Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.12875
Peer-review started: September 20, 2022
First decision: October 21, 2022
Revised: November 13, 2022
Accepted: November 17, 2022
Article in press: November 17, 2022
Published online: December 16, 2022
Processing time: 85 Days and 0.6 Hours
Cardiovascular complications of patients with type 2 diabetes mellitus (T2DM) threaten the health and life of numerous individuals. Recently, growth factor receptor-binding protein 10 (GRB10) was found to play a pivotal role in vascular complications of T2DM, which participates in the regulation of lipid metabolism of T2DM patients. The genetic variation of GRB10 rs1800504 is closely related to the risk of coronary heart disease in patients with T2DM. The development of GRB10 as a key mediator in the association of lipid metabolism with cardiova
Core Tip: Growth factor receptor-binding protein 10 (GRB10) performs a vital role in vascular complications of type 2 diabetes mellitus (T2DM). GRB10 variant may be associated with the blood lipids and then may also related to the risk of coronary heart disease in patients with T2DM. GRB10 is expected to be a potential target for the prevention and treatment of vascular complications in patients with T2DM.
- Citation: Yang Y, Yao HJ, Lin WJ, Huang SC, Li XD, He FZ. Real role of growth factor receptor-binding protein 10: Linking lipid metabolism to diabetes cardiovascular complications. World J Clin Cases 2022; 10(35): 12875-12879
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/12875.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.12875
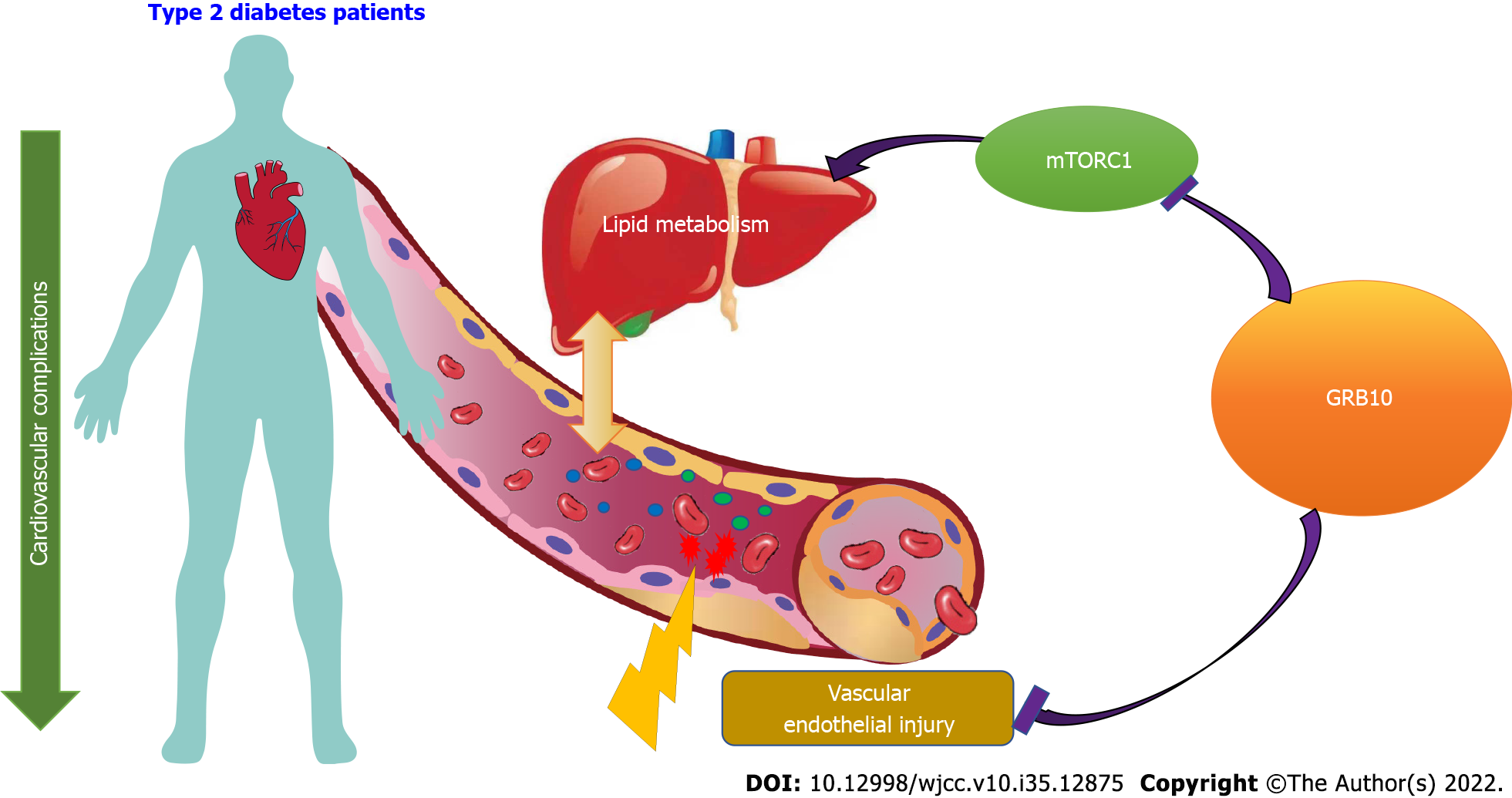
Growth factor receptor-binding (GRB) proteins belong to a class of multi-structural domain adaptor proteins with various cellular functions. Among all GRB proteins, GRB7, GRB10, and GRB14 have similar overall structure, including an N-terminal proline-rich domain, a central pleckstrin-homologous PH domain, a C-terminal SH2 domain, and a family-specific BPS domain (between PH and SH2 domains)[1-3]. GRB10 is an adaptor protein in the GRB7/GRB10/GRB14 protein family. It has three subtypes, GRB10α, GRB10β and GRB10γ, of which GRB10γ subtype has the longest amino acid chain in length, while GRB10α subtype has the shortest amino acid chain in length[4,5]. Molecular biology studies have confirmed that GRB10 can interact with multiple tyrosine kinase receptors and affect a variety of intracellular signaling pathways. In addition, GRB10 plays a key regulatory role in myriad cellular functions such as proliferation, apoptosis and metabolism[6-8]. Besides, Di Paola et al[9] found that the minor allele variant of GRB10 rs4947710 was associated with the low risk of type 2 diabetes mellitus (T2DM) in Caucasian subjects from Italy, while GRB10 rs2237457 gene variant was related to the development of T2DM in the Amish population. A recent study found the GRB10 rs1800504 gene variant was associated with coronary heart disease (CHD) susceptibility in T2DM patients. Relative to the TT genotype, the CC + CT genotype may contribute to a significant increase of CHD incidence in T2DM and the mechanism may be achieved by regulating the levels of circulating blood lipids. In vitro experiments further confirmed that GRB10 rs1800504 genetic variation is related to lipid metabolism in hepatocytes[10]. Therefore, this article will focus on the role and mechanism of GRB10 in regulating vascular endothelial function and lipid metabolism, aiming to clarify the important role of GRB10 in the occurrence and development of vascular complications of T2DM.
Vascular complications of diabetes are the leading reason for death of patients with T2DM, which include macrovascular and microvascular complications (Figure 1). Diabetic macroangiopathy includes cardiovascular disease, cerebrovascular disease and peripheral arterial disease, among which cardiovascular disease is the most common cause of death in diabetic patients[11,12]. One of the major hallmarks of diabetic macrovascular disease is the imbalance of vascular homeostasis caused by dysfunction of endothelial cells and smooth muscle cells, which eventually leads to atherosclerosis and thrombosis[13,14]. Reddy et al[15] discovered that GRB10 a critical downstream mediator of miR-504 in vascular smooth muscle cell (VSMC), and miR-504 was closely associated with VSMC dysfunction in mice with T2DM. Furthermore, as a key regulator of angiogenesis, vascular endothelial growth factor (VEGF) is widely known to be involved in the development of numerous angiogenesis-related disorders. Activation of VEGF receptor leads to recruitment of SH2 containing protein, so VEGF stimulation can increase the expression level of GRB10, while the overexpression of GRB10 leads to the increase of VEGFR2 and tyrosine phosphorylation[16,17]. It is revealed that GRB10 might participate in the positive feedback loop of the VEGF signaling pathway. Therefore, GRB10 may be a key gene in the process of VEGF regulating diabetic vascular complications.
Insulin resistance and lipid metabolism disorder are the main causes of T2DM, and they are also crucial factors leading to diabetic vascular complications. Holt et al[18] found that GRB10 was highly expressed in tissues involving insulin action and glucose metabolism, such as muscle, pancreas and adipose tissue. Relevant data show that severe obesity (Body mass index > 35 kg/m2) can increase the risk of diabetes and coronary heart disease by about two times[19]. Wang et al[20] demonstrated that GRB10 could interact with IGFR to negatively modulate IGF/IGFR signaling pathway. IGF overexpressed in diabetes, and may lead to insulin resistance by blocking insulin signal transduction. Insulin resistance can result in lipid metabolism disorder and obesity. On the contrary, obesity further aggravates insulin resistance. Obesity leads to heterotopic deposition of fat and insufficient insulin signal transduction, followed by insulin resistance, inhibition of hepatic glycogen synthesis and increase of hepatic gluconeogenesis, which eventually leads to abnormal lipid metabolism in peripheral tissues such as adipocytes[21,22]. Insulin can enhance the intake of FFA by adipocytes and the synthesis of triglycerides[23]. However, insulin resistance leads to the increase of FFA, which in turn promotes the occurrence of atherosclerosis[24]. Moreover, Trayhurn et al[25] found that the cytokines [such as tumor necrosis factor-α, interleukin (IL)-1β, IL-6 and plasminogen activator inhibitor-1] released by adipose tissue of T2DM patients can promote chronic inflammation and thrombosis. About 97% of T2DM patients have dyslipidemia, which is closely related to atherosclerosis[26].
GRB10 is closely related to obesity and participates in lipid metabolism. Studies have shown that specific knock-down of GRB10 in peripheral tissues leads to significant overgrowth of mice[20]. The mutation of GRB 10/14 is also related to obesity and insulin resistance[27]. The silenced GRB10 gene can be reactivated by acute ER stress, and its reactivation plays an important role in the early development of hepatic steatosis[28]. A study by Madon-Simon et al[29] found that GRB10 affected the fat ratio, glucose metabolism regulation and lipid storage in the liver. Besides, recent clinical study found that patients with TT genotype of GRB10 rs1800504 had significantly lower levels of cholesterol and low-density lipoprotein cholesterol than patients with CC + CT genotype. Compared to GRB10-WT, the total cholesterol level was notably reduced in GRB10-Mut of MIHA cells[10]. Cold exposure obviously induced the expression of GRB10 in adipose tissues. In addition, fat-specific knockout of GRB10 inhibits the expression of lipogenic and thermogenic genes, reduces energy consumption, and aggravates diet-induced obesity and insulin resistance[30]. In conclusion, GRB10 plays an important role in lipid metabolism.
The mammalian target of rapamycin complex 1 (mTORC1) signaling pathway is an important regulatory pathway for cell growth and metabolism. The deletion of GRB 10 and the over-activation of mTORC1 lead to the increase of insulin over-expression, thus inhibiting the activation of PI3K/Akt signal pathway mediated by insulin. GRB10 negatively regulates insulin and mTORC1 signals in insulin target cells[31,32]. mTORC1 is highly active in tissues of rodents fed an obese and high-fat diet[33]. In vitro, activation of the mTORC1 signaling pathway has been shown to inhibit lipolysis, stimulate lipogenesis, and promote lipid accumulation in cells[34]. On the other hand, inhibition of mTORC1 promotes triacylglycerol glycolysis and the release of free fatty acids, blocking fat production and impairing the maintenance of adipocytes[35,36]. GRB10 can promote the metabolism of thermogenic brown adipose cells, inhibit lipogenesis, regulate the expression of thermogenic genes and energy consumption by negatively regulating the mTORC1 signaling pathway[30]. Therefore, GRB10 may negatively regulate lipid metabolism by inhibiting mTORC1 pathway, and then participate in the pathogenesis of diabetic vascular complications.
T2DM is a major risk factor for cardiovascular disease. In recent years, the therapeutic strategy for T2DM has shifted from lowering blood glucose to reducing target organ damage and improving clinical outcomes for patients. The management of cardiovascular complications in patients with T2DM is widely respected for improving the quality of life for T2DM patients. Here we discussed that GRB10 is associated with the risk of cardiovascular complications in T2DM patients by affecting lipid metabolism of T2DM patients. Its potential mechanism may be mediated through the VEGF and mTORC1 pathway. However, our conclusion needs to be confirmed by more studies. In summary, GRB10 might be a new potential target for the prevention and therapy of vascular complications in T2DM patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dalpatadu KPC, Sri Lanka; Xiao XH, China S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Margolis B, Silvennoinen O, Comoglio F, Roonprapunt C, Skolnik E, Ullrich A, Schlessinger J. High-efficiency expression/cloning of epidermal growth factor-receptor-binding proteins with Src homology 2 domains. Proc Natl Acad Sci U S A. 1992;89:8894-8898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Frantz JD, Giorgetti-Peraldi S, Ottinger EA, Shoelson SE. Human GRB-IRbeta/GRB10. Splice variants of an insulin and growth factor receptor-binding protein with PH and SH2 domains. J Biol Chem. 1997;272:2659-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Ooi J, Yajnik V, Immanuel D, Gordon M, Moskow JJ, Buchberg AM, Margolis B. The cloning of Grb10 reveals a new family of SH2 domain proteins. Oncogene. 1995;10:1621-1630. [PubMed] |
| 4. | Daly RJ. The Grb7 family of signalling proteins. Cell Signal. 1998;10:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Dong LQ, Du H, Porter SG, Kolakowski LF Jr, Lee AV, Mandarino LJ, Fan J, Yee D, Liu F. Cloning, chromosome localization, expression, and characterization of an Src homology 2 and pleckstrin homology domain-containing insulin receptor binding protein hGrb10gamma. J Biol Chem. 1997;272:29104-29112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Holt LJ, Siddle K. Grb10 and Grb14: enigmatic regulators of insulin action--and more? Biochem J. 2005;388:393-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Riedel H. Grb10 exceeding the boundaries of a common signaling adapter. Front Biosci. 2004;9:603-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lim MA, Riedel H, Liu F. Grb10: more than a simple adaptor protein. Front Biosci. 2004;9:387-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Di Paola R, Wojcik J, Succurro E, Marucci A, Chandalia M, Padovano L, Powers C, Merla G, Abate N, Sesti G, Doria A, Trischitta V. GRB10 gene and type 2 diabetes in Whites. J Intern Med. 2010;267:132-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yang Y, Qiu W, Meng Q, Liu M, Lin W, Yang H, Wang R, Dong J, Yuan N, Zhou Z, He F. GRB10 rs1800504 Polymorphism Is Associated With the Risk of Coronary Heart Disease in Patients With Type 2 Diabetes Mellitus. Front Cardiovasc Med. 2021;8:728976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, Standl E, Beulens JW. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 12. | Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1295] [Cited by in RCA: 1309] [Article Influence: 187.0] [Reference Citation Analysis (2)] |
| 13. | Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J. 2013;34:2444-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Ceramides. N Engl J Med. 2019;381:1866-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Reddy MA, Das S, Zhuo C, Jin W, Wang M, Lanting L, Natarajan R. Regulation of Vascular Smooth Muscle Cell Dysfunction Under Diabetic Conditions by miR-504. Arterioscler Thromb Vasc Biol. 2016;36:864-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Giorgetti-Peraldi S, Murdaca J, Mas JC, Van Obberghen E. The adapter protein, Grb10, is a positive regulator of vascular endothelial growth factor signaling. Oncogene. 2001;20:3959-3968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Murdaca J, Treins C, Monthouël-Kartmann MN, Pontier-Bres R, Kumar S, Van Obberghen E, Giorgetti-Peraldi S. Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J Biol Chem. 2004;279:26754-26761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Holt LJ, Brandon AE, Small L, Suryana E, Preston E, Wilks D, Mokbel N, Coles CA, White JD, Turner N, Daly RJ, Cooney GJ. Ablation of Grb10 Specifically in Muscle Impacts Muscle Size and Glucose Metabolism in Mice. Endocrinology. 2018;159:1339-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Zarzour A, Kim HW, Weintraub NL. Understanding Obesity-Related Cardiovascular Disease: It's All About Balance. Circulation. 2018;138:64-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Wang L, Balas B, Christ-Roberts CY, Kim RY, Ramos FJ, Kikani CK, Li C, Deng C, Reyna S, Musi N, Dong LQ, DeFronzo RA, Liu F. Peripheral disruption of the Grb10 gene enhances insulin signaling and sensitivity in vivo. Mol Cell Biol. 2007;27:6497-6505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1127] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 22. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1601] [Article Influence: 400.3] [Reference Citation Analysis (33)] |
| 23. | Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 981] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 24. | Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 1204] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 25. | Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 336] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 26. | Rosenson RS. Clinical role of LDL and HDL subclasses and apolipoprotein measurement. ACC Curr J Rev. 2004;13:33-37. |
| 27. | Desbuquois B, Carré N, Burnol AF. Regulation of insulin and type 1 insulin-like growth factor signaling and action by the Grb10/14 and SH2B1/B2 adaptor proteins. FEBS J. 2013;280:794-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Luo L, Jiang W, Liu H, Bu J, Tang P, Du C, Xu Z, Luo H, Liu B, Xiao B, Zhou Z, Liu F. De-silencing Grb10 contributes to acute ER stress-induced steatosis in mouse liver. J Mol Endocrinol. 2018;60:285-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Madon-Simon M, Cowley M, Garfield AS, Moorwood K, Bauer SR, Ward A. Antagonistic roles in fetal development and adult physiology for the oppositely imprinted Grb10 and Dlk1 genes. BMC Biol. 2014;12:771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Liu M, Bai J, He S, Villarreal R, Hu D, Zhang C, Yang X, Liang H, Slaga TJ, Yu Y, Zhou Z, Blenis J, Scherer PE, Dong LQ, Liu F. Grb10 promotes lipolysis and thermogenesis by phosphorylation-dependent feedback inhibition of mTORC1. Cell Metab. 2014;19:967-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Yea SS, Fruman DA. Cell signaling. New mTOR targets Grb attention. Science. 2011;332:1270-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Cai Z, Liu F, Yang Y, Li D, Hu S, Song L, Yu S, Li T, Liu B, Luo H, Zhang W, Zhou Z, Zhang J. GRB10 regulates β-cell mass by inhibiting β-cell proliferation and stimulating β-cell dedifferentiation. J Genet Genomics. 2022;49:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 34. | Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59:775-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 35. | Polak P, Cybulski N, Feige JN, Auwerx J, Rüegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 399] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 36. | Soliman GA. The integral role of mTOR in lipid metabolism. Cell Cycle. 2011;10:861-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |