Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13028
Peer-review started: August 14, 2022
First decision: September 5, 2022
Revised: October 26, 2022
Accepted: November 8, 2022
Article in press: November 8, 2022
Published online: December 16, 2022
Processing time: 115 Days and 19.7 Hours
Multiple myeloma (MM) can be accompanied by amyloidosis, which occurs in a small number of patients and is characterized by deposition of light chains in the joints, leading to multiple myeloma-associated amyloid arthropathy (MAA). As a rare complication of MM, clinical manifestations of MAA are often similar to those of rheumatoid arthritis, and the two are easily confused.
In recent years, our center treated two patients of MM with amyloid arthropathy as the first manifestation, both of whom presented with polyarthritis. After treatment for MM, both patients achieved complete remission. However, subsequently, the two patients underwent hip arthroplasty for femoral neck fractures. Congo red staining and immunofluorescence of the joint tissues confirmed MAA after surgery. Eventually, one of the patients died of MM recurrence, while the other survived.
MAA should be regarded as an initial symptom of MM and should be taken seriously.
Core Tip: Multiple myeloma-associated amyloid arthropathy is easily confused with other types of arthritis when it appears as the first manifestation of MM. Delayed treatment may affect prognosis. We report two cases of MM presenting with amyloid arthropathy as the first manifestation.
- Citation: He C, Ge XP, Zhang XH, Chen P, Li BZ. Multiple myeloma presenting with amyloid arthropathy as the first manifestation: Two case reports. World J Clin Cases 2022; 10(35): 13028-13037
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13028.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13028
Immunoglobulin light chains can be deposited in the joints and bones, leading to amyloid arthropathy, which is mainly observed in monoclonal gammopathies. Approximately 20% of multiple myeloma (MM) cases can be accompanied by immunoglobulin light chain (AL) amyloidosis. Among them, 3.7%-9.2% of patients have AL amyloidosis involving the joints, leading to multiple myeloma-associated amyloid arthropathy (MAA)[1-3]. MAA mainly manifests as symmetric rheumatoid factor-negative non-erosive polyarthritis, which is easily confused with rheumatoid arthritis (RA). In recent years, our department admitted and treated two patients with MM with amyloid arthropathy as the initial manifestation. Here, we have described the two cases.
Case one: A 74-year-old man, developed pain in both knee joints in March 2018, followed by gradual development of pain in both elbows, shoulders, and hips.
Case two: A 68-year-old man visited the orthopedics department in November 2017 due to numbness in his left hand.
Case one: In August 2018, the patient visited the rheumatology department because of increased joint pain. Laboratory examination was negative for rheumatoid factor (RF), anti-cyclic citrullinated peptide antibodies (ACPA), anti-nuclear antibody, anti-neutrophil cytoplasmic antibodies (ANCA), and HLA-B27, while hemoglobin was 99 g/L, serum creatinine 175 μmol/L, serum globulin 38.2 g/L, lactate dehydrogenase 272 U/L, C-reactive protein 58.7 mg/L, and erythrocyte sedimentation rate (ESR) 71 mm/h. According to imaging examinations (see below), the patient was diagnosed with seronegative RA and treated with methylprednisolone, leflunomide, technetium methylene bisphosphate, and triamcinolone acetonide injected into the joint cavities of both knees and shoulders. The patient’s symptoms improved to a certain extent. In November 2018, the patient presented again due to worsening right hip pain.
Case two: His hemoglobin level was 116 g/L, serum creatinine 162 μmol/L, urine protein was positive, and serum globulin and lactate dehydrogenase levels were normal. According to magnetic resonance imaging (MRI) results (see below), the patient was diagnosed with carpal tunnel syndrome and underwent median nerve release and decompression surgery, followed by an improvement in the symptoms. In November 2018, the patient developed numbness and stiffness in both hands and went to the rheumatology department.
Case one: The patient had hypertension for the previous 10 years, which was well controlled by drug therapy.
Case two: The patient had hypertension for the previous 2 years, which was well controlled by drug therapy.
Two patients had no previous or family history of similar illnesses.
Case one: Physical examination revealed tenderness in the shoulders, elbows, and knees with limited mobility, swelling of the knees on both sides with elevated skin temperature, and the right knee joint was more severe.
Case two: Physical examination showed that the metacarpophalangeal joints (MCP) and proximal interphalangeal joints (PIP) of both hands were stiff in a fixed position and unable to flex, along with limited movement of the wrist and knee joints on both sides.
Case one: In November 2018, the patient’s hemoglobin was 93 g/L, serum creatinine 391 μmol/L, serum globulin 28.5 g/L, serum β2-microglobulin 12192.1 ng/mL, with normal lactate dehydrogenase. His 24-hour urine protein was 5.41 g. Immunofixation electrophoresis showed that IgG-λ type M protein accounted for 14.9%, urine λ light chain concentration was 574 mg/L (κ light chain 20.4 mg/L), and peripheral blood λ free light chain concentration was > 735 mg/L (κ free light chain 463 mg/L). Bone marrow aspiration revealed that plasma cells accounted for 62% of the cells. Immunophenotyping suggested that the cells were clonal plasma cells. Karyotype analysis revealed a superdiploid complex karyotype. Fluorescence in situ hybridization (FISH) [including del(17p), IgH translocations, del13, 1q21 gain, and Rb1 deletion] were negative.
Case two: On laboratory examination, RF, ACPA, anti-nuclear antibody, ANCA, HLA-B27 were negative, while hemoglobin was 60 g/L, serum creatinine 560 μmol/L, serum globulin 23.5 g/L, lactate dehydrogenase 156 U/L, and serum β2-microglobulin 11757.8 ng/mL. Serum immunofixation electrophoresis showed κ-type M protein accounted for 6.3%, urine κ light chain concentration was 2610 mg/L (λ light chain was less than the lowest limit), serum κ free light chain > 1940 mg/L, λ free light chain 35.6 mg/L, and к:λ > 54.494. Bone marrow aspiration showed that plasma cells accounted for 70% of the cells. Immunophenotyping suggested that the cells were clonal plasma cells. Chromosomes were normal, and MM-FISH (same as case one) were negative.
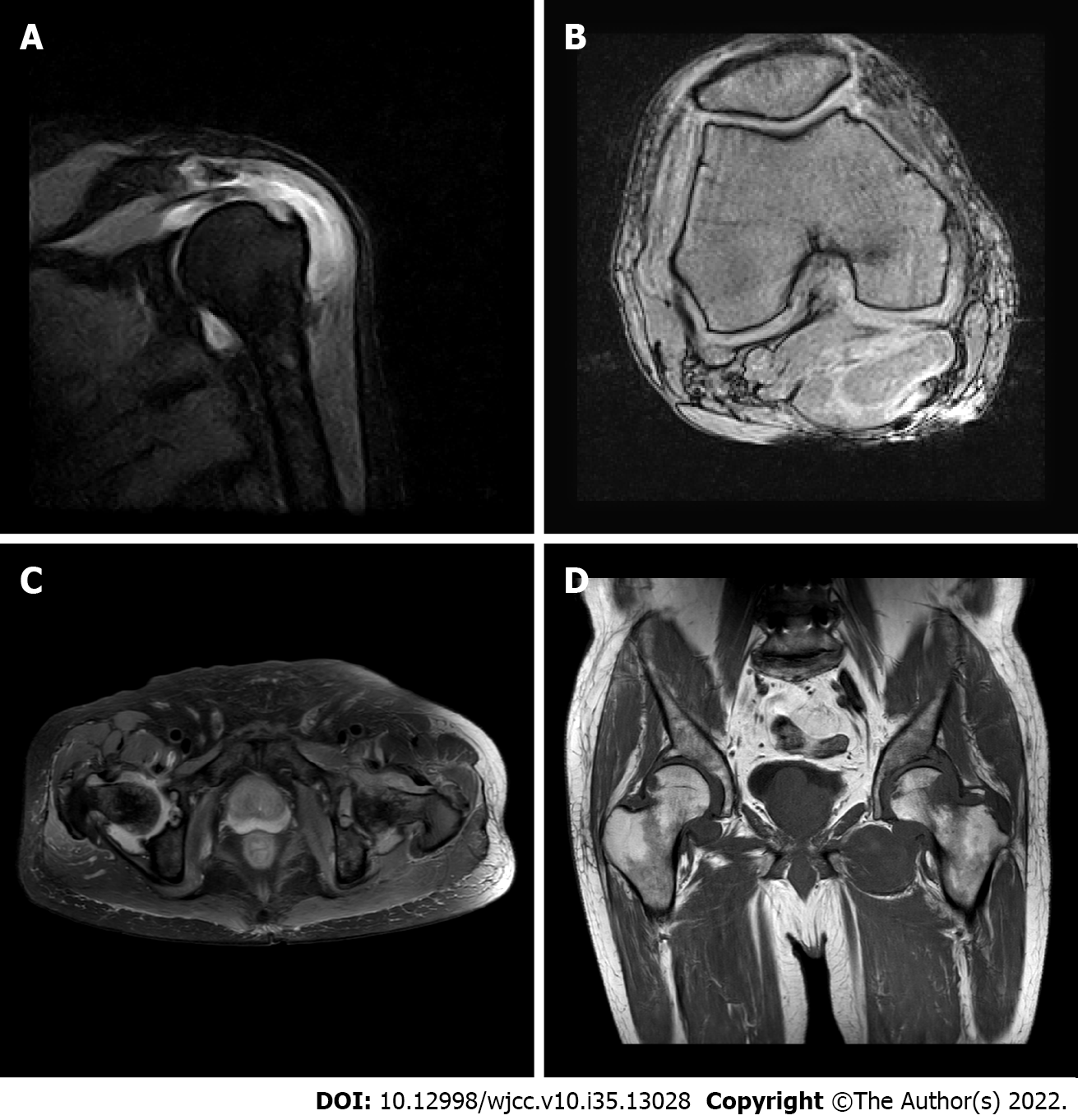
Case one: MRI of the shoulder and knee joints (Figure 1) revealed full-thickness tear of the supraspinatus tendon of the right shoulder, grade II abrasion of the glenohumeral articular cartilage, acromion-deltoid sac, and long head tendon sheath effusion of the biceps tendon, along with a large amount of synovial tissue hyperplasia, coracohumeral ligament degeneration, left shoulder joint acromion-deltoid subcapsule, and biceps tendon long head tendon sheath effusion, accompanied by a large amount of abnormal synovial tissue. The MRI scans also revealed a right knee medial meniscus posterior angle injury grade III, double hip joint capsule slip membrane hyperplasia (obvious on the left side and invading the femoral head and adjacent soft tissue masses), secondary osteoarthritis of both hips, and effusion of the right hip joint. Color Doppler ultrasound of the right shoulder joint showed a small amount of viscous fluid in the articular cavity of the right shoulder joint, bone erosion of the right shoulder joint, severe synovial hyperplasia of the right shoulder joint with severe synovitis, and tenosynovitis of the long head of the right biceps.
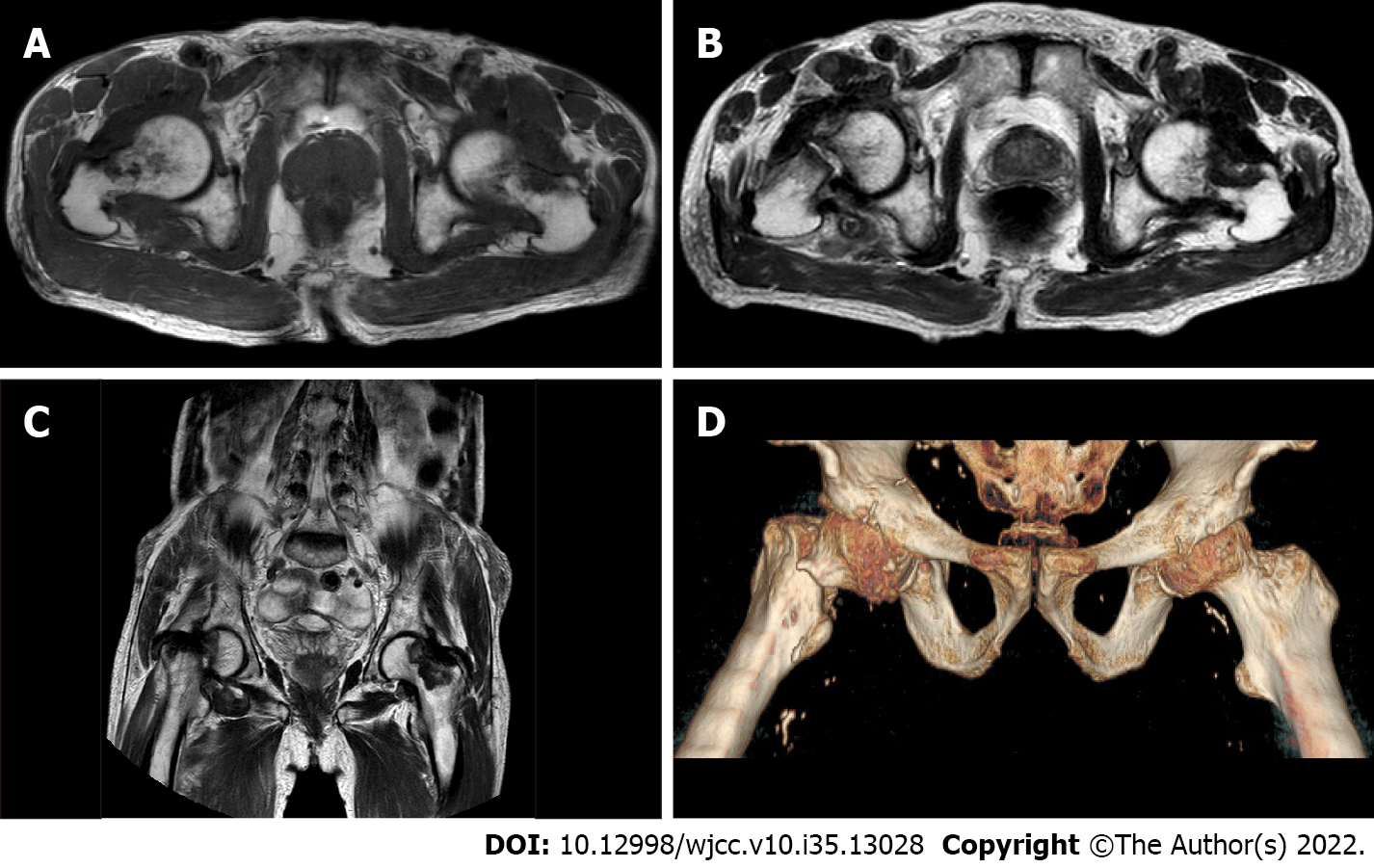
Case two: In November 2017, MRI of the left shoulder and wrist joints (Figure 2) showed partial injury of the left supraspinatus tendon, long head tendon sheath effusion of the biceps, effusion of the acromion-deltoid muscle, bursa effusion around the subscapularis, subcoracoid capsule, glenohumeral joint effusion, left wrist joint effusion with synovial tissue hyperplasia, carpal tunnel horizontal flexor tenosynovitis, and mild edema in the median nerve running area.
In November 2018, MRI of the whole spine and pelvis revealed multiple patchy T2WI/T1WI hyperintensity shadows in each vertebral body and multiple abnormal signal foci in the soft tissue of the pelvis involving bilateral junctions of the femoral head and neck (Figure 3A).
Case one: The patient was diagnosed with multiple myeloma (IgG-λ type, D-S stage IIIB, ISS stage III, R-ISS stage II).
Case two: The patient was diagnosed with multiple myeloma (κ light chain type, D-S stage IIIB, ISS stage III, R-ISS stage II).
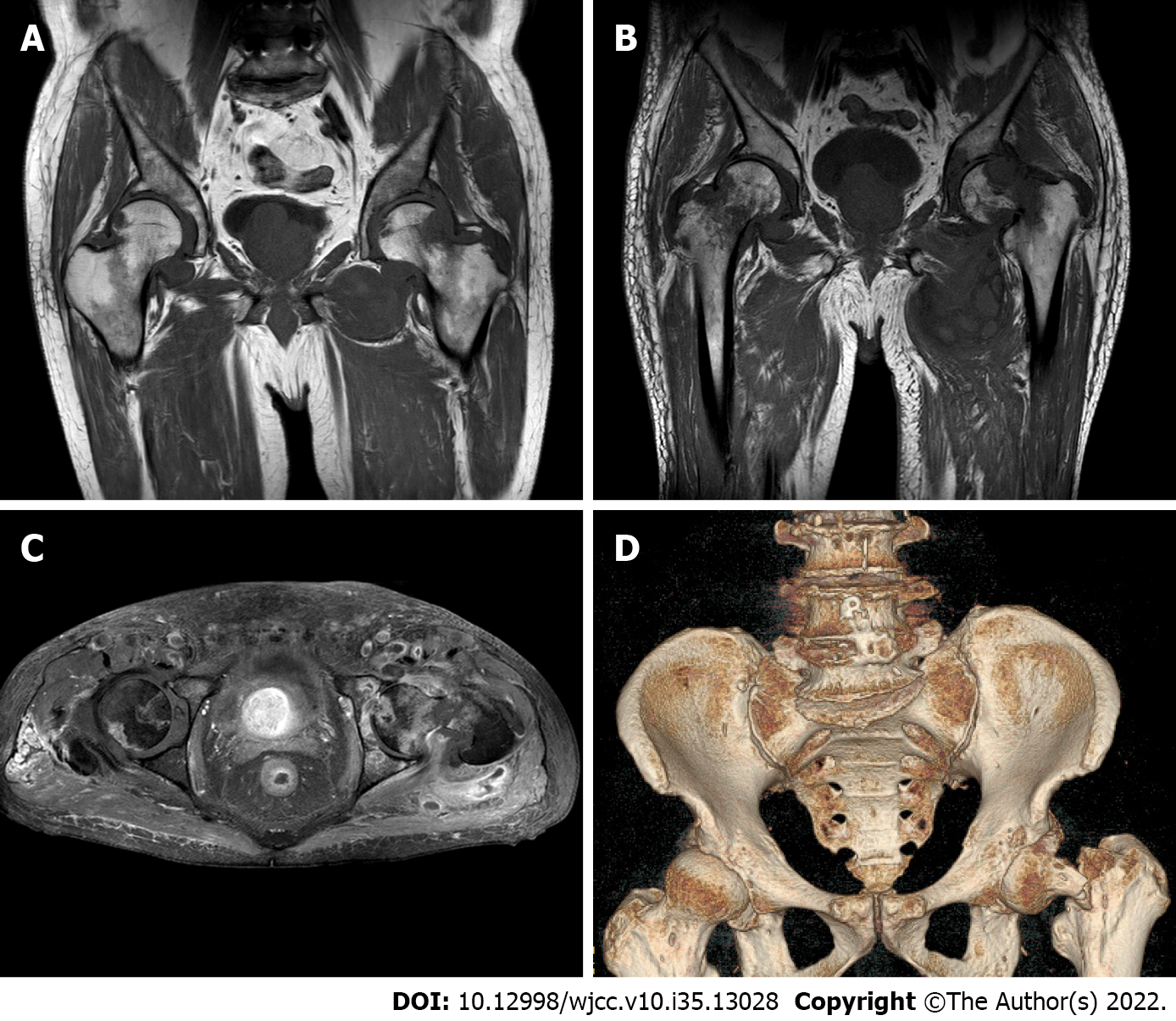
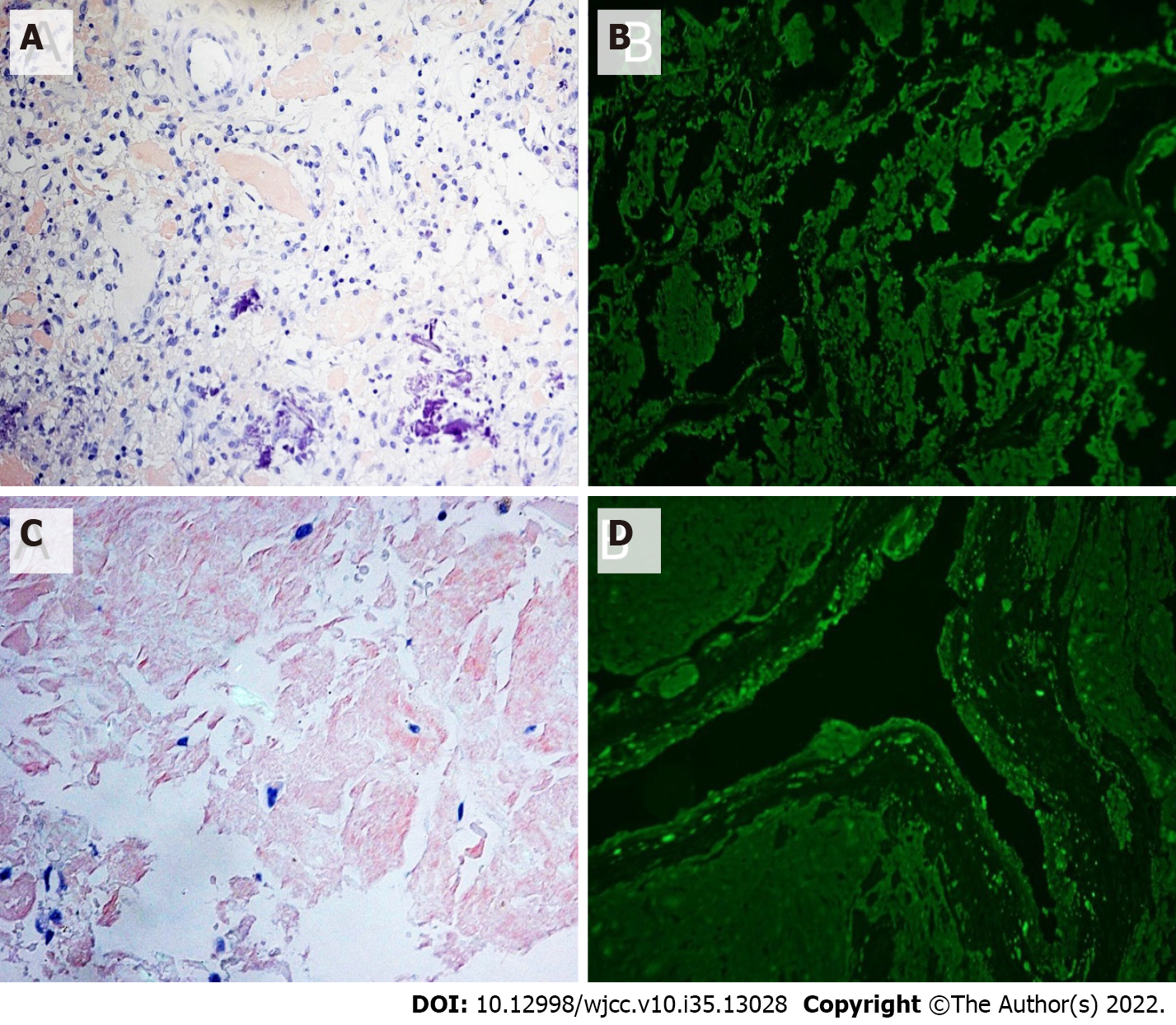
He was administered the VCD (bortezomib + cyclophosphamide + dexamethasone) regimen for 3 cycles. After the last chemotherapy, the disease response (according to the International Myeloma Working Group criteria) reached CR (complete remission), and the serum creatinine level stabilized at 120 μmol/L. After the treatment, the patient's knee joint pain improved. However, symptoms in both hip joints were not alleviated. MRI of the hip joints (Figure 4) revealed an old fracture of the left femoral neck with joint effusion and synovial hyperplasia involving the humeral head and acetabulum; a huge cystic solid mass in the soft tissue space around the left hip joint, which was considered a synovial cyst with pigmented villonodular synovitis that is connected to the joint cavity; abnormal changes in the right femoral neck; right hip joint effusion with synovial hyperplasia; and edema in the soft tissue space around the left hip joint (the gap between the gluteus maximus and gluteus medius). Puncture of the left hip joint demonstrated amyloidosis and fibrogranulation hyperplasia in the tissues. Congo red and crystal violet staining were positive (Figure 5A and B), supporting polyarticular amyloidosis arthropathy. Subsequently, left hip replacement was performed, and the pain improved. Postoperative pathology showed a large amount of amyloid deposition and calcification in the femoral head and synovial tissue. Under polarized light, there was apple green double refraction, while κ light chain was negative and λ light chain was positive. A clear diagnosis of joint amyloidosis was made, and MAA was considered.
The VCD regimen was administered in four cycles. After the last chemotherapy, CR was achieved, numbness and pain in the hands disappeared, joint stiffness was partially improved (MCP and PIP of both hands had limited mobility but returned to a bendable angle of approximately 45°), and the serum creatinine level stabilized at 280 μmol/L. However, the patient developed bilateral hip pain during the third course of treatment, which progressively worsened, mainly on the right side. MRI of both hips (Figure 3B-D) demonstrated a fracture of the right hip and femoral neck with abnormal signal shadow at the broken end, pathological fractures, fluid in the right joint cavity with a large amount of synovial tissue hyperplasia, and abnormal signal shadow on the femoral neck and head of the left hip. Right hip replacement was performed. Postoperative pathology showed that the joint tissue was positive for Congo red staining (Figure 5C and D). Under polarized light, apple green birefringence was observed. On immunofluorescence, k light chain was positive, while lambda light chain was negative (Figure 5C and D). A clear diagnosis of joint amyloidosis was made, and MAA was considered.
The patient's left hip pain was relieved after surgery. Two months later, pain in the right hip joint increased again. MRI revealed a fracture of the right hip joint. The patient underwent a right hip replacement. Pathological analysis showed focal amyloidosis. There was no further treatment after surgery, and the patient died of MM relapse in October 2019.
The patient recovered from the surgery without complications. Later, he completed five follow-up cycles of the VCD regimen and obtained sCR (strictly complete remission) after the 8th course. However, the patient's restricted movement of the joints of both hands did not recover further.
Osteolytic bone lesions in MM patients can involve joints and are often associated with light chain amyloid protein deposition, namely MAA, which is a rare complication of MM. No sex difference was noted in the onset of MAA, and the average age at diagnosis was 58.6 years[4]. Clinical manifestations are nonspecific and are often similar to RA[5]. The main manifestations are morning stiffness, swelling, and tenderness of the soft tissues around the joints with limited joint movement. The shoulder joints are the most commonly affected joints, followed by the knee, wrist, hip, and metacarpophalangeal joints[6]. Some patients undergo fractures due to joint bone damage[4]. The "shoulder pad sign" may appear on imaging, and it may also be accompanied by other manifestations related to amyloid deposition such as carpal tunnel syndrome, macroglossia, soft tissue swelling, and subcutaneous nodules.
A review of relevant data revealed that there were approximately 122 MAA cases reported from countries other than China. The two cases described in this article may be the first reported in China. A study found that 62% of patients had a history of arthritis of 1-84 mo (median 11 mo) before the diagnosis of MM, 30% of the patients were diagnosed early with RA, and only in eight patients, arthritis and MM were diagnosed at the same time[4,7]. The vast majority of MAA cases were reported by rheumatologists or orthopedic surgeons, and only five patients were found by hematologists, including the two patients in this study. Moreover, the two patients in this article still first presented to rheumatology and orthopedics[4]. A general laboratory examination in MAA is nonspecific. One-third of the patients demonstrated elevated ESR, while RF and cyclic citrullinated peptide (CCP) were negative[4,8]. Two-thirds of the patients had renal insufficiency and anemia. The median age of the two patients in this study was 71 years, and the median time from symptom onset to diagnosis was approximately 48 mo. At the time of diagnosis, both had polyarthritis, and all rheumatism-related indicators were negative. Case 1 initially had knee joint pain, which progressed to multiple large joints. Case 2 mainly showed numbness and stiffness of both hands, and gradually developed shoulder, wrist, and hip pain. Although both patients had manifestations of polyarthritis, the first sites were the finger and knee joints, which were slightly different from those reported in the literature. After the two patients were given RA-related treatments (including glucocorticoids), they achieved a certain degree of relief, but the maintenance time was short. Both patients were found to have mild anemia and renal insufficiency at the first visit, but due to the presence of hypertension and normal urine output, they were not taken seriously.
MRI is the most sensitive tool for the early detection of RA lesions, which mainly manifest as synovial thickening, bone marrow edema, and slight articular surface erosion. Previous reports have found that early MRI manifestations of MAA have many similarities with RA. MAA shows low to moderate signal intensity on both T1-and T2-weighted images, nodular synovial soft tissue thickening, subchondral erosion changes, and innervation-related muscle edema. A high T2 signal may be observed in the cyst-like area. The amyloid signal around the joint may be slightly enhanced when the contrast agent is enhanced[4,9,10]. A computerized tomography (CT) scan was consistent with amyloidosis. Typical imaging features include swelling of the soft tissue near the joints, osteoporosis around the joints, and subchondral cystic degeneration with hardened edges[11]. The use of positron emission tomography–computed tomography (PET-CT) in amyloidosis is limited, and only two cases have been reported that showed a moderate increase in the fluorodeoxyglucose uptake at the amyloid deposition site in the tissues around the joints[12]. The two patients in this study were examined by MRI at an early stage, which clearly indicated the presence of synovial hyperplasia, joint effusion, and surrounding inflammation, and is consistent with the literature. In particular, when the two patients in this study were diagnosed with MM, their hip joint MRI showed femoral neck destruction, which is different from that reported in the literature. The underlying reason requires further research.
Synovial biopsy is an important technique for diagnosing MAA, especially when the diagnosis of RA is uncertain. In a study by Elsaman et al[4], 60% of MAA patients were diagnosed by pathological examination of the synovial tissue, while one patient was diagnosed by detection of amyloid in the synovial fluid deposits. Many studies have shown the presence of B cells and plasma cells in the bone marrow and synovial tissues of RA patients, and their roles include antibody production, CD4+ T cell activation, and changing the cytokine profile to promote inflammation[11]. Interestingly, histological analysis of the MAA synovial tissue showed the presence of CD68+ and CD3+ mononuclear inflammatory cells (synovial macrophages) in the synovium and synovial fluid deposits, instead of CD20+ B cells and CD38+ plasma cells. Electron microscopy has revealed that synovial macrophages engulf the amyloid[9]. Elsaman et al[4] further found that in all patients who had undergone synovial biopsy, no plasma cell phenotype was found in any MAA inflammatory infiltrate, and some specimens had plasma cell depletion. This is different from the synovial pathology of RA. This proves that MAA is a chronic synovitis, rather than B cell or plasma cell invasion, and is characterized by mild synovitis without plasma cell infiltration. Although the two patients reported in this article did not undergo a synovial biopsy, they underwent joint biopsy after surgery. Congo red staining was positive with apple-green birefringence under polarized light. Immunofluorescence was positive for κ and λ light chains. These findings confirmed amyloidosis of the joint tissue.
The current treatment of AL amyloidosis is similar to that of MM. Since the number of reported MAA cases is relatively small, there is no uniform treatment regimen. The literature mainly focuses on the treatment of MM. Initially, melphalan and immunomodulator-based regimens were chosen, including MP (melphalan and prednisone) and TD (thalidomide and dexamethasone)[13,14]. As proteasome inhibitors have become the cornerstone of the treatment of MM, new immunomodulators have been developed, such as VCD (bortezomib, cyclophosphamide, and dexamethasone) and VRD (bortezomib, lenalidomide, and dexamethasone). Additionally, other therapies are also used for the treatment of MAA[3,15-18]. In the reports included in this article, 10 patients received bortezomib-based treatment, of which six experienced improvement in the joint symptoms, while four underwent hip replacement due to no improvement or aggravation of symptoms. All patients undergoing joint replacement had symptoms of arthritis for more than 2 years before the diagnosis of MM. Therefore, we infer that the occurrence of fractures may be related to the length of the disease. Three studies reported two patients who used anti-interleukin-6 receptor monoclonal antibody (tocilizumab) and one patient who received anti-human tumor necrosis factor monoclonal antibody (adalimumab) to treat MAA. Interestingly, all three patients showed symptom improvement, and the molecular basis may be related to the involvement of the interleukin-6 receptor and tumor necrosis factor in the pathogenesis of MM[19,20]. In the present study, two patients were treated with the VCD regimen and achieved the ideal curative effect. However, since obvious joint disease was already present at the time of initial diagnosis, and the disease gradually progressed with occurrence of fractures during treatment, the two patients finally had to undergo surgery instead of medical treatment. In this regard, it is recommended that during the initial assessment of MM, attention should be paid to painful joints. If abnormal weight-bearing joints or bones are found, early braking can be used to avoid fractures and improve the patient’s quality of life. No fracture was found in the two patients during the initial diagnosis of MM, but they demonstrated different progression and prognoses in the later period. Although the chromosomal karyotype of Case 1 showed polyploid abnormalities, there were no genetic indicators of poor prognosis related to myeloma; therefore, this cannot be considered as one of the reasons for the poor prognosis.
MM often presents with osteolytic lesions, but involvement of the joints is often associated with light chain amyloid deposition. Amyloid deposition in the joint and surrounding tissues causes arthropathy and is a rare complication of MM. The clinical presentation of MAA lacks specificity and is similar to RA. However, RF and CCP of MAA are usually negative; therefore, MAA in particular should be adequately excluded before the diagnosis of seronegative RA. MAA is characterised by plasma cell tumors and patients should be screened accordingly. In the presence of CRAB symptoms, serum and urine M protein screening can identify the vast majority of plasma cell diseases. Moreover, if patients are positive for the M protein, the likelihood of MM should be further evaluated. MRI can evaluate both arthritis and MM-associated bone lesions. Currently, synovial biopsy is the only means of confirming MAA. In addition, although cases in which MM precedes the diagnosis of MAA are rare in literature reports, secondary MAA should be considered when arthritis manifestations occur in MM patients. In terms of treatment, we still mainly treat multiple myeloma with medications. Surgical intervention is performed only when serious complications occur in the involved joints.
We appreciate all participants associated with this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Du X, China; Giudice MLD, Italy; Ishida T, Japan; Salimi M, Iran S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Goldberg A, Brodsky I, Mccarty D. Multiple myeloma with paramyloidosis presenting as rheumatoid disease. Am J Med. 1964;37:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Gordon DA, Pruzanski W, Ogryzlo MA, Little HA. Amyloid arthritis simulating rheumatoid disease in five patients with multiple myeloma. Am J Med. 1973;55:142-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Alpay N, Artim-Esen B, Kamali S, Gül A, Kalayoğlu-Beşişik S. Amyloid arthropathy mimicking seronegative rheumatoid arthritis in multiple myeloma: case reports and review of the literature. Amyloid. 2009;16:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Elsaman AM, Radwan AR, Akmatov MK, Della Beffa C, Walker A, Mayer CT, Dai L, Nativ S, Rygg M, Atsali E, Saijo K, Ogdie AR, Srinivasulu N, Fathi N, Schumacher HR, Pessler F. Amyloid arthropathy associated with multiple myeloma: a systematic analysis of 101 reported cases. Semin Arthritis Rheum. 2013;43:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Fautrel B, Fermand JP, Sibilia J, Nochy D, Rousselin B, Ravaud P. Amyloid arthropathy in the course of multiple myeloma. J Rheumatol. 2002;29:1473-1481. [PubMed] |
| 6. | Diaz-Perez JA, Conway SA, Zuo Y, Nielsen GP, Selig M, Rosenberg AE. Amyloid Arthropathy: A Review. Adv Anat Pathol. 2021;28:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Yang Y, Chen L, Jia Y, Liu Y, Wen L, Liang Y, An Y, Chen S, Su Y, Li Z. Monoclonal gammopathy in rheumatic diseases. Clin Rheumatol. 2018;37:1751-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Weyand CM, Seyler TM, Goronzy JJ. B cells in rheumatoid synovitis. Arthritis Res Ther. 2005;7 Suppl 3:S9-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Pessler F, Ogdie AR, Mayer CT, Kretzschmar WW, Dai L, Elsaman AM, Einhorn E, Krenn V, Schumacher HR. Amyloid arthropathy associated with multiple myeloma: polyarthritis without synovial infiltration of CD20+ or CD38+ cells. Amyloid. 2014;21:28-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | McQueen FM, Issa S. Is rheumatoid arthritis a B-cell haematological disease with a predilection for the joints? Med Hypotheses. 2014;82:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Askling J, Fored CM, Baecklund E, Brandt L, Backlin C, Ekbom A, Sundström C, Bertilsson L, Cöster L, Geborek P, Jacobsson LT, Lindblad S, Lysholm J, Rantapää-Dahlqvist S, Saxne T, Klareskog L, Feltelius N. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Sabaté-Llobera A, Llinares E, Vallansot R, Landeyro J, Gámez-Cenzano C. Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Amyloid Arthropathy Associated to Multiple Myeloma. J Clin Rheumatol. 2020;26:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Sahin F, Soyer NA, Saydam G, Vural F, Tombuloglu M, Argin M, Ertan Y. Amyloid deposition in knee and ankle joints in the course of multiple myeloma. Joint Bone Spine. 2007;74:209-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Cho YJ, Chun YS, Rhyu KH, Park YK, Ryu KN, Park JS, Liang H, Jung GY, Shin WJ. Amyloid Arthropathy of the Hip Joint Associated with Multiple Myeloma: A Case Report. Hip Pelvis. 2016;28:127-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Khoo HW, Ding CSL, Tandon AA. Radiologic Findings in Polyarticular Amyloid Arthropathy and Myopathy in Multiple Myeloma: A Case Report. Am J Case Rep. 2018;19:1398-1404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Guidelli GM, Bardelli M, Berti G, Selvi E. Amyloid Arthropathy: When the Rheumatologist Meets the Hematologist. J Clin Rheumatol. 2016;22:285-286. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Yamada S, Takahashi W, Maruyama H, Mochizuki K, Yoshida A, Kaya H, Okumura H. [Multiple myeloma diagnosed due to development of amyloid arthritis]. Rinsho Ketsueki. 2019;60:791-796. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Mielke F, Schweigert M. Safe adalimumab therapy for rheumatoid arthritis in a patient with pre-existing multiple myeloma. Nat Clin Pract Rheumatol. 2008;4:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Matsuyama Y, Nagashima T, Honne K, Kamata Y, Iwamoto M, Okazaki H, Sato K, Ozawa K, Minota S. Successful treatment of a patient with rheumatoid arthritis and IgA-κ multiple myeloma with tocilizumab. Intern Med. 2011;50:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Schütz N, Märker-Hermann E. [Rheumatoid arthritis and multiple myeloma as comorbidity. Is tocilizumab a therapy option? Z Rheumatol. 2012;71:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |