody, washed, colored, and analyzed. The optical density was read using a 450-nm wavelength and substituted into the standard curve to calculate the concentration (the kit was from Wuhan Youersheng Technology Co., Ltd., Wuhan, China; the test instrument was the Elx88 automatic enzyme label instrument from Bertin Corporation, Rockville, MD, USA).
The bone mineral density values of the lumbar spine (L1-L4) and hip joint and levels of serum bone alkaline phosphatase (BALP), type 1 procollagen amino-terminal propeptide (P1NP), osteocalcin (BGP), and special sequence of carboxy-terminal peptide β of type 1 collagen (β-CTX) of the three groups were measured.
Venous blood samples (5 mL) were extracted from all participants during the morning after fasting for more than 8 h; they were kept at room temperature for 2 h after centrifugation. After centrifugation at 3000 rpm for 10 min, the serum was obtained. BALP, P1NP, BGP, and β-CTX were detected using electrochemiluminescence (the kit was from Beijing Boorsen Biological Co., Ltd., Beijing, China; the detection instrument was the Unicel DxI800 automatic chemiluminescence immunoassay analyzer from Beckman-Coulter, Brea, CA, USA).
All participants underwent testing to determine the bone mineral density values of the lumbar spine (L1-L4) and hip joint. Low-energy and high-energy photon peaks were obtained using an X-ray tube ball, and the data were transferred to the computer for processing and converted to bone mineral density values (DiscoveryA dual-energy X-ray bone mineral density instrument; GE, Madison, WI, USA).
Data processing software SPSS (v21.0; IBM, Cary, NC, USA) was used. The measurement indexes, including body mass index (BMI), fasting plasma glucose (FPG) level, and HbA1c level, during this study were tested using the normal distribution test, and the results were in line with the approximate normal distribution or normal distribution represented by mean ± SD. The t-test or single-factor analysis was used for comparisons between groups, and the least significant difference t-test was used for comparisons at any time point. The enumeration data were analyzed and compared using the χ2 test. The Pearson linear correlation method was used for correlation analyses and the logistic regression model was used for multivariate analyses (α = 0.05).
Differences in sex, smoking, and drinking among the case group, control group, and healthy group were not statistically significant (P > 0.05). The mean age of the case group was higher than those of the control and healthy groups (P < 0.05). The BMI, FPG level, HbA1c level, hypertension rate, and coronary heart disease rate of the case and control groups were higher than those of the healthy group (P < 0.05) (Table 1).
The serum GLP-1 and MGP levels of the case group were lower than those of the control and healthy groups; these differences were statistically significant (P < 0.05). The serum GLP-1 and MGP levels of the control group were lower than those of the healthy group; these differences were statistically significant (P < 0.05) (Table 2).
| Group | n | GLP-1 (pmol/L) | MGP (nmol/L) |
| Case group | 60 | 9.19 ± 1.10 | 7.88 ± 0.92 |
| Control group | 60 | 13.88 ± 1.65 | 9.77 ± 1.52 |
| Healthy group | 60 | 17.07 ± 2.48 | 10.79 ± 1.63 |
| F | 280.527 | 67.487 | |
| P value | 0.000 | 0.000 |
The bone mineral density values of the lumbar vertebrae (L1-L4) and hip joint of the case group were lower than those of the control and healthy groups; these differences were statistically significant (P < 0.05). The bone mineral density values of the lumbar vertebrae (L1-L4) and hip joint of the control group were lower than those of the healthy group; these differences were statistically significant (P < 0.05). The serum P1NP, BGP, and β-CTX levels of the case group were higher than those of the control and healthy group; these differences were statistically significant (P < 0.05). The serum P1NP, BGP, and β-CTX levels of the control group were higher than those of the healthy group; these differences were statistically significant (P < 0.05) (Table 3).
| Group | n | Lumbar spine (g/m2) | Hip joint (g/m2) | BALP (μg/mL) | P1NP (ng/mL) | BGP (μgmL) | β-CTX (ng/mL) |
| Case group | 60 | 0.69 ± 0.08 | 0.66 ± 0.09 | 4.08 ± 1.20 | 49.16 ± 4.08 | 15.92 ± 4.08 | 0.49 ± 0.08 |
| Control group | 60 | 0.95 ± 0.14 | 0.83 ± 0.10 | 4.03 ± 0.84 | 44.41 ± 2.75 | 14.40 ± 2.75 | 0.44 ± 0.08 |
| Healthy group | 60 | 1.08 ± 0.16 | 0.99 ± 0.14 | 4.07 ± 0.82 | 31.59 ± 2.38 | 12.14 ± 2.38 | 0.41 ± 0.10 |
| F | 142.637 | 130.422 | 0.036 | 122.211 | 21.822 | 14.496 | |
| P value | 0.000 | 0.000 | 0.965 | 0.000 | 0.000 | 0.000 |
The serum GLP-1 and MGP levels of the case group were significantly positively correlated with the bone mineral density values of the hip joint and lumbar vertebrae (P < 0.05) (Table 4).
| Index | Relativity | Lumbar spine (g/m2) | Hip joint (g/m2) |
| GLP-1 (pmol/L) | r | 0.707 | 0.691 |
| P value | 0.000 | 0.000 | |
| MGP (nmol/L) | r | 0.571 | 0.546 |
| P value | 0.000 | 0.000 |
The serum GLP-1 and MGP levels of the case group were significantly negatively correlated with the serum P1NP, BGP, and β-CTX levels (P < 0.05) (Table 5).
| Index | Relativity | BALP (μg/mL) | P1NP (ng/mL) | BGP (μgmL) | β-CTX (ng/mL) |
| GLP-1 (pmol/L) | r | 0.192 | -0.401 | -0.386 | -0.377 |
| P value | 0.163 | 0.000 | 0.003 | 0.005 | |
| MGP (nmol/L) | r | 0.155 | -0.421 | -0.419 | -0.351 |
| P value | 0.227 | 0.000 | 0.000 | 0.010 |
The logistic regression model was established with osteoporosis as the dependent variable and BMI, FPG level, HbA1c level, hypertension, coronary heart disease, GLP-1 Level, MGP level, P1NP level, BGP level, and β-CTX level as the independent variables. The results showed that older age and duration of diabetes were independent risk factors for osteoporosis in diabetic patients (P < 0.05) and that increased GLP-1 and MGP levels were protective factors against osteoporosis in diabetic patients (P < 0.05) (Table 6).
| Index | SE | Walds | P value | OR | 95%CI | ||
| Age | 0.551 | 0.225 | 5.997 | 0.007 | 1.735 | 1.116 | 2.697 |
| Course of disease | 0.494 | 0.240 | 4.237 | 0.048 | 1.639 | 1.024 | 2.623 |
| BMI | 0.381 | 0.211 | 3.261 | 0.094 | 1.464 | 0.968 | 2.213 |
| FPG | 0.307 | 0.184 | 2.784 | 0.116 | 1.359 | 0.948 | 1.950 |
| HbA1c | 0.502 | 0.316 | 2.524 | 0.131 | 1.652 | 0.889 | 3.069 |
| Hypertension | 0.484 | 0.408 | 1.407 | 0.248 | 1.623 | 0.729 | 3.610 |
| Coronary heart disease | 0.490 | 0.411 | 1.421 | 0.241 | 1.632 | 0.729 | 3.653 |
| GLP-1 | -0.616 | 0.277 | 4.945 | 0.039 | 0.540 | 0.314 | 0.930 |
| MGP | -0.573 | 0.254 | 5.089 | 0.037 | 0.564 | 0.343 | 0.928 |
| β-CTX | 0.184 | 0.152 | 1.465 | 0.237 | 1.202 | 0.892 | 1.619 |
| BALP | 0.207 | 0.182 | 1.294 | 0.301 | 1.230 | 0.861 | 1.757 |
| BGP | 0.118 | 0.104 | 1.287 | 0.316 | 1.125 | 0.918 | 1.380 |
T2DM is characterized by reduced insulin secretion or decreased insulin sensitivity resulting in elevated blood glucose levels; it is often accompanied by metabolic disorders involving fat, protein, water, and electrolytes[7]. An epidemiological survey found that the worldwide incidence of T2DM exceeded 6.87%. Furthermore, diabetic complications result from disease progression and can cause disability or death[8]. The bone metabolism of patients with osteoporosis is abnormal, and their bone loss is aggravated, thus leading to decreased bone strength and increasing their risk of fractures. If patients with T2DM develop osteoporosis, then their risk of disability is increased, thereby adversely affecting their prognosis[9].
During this study, the lumbar vertebrae (L1-L4) and hip bone mineral density values were grouped and compared with the general data. Patients with T2DM complicated with osteoporosis were older than patients with T2DM complicated with decreased bone mass and healthy participants. The BMI, FPG level, HbA1c level, hypertension rate, and coronary heart disease rate of diabetic patients were higher than those of healthy participants, suggesting that elderly patients with T2DM are more likely to have osteoporosis. Diabetes patients have a higher risk of obesity, hypertension, coronary heart disease, and other complications. P1NP is a peptide secreted by osteoblasts that can sensitively reflect the synthesis rate of type 1 collagen. BGP is a non-collagen acidic glycoprotein synthesized by osteoblasts and chondrocytes. P1NP and BGP are bone formation markers recommended by the International Osteoporosis Foundation[10]. BALP can directly reflect the osteoblast activity, which is the best indicator of human bone mineralization disorders[11]. β-CTX is an index of bone absorption and collagen degradation during bone remodeling[12]. During this study, the serum P1NP, BGP, BALP, and β-CTX levels of the three groups were compared. These levels were higher in patients with T2DM complicated with osteoporosis than in those of patients with T2DM complicated with decreased bone mass and healthy participants. The serum P1NP, BGP, and β-CTX levels of patients with T2DM complicated with bone loss were higher than those of healthy participants, suggesting that diabetic patients with osteoporosis have more severe bone metabolism disorders related to their lack of insulin, increased thyroid hormone secretion, insufficient collagen synthesis, and decreased osteoblast function. The abnormal bone metabolism in diabetic patients is one of the important mechanisms underlying their osteoporosis.
GLP-1 is a hormone secreted by ileal endocrine cells that can protect islet β cells, stimulate insulin secretion, and inhibit glucagon secretion to reduce blood glucose levels. GLP-1 exhibits low expression in patients with T2DM because of the impaired incretin effect[13-15]. GLP-1 receptor agonists promote the secretion of GLP-1 by binding to the receptor, thereby producing a hypoglycemic effect[16]. MGP is a strong inhibitor of calcium and phosphorus deposition that can bind to bone morphogenetic protein-2 to inhibit its biological activity and affect the transformation of mesenchymal cells into chondrocytes or osteoid cells[17-20]. In this study, serum GLP-1 and MGP levels were compared among the three groups. It was found that serum GLP-1 and MGP levels of patients with T2DM complicated with osteoporosis were lower than those of patients with T2DM complicated with bone loss and healthy participants. Furthermore, serum GLP-1 and MG levels of patients with T2DM and bone loss were lower than those of healthy participants, suggesting that decreased serum GLP-1 and MGP levels may be related to osteoporosis in patients with T2DM. GLP-1 can regulate bone metabolism by regulating calcium balance and affecting the proliferation and apoptosis of osteoclasts and osteoblasts. The decrease in serum GLP-1 Levels can lead to abnormal bone metabolism and osteoporosis. MGP is an inhibitor of vascular and cartilage calcification. MGP deficiency can trigger the differentiation of chondrocytes and cartilage formation in the middle layer of blood vessels and affect bone formation.
Furthermore, the correlation analysis results indicated that serum GLP-1 and MGP levels were significantly positively correlated with the bone mineral density values of the hip joint and lumbar vertebrae. The serum GLP-1 and MGP levels were significantly negatively correlated with the serum P1NP, BGP, and β-CTX levels. The results of the logistic regression model indicated that older age and duration of diabetes were independent risk factors for osteoporosis in diabetic patients and that increased GLP-1 and MGP levels were protective factors against osteoporosis in diabetic patients. Future clinical studies should involve osteoporosis screening for elderly patients with long durations of diabetes, and the serum GLP-1 and MGP levels should be used as auxiliary evaluation indexes to evaluate the risk of osteoporosis in patients with T2DM to allow early detection of and intervention for diabetes with osteoporosis and improve the prognosis.
In summary, serum GLP-1 and MGP levels of diabetic patients with osteoporosis were markedly decreased and significantly positively correlated with bone mineral density. Furthermore, they were independent risk factors for osteoporosis in patients with diabetes.
Osteoporosis is a systemic bone disease characterized by decreased bone mass, damaged bone mass, and decreased bone strength, leading to increased bone fragility and fractures. Type 2 diabetes (T2DM) complicated by osteoporosis is a common systemic metabolic bone disease. The reduction of bone mass and bone strength is considered to be the main clinical feature; its occurrence is considered to be related to gender, age and genetic factors.
Explore the risk factors of T2DM complicated with osteoporosis, and provide reasonable guidance for preventing this problem.
This study aimed to investigate the relationships among serum glucagon-like peptide-1 (GLP-1) levels, matrix Gla protein (MGP) levels, and diabetes with osteoporosis.
Sixty T2DM patients with osteoporosis were selected as the case group, and 60 T2DM patients with bone loss were selected as the control group. Sixty healthy subjects were selected as the healthy group for the study.
Serum GLP-1 and MGP levels in diabetic osteoporosis patients are independent risk factors for osteoporosis in diabetic patients.
Serum GLP-1 and MGP levels of diabetic patients with osteoporosis were significantly decreased and positively correlated with bone mineral density and were independent risk factors for osteoporosis in diabetic patients.
Provide for the prevention of osteoporosis in diabetic patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and Metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Botta A, Ward LM S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Elena C, Chiara M, Angelica B, Chiara MA, Laura N, Chiara C, Claudio C, Antonella F, Nicola G. Hyperglycemia and Diabetes Induced by Glucocorticoids in Nondiabetic and Diabetic Patients: Revision of Literature and Personal Considerations. Curr Pharm Biotechnol. 2018;19:1210-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Cortet B, Lucas S, Legroux-Gerot I, Penel G, Chauveau C, Paccou J. Bone disorders associated with diabetes mellitus and its treatments. Joint Bone Spine. 2019;86:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Bird GH, Fu A, Escudero S, Godes M, Opoku-Nsiah K, Wales TE, Cameron MD, Engen JR, Danial NN, Walensky LD. Hydrocarbon-Stitched Peptide Agonists of Glucagon-Like Peptide-1 Receptor. ACS Chem Biol. 2020;15:1340-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Sasaki M, Hasegawa T, Yamada T, Hongo H, de Freitas PH, Suzuki R, Yamamoto T, Tabata C, Toyosawa S, Oda K, Li M, Inoue N, Amizuka N. Altered distribution of bone matrix proteins and defective bone mineralization in klotho-deficient mice. Bone. 2013;57:206-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Cui JY, Zhou RR, Han S, Wang TS, Wang LQ, Xie XH. Statin therapy on glycemic control in type 2 diabetic patients: A network meta-analysis. J Clin Pharm Ther. 2018;43:556-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Pignone M, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:2521-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 430] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 7. | Gortázar AR, Ardura JA. Osteocytes and Diabetes: Altered Function of Diabetic Osteocytes. Curr Osteoporos Rep. 2020;18:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Pothof AB, O'Donnell TFX, Swerdlow NJ, Liang P, Li C, Varkevisser RRB, de Borst GJ, Schermerhorn ML. Risk of insulin-dependent diabetes mellitus in patients undergoing carotid endarterectomy. J Vasc Surg. 2019;69:814-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Minami M, Ikoma K, Horii M, Sukenari T, Onishi O, Fujiwara H, Ogi H, Itoh K, Kubo T. Usefulness of Sweep Imaging With Fourier Transform for Evaluation of Cortical Bone in Diabetic Rats. J Magn Reson Imaging. 2018;48:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Hansen S, Shanbhogue VV, Jørgensen NR, Beck-Nielsen SS. Elevated Bone Remodeling Markers of CTX and P1NP in Addition to Sclerostin in Patients with X-linked Hypophosphatemia: A Cross-Sectional Controlled Study. Calcif Tissue Int. 2019;104:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Maly IP, Eppler E, Müller-Gerbl M. High metabolic activity of tissue-nonspecific alkaline phosphatase not only in young but also in adult bone as demonstrated using a new histochemical detection protocol. Gen Comp Endocrinol. 2018;258:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Vallet S, Hoyle NR, Kyle RA, Podar K, Pecherstorfer M. A role for bone turnover markers β-CrossLaps (CTX) and amino-terminal propeptide of type I collagen (PINP) as potential indicators for disease progression from MGUS to multiple myeloma. Leuk Lymphoma. 2018;59:2431-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Singh P, Taufeeq M, Pesavento TE, Washburn K, Walsh D, Meng S. Comparison of the glucagon-like-peptide-1 receptor agonists dulaglutide and liraglutide for the management of diabetes in solid organ transplant: A retrospective study. Diabetes Obes Metab. 2020;22:879-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Frías JP. Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab. 2020;15:379-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Andrikou E, Tsioufis C, Andrikou I, Leontsinis I, Tousoulis D, Papanas N. GLP-1 receptor agonists and cardiovascular outcome trials: An update. Hellenic J Cardiol. 2019;60:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Granhall C, Donsmark M, Blicher TM, Golor G, Søndergaard FL, Thomsen M, Bækdal TA. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of the Novel Oral Human GLP-1 Analogue, Oral Semaglutide, in Healthy Subjects and Subjects with Type 2 Diabetes. Clin Pharmacokinet. 2019;58:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 17. | Mhalhal TR, Washington MC, Newman KD, Heath JC, Sayegh AI. Combined gastrin releasing peptide-29 and glucagon like peptide-1 reduce body weight more than each individual peptide in diet-induced obese male rats. Neuropeptides. 2018;67:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Buyukterzi Z, Can U, Alpaydin S, Guzelant A, Karaarslan S, Mustu M, Kocyigit D, Gurses KM. Enhanced serum levels of matrix Gla protein and bone morphogenetic protein in acute coronary syndrome patients. J Clin Lab Anal. 2018;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Cai S, Tseng SCG, Zhu YT. Isolation and Expansion of Multipotent Progenitors from Human Trabecular Meshwork. Sci Rep. 2018;8:2814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Kühnisch J, Seto J, Lange C, Stumpp S, Kobus K, Grohmann J, Elefteriou F, Fratzl P, Mundlos S, Kolanczyk M. Neurofibromin inactivation impairs osteocyte development in Nf1Prx1 and Nf1Col1 mouse models. Bone. 2014;66:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |